Abstract
As hypertrophic cardiomyopathy is a common cause of sudden deaths among athletes, differentiating this condition from the non-pathological “athlete’s heart” presents an important challenge
Keywords: cardiomyopathy, athletes, echocardiography, sudden death
Sudden unexpected deaths of young trained athletes are highly visible events with substantial impact on the physician and lay communities.1 Although a focal point first in the USA in the early 1980s,2 this issue has now become one of increasing concern in many parts of the industrialised world (particularly Europe).3,4 The visibility accorded this medical issue is underscored by the highly publicised sudden deaths of several elite professional athletes, including Hank Gathers and Reggie Lewis in the USA, and more recently several athletes from Europe, South America, and Africa including Marc-Vivian Foe (Cameroon; soccer) who was known to have hypertrophic cardiomyopathy (HCM).5
IMPLICATIONS OF DIFFERENTIATING HEART DISEASE FROM ATHLETE’S HEART
Interest in athletic field deaths has also accelerated because of the recognition that these catastrophic events are probably more common than previously thought, occur in young people of both sexes and under-served minorities, and that the responsible but usually unsuspected structural cardiovascular diseases are often clinically identifiable—including by pre-participation screening.4 Furthermore, application of the implantable cardioverter-defibrillator to selected high risk patients has made diagnosis of these diseases, and disqualification from certain sports,1,6–8 particularly relevant.
The cardiac diseases that predispose athletes to lethal ventricular tachyarrhythmias have been documented in several necropsy based studies.1,3,9,10 In the USA, the single most common cause of athletic field deaths is HCM, accounting for about one third.1,3,9 Consequently, differential diagnosis between HCM and physiologic non-pathologic left ventricular (LV) hypertrophy associated with systematic training (commonly referred to as “athlete’s heart”) has become a not uncommon clinical dilemma.11 This diagnostic distinction has important implications since identification of cardiovascular diseases associated with sudden death may be the basis for disqualification from competition to minimise risk.8 Conversely, improper over-diagnosis of cardiac disease may lead to unnecessary withdrawal from athletics, thereby depriving that individual of the varied psychological and sometimes economic benefits of competitive sports. Interest in application of non-invasive markers that may resolve such differential diagnosis has increased with the greater visibility of athletic field deaths1–3,5 and focus on pre-participation screening in both the USA and Europe.3,5 Furthermore, legal liability incurred by evaluating competitive athletes with cardiovascular disease (presently most substantial in the USA) has raised the general level of anxiety surrounding these clinical situations.
STRATEGIES FOR DIFFERENTIAL DIAGNOSIS
An algorithm for differential diagnosis between HCM (with mild phenotypic expression) and extreme physiologic hypertrophy of athlete’s heart has evolved, with particular relevance to trained athletes in whom left ventricular (LV) wall thickness falls into a “grey zone” of overlap between these clinical scenarios (fig 1)—that is, ventricular septal thickness of 13–15 mm present in 2% of highly trained male athletes.1,11 Of note, this clinical distinction is relevant only to non-obstructive HCM under resting (basal) conditions, in which mitral valve systolic anterior motion (SAM) is absent, since SAM is inconsistent with athlete’s heart and strongly suggests HCM.
Figure 1.
