Figure 5.
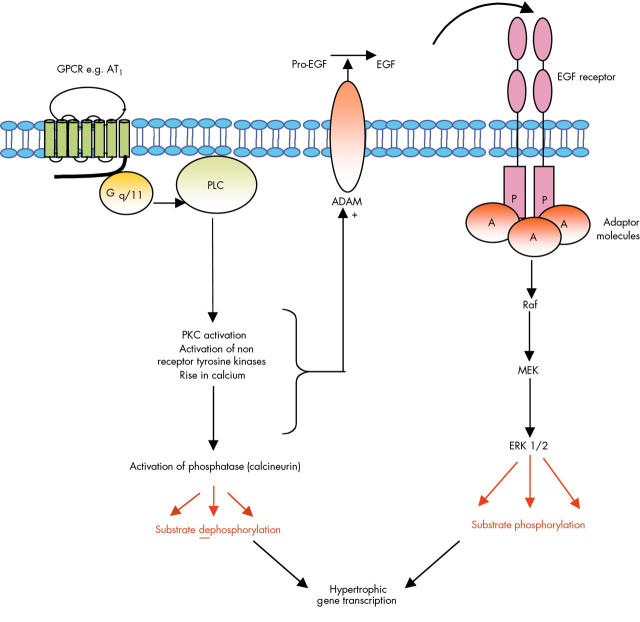
Kinases and phosphatases contribute to cardiac myocyte hypertrophy. Raised concentrations of angiotensin II, a powerful vasoconstrictor, have been implicated in a number of pathologies associated with hypertension including atherosclerosis and cardiac hypertrophy.16 In the heart, angiotensin II acts through the G protein Gq/11 to stimulate phospholipase C (PLC) and generate IP3 and DAG. These events increase the intracellular concentration of calcium and activate PKC. Non-receptor tyrosine kinases such as Src and Pyk2 are also implicated in the hypertrophic response to angiotensin II, along with activation of the calcium dependent phosphatase calcineurin. GPCRs in a number of cardiovascular cells can make extensive use of growth factor receptors to initiate signalling programmes and this is particularly evident in cardiac myocytes where several of these intermediary molecules are involved in controlling angiotensin II mediated transactivation of the epidermal growth factor (EGF) receptor. Current evidence suggests that AT1 receptor mediated transactivation of the EGF receptor on cardiac myocytes involves stimulation of the activities of a family of membrane associated metalloprotease enzymes (ADAMs). These ultimately cleave EGF receptor ligands (such as heparin binding EGF) from their membrane associated precursors which releases them for interaction with their receptors. Stimulation of EGF receptors then triggers activation of several other signalling pathways in the myocytes including the MAP kinases. Phosphatase and kinase activities regulate a number of cytosolic and nuclear phosphorylation events which together control the hypertrophic gene transcription responsible for driving ventricular hypertrophy.