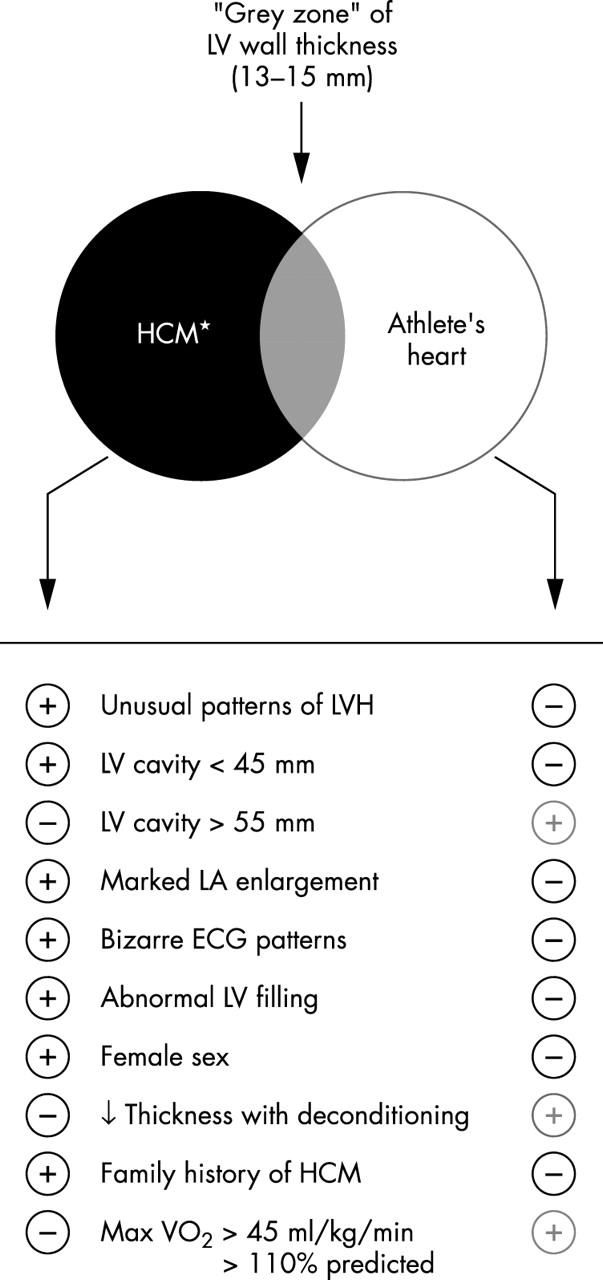
Criteria used to distinguish hypertrophic cardiomyopathy (HCM) from athlete’s heart when the left ventricular (LV) wall thickness is within the shaded “grey zone” of overlap, consistent with both diagnoses. ↓ indicates decreased; LA, left atrial; LVH, left ventricular hypertrophy. Reproduced from Maron et al,11 with permission of American Heart Association.
Genetic testing
The most definitive resolution of this important differential diagnosis can come from genetic testing. Indeed, a rapid genetic test is now available,12 analysing by direct DNA sequencing mutations in the eight most common HCM causing genes. While a positive test result in an athlete can resolve the diagnostic ambiguity between athlete’s heart and HCM, there is however significant potential for false negative test results in which a HCM diagnosis cannot be excluded.
Cavity dimension
In lieu of genetic testing, other echocardiographic parameters may be useful to distinguish between athlete’s heart and HCM, including LV end diastolic cavity enlargement > 55 mm. LV dimensions in this range are common in trained athletes, but rare in HCM in which cavity size is often < 45 mm and dimensions of > 55 mm usually occur only in the end stage phase with progressive heart failure and systolic dysfunction.1 Therefore, in some instances, LV cavity dimension is useful in distinguishing athlete’s heart from HCM.
LV filling
Most HCM patients show abnormal pulsed or tissue Doppler diastolic indexes of LV filling, independent of whether heart failure or outflow obstruction are present.1 In contrast, trained athletes invariably demonstrate normal LV filling patterns.1 Consequently, in a trained athlete suspected of having HCM, a distinctly abnormal Doppler filling pattern supports this diagnosis, while a normal study is consistent with either HCM or athlete’s heart.
Sex
Sex differences in cardiac dimensions are often relevant to differential diagnosis. Highly trained female athletes rarely show absolute LV wall thicknesses > 11 mm.1 Indeed, of 600 elite women athletes, none had LV thickness in the range compatible with the diagnosis of HCM (⩾ 13 mm).13 Therefore, female athletes presenting with “grey zone” LV wall thickness of 13–15 mm (and normal cavity size) are most likely to have HCM.
Deconditioning
Forced detraining may be a useful manoeuvre to resolve differential diagnosis between athlete’s heart and HCM. Elite athletes with LV hypertrophy may show reduction in wall thickness (of about 2–5 mm) over short deconditioning periods (about three months).1 However, identification of such changes in wall thickness with deconditioning requires: (1) substantial compliance to interrupt the training of highly motivated athletes; and (2) serial echocardiographic or magnetic resonance imaging studies of technical quality suitable for comparison. Decreased LV wall thickness associated with deconditioning substantiates that LV hypertrophy is a physiologic consequence of athletic training, as such changes in wall thickness are inconsistent with pathologic hypertrophy of HCM.14
Type of sport training
The specific nature of athletic training has a major influence on LV dimension. For example, rowing sports and cycling are most commonly associated with LV wall thickness ⩾ 13 mm. Conversely, isometric (or power) sports, such as weightlifting or wrestling, are not associated with absolute increases in LV wall thickness > 12 mm.15 Therefore, in assessing whether an athlete with LV hypertrophy has HCM, detailed knowledge of the training regimen is important.
In this issue of Heart, Anastasakis et al16 use cardiopulmonary exercise testing to differentiate between HCM patients and elite male athletes. These authors specifically offer novel observations regarding endurance compared to strength training in athletes without structural heart disease. Cardiopulmonary exercise parameters, such as peak oxygen consumption, reliably distinguished HCM from endurance athletes (middle/long distance runners), but not from strength trained athletes (weightlifters). This observation adds an important and much needed piece of information to this area of investigation, contributing a measure of clarity to clinical strategies for differentiating pathologic from physiologic hypertrophy.
CONCLUSIONS
In highly trained athletes, it is often critically important to determine whether LV hypertrophy is an expression of a physiologic and benign adaptation to training or rather a pathologic process with risk for sudden death, such as HCM. Because there is presently no single testing approach available that will definitively resolve this question in all such athletes, several non-invasive strategies have evolved, including that reported here by Anastasakis et al16 which offers a measure of resolution to this compelling diagnostic dilemma, so important to the highly visible problem of sudden death in young competitive athletes.
Abbreviations
HCM, hypertrophic cardiomyopathy
LV, left ventricular
SAM, systolic anterior motion
I have no competing interests to declare.
REFERENCES
- 1.Maron BJ. Sudden death in young athletes. N Engl J Med 2000;349:1064–75. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ. Sudden death in young athletes: lessons from the Hank Gathers affair. N Engl J Med 1993;329:55–7. [DOI] [PubMed] [Google Scholar]
- 3.Corrado D, Pelliccia A, Bjørnstad HH, et al. Cardiovascular preparticipation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Eur Heart J 2005;26:516–24. [DOI] [PubMed] [Google Scholar]
- 4.Corrado D, Basso C, Schiavon M, et al. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med 1998;339:364–9. [DOI] [PubMed] [Google Scholar]
- 5.Maron BJ. How should we screen competitive athletes for cardiovascular disease? [editorial]. Eur Heart J 2005;26:428–30. [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ, Shen W-K, Link MS, et al. Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med 2000;342:365–73. [DOI] [PubMed] [Google Scholar]
- 7.Corrado D, Leoni L, Link MS, et al. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2003;108:3084–91. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ, Zipes DP. 36th Bethesda Conference: eligibility recommendations for competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol 2005;45:1312–75. [DOI] [PubMed] [Google Scholar]
- 9.Maron BJ, Shirani J, Poliac LC, et al. Sudden death in young competitive athletes: Clinical, demographic and pathological profiles. JAMA 1996;276:199–204. [PubMed] [Google Scholar]
- 10.Maron BJ, Carney KP, Lever HM, et al. Relationship of race to sudden cardiac death in competitive athletes with hypertrophic cardiomyopathy. J Am Coll Cardiol 2003;41:974–80. [DOI] [PubMed] [Google Scholar]
- 11.Maron BJ, Pelliccia A, Spirito P. Cardiac disease in young trained athletes. Insights into methods for distinguishing athlete’s heart from structural heart disease, with particular emphasis on hypertrophic cardiomyopathy. Circulation 1995;91:1596–601. [DOI] [PubMed] [Google Scholar]
- 12.Maron BJ, Seidman JG, Seidman CE. Proposal for contemporary screening strategies in families with hypertrophic cardiomyopathy. J Am Coll Cardiol 2004;44:2125–32. [DOI] [PubMed] [Google Scholar]
- 13.Pelliccia A, Maron BJ, Culasso F, et al. Athlete’s heart in women: echocardiographic characterization of highly trained elite female athletes. JAMA 1996;276:211–15. [DOI] [PubMed] [Google Scholar]
- 14.Sarto P, Merlo L, Noventa D, et al. Electrocardiographic changes associated with training and discontinuation of training in an athlete with hypertrophic cardiomyopathy. Am J Cardiol 2004;93:518–19. [DOI] [PubMed] [Google Scholar]
- 15.Pelliccia A, Maron BJ, Spataro A, et al. Absence of left ventricular hypertrophy in athletes engaged in intense power training. Am J Cardiol 1993;72:1048–54. [DOI] [PubMed] [Google Scholar]
- 16.Anastasakis A, Kotsiopoulou C, Rigopoulos A, et al. Similarities in the profile of cardiopulmonary exercise testing between patients with hypertrophic cardiomyopathy and strength athletes. Heart 2005;91:1477–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


