Abstract
Objective: To identify fetal echocardiographic features, associations, and outcome of atrioventricular and ventriculoarterial discordance.
Design: Retrospective analysis of videotape recordings of fetal echocardiograms and examination of departmental database and patient records to establish outcome.
Setting: Tertiary centre for fetal cardiology.
Patients: 34 consecutive fetuses with a prenatal diagnosis of double discordance seen between January 1993 and December 2003.
Main outcome measures: Echocardiographic features including reversed differential insertion, identification of the right ventricle through the moderator band, and the origin and orientation of the great arteries. All associated cardiac abnormalities and outcome.
Results: Reversed differential insertion was identified in 26 fetuses. The right ventricle could be identified by the moderator band in 30 fetuses and was left sided in all but one. A parallel or abnormal orientation of the great arteries was seen in all fetuses. Only five of 34 cases were isolated from a cardiac viewpoint. There was a ventricular septal defect in 21, Ebstein’s anomaly in 8, pulmonary stenosis in 12, coarctation of the aorta in 6, tricuspid atresia in 2, congenital heart block in 2 fetuses prenatally, and dextrocardia in 6. Nineteen babies survived; one baby died in the neonatal period and three in infancy. Eleven pregnancies were interrupted.
Conclusions: A left sided or posterior right ventricle, identified by reversed differential insertion or the moderator band, in association with abnormal orientation of the great arteries should alert a sonographer to the diagnosis of double discordance. Isolated cases are rare prenatally (14%) but the short term survival in our fetal series is good. Where pregnancy was continued, 19 of 23 babies (82%) remained alive.
Keywords: corrected transposition, double discordance, fetal heart malformation, fetal heart defect
Atrioventricular and ventriculoarterial discordance, also known as congenitally corrected transposition of the great arteries (CCTGA), is a complex type of heart lesion that accounts for less than 1% of infants with congenital heart disease.1–3 One of the original descriptions of CCTGA was by Karl von Rokitansky in 1875,4 who described the hearts of a 4 month old girl and an 11 month old boy. In these hearts the great arteries were transposed, but the ventricles and the ventricular septum were arranged such that the transposition was physiologically corrected by allowing venous blood to enter the pulmonary artery and arterial blood to enter the aorta.
This type of cardiac malformation encompasses a very heterogeneous group of abnormalities, but the characteristic feature of all cases is discordance at both the atrioventricular and ventriculoarterial junctions. This double discordance results in a physiologically corrected circulation, but the left ventricle supports the pulmonary circulation and the right ventricle supports the systemic circulation. The goal of this study was to highlight features of CCTGA that should be recognisable during fetal heart scanning to improve the prenatal detection of this condition. In addition, the spectrum of this abnormality seen during fetal life has been documented and the outcome associated with both the isolated cases and cases occurring with other cardiac malformations has been evaluated.
METHODS
This study was a retrospective analysis and audit of data and echocardiograms already performed for clinical reasons, so ethical approval was not necessary.
Patient population
The study population was selected from patients referred to a tertiary centre for fetal cardiology between January 1993 and December 2003. During this period, 15 861 pregnant women at high risk of having a baby with congenital heart disease were scanned in our unit and structural congenital heart disease was diagnosed prenatally in 1913 fetuses. In 34 fetuses (1.8%) seen sequentially, CCTGA was diagnosed prenatally and these form the study group. The majority of fetuses, 31 of 34 (91%), were referred because of a suspected cardiac abnormality noted during an obstetric anomaly scan. One fetus was referred because of a family history of congenital heart disease, one because of maternal diabetes, and one because of an increased nuchal translucency measurement. The median gestational age at presentation was 20 weeks (range 15–31 weeks). In 28 of 34 fetuses (82%) the diagnosis was confirmed either postnatally or, where pregnancy was interrupted, at post mortem examination. However, for six fetuses, parental permission for necropsy was refused. During the study period a complex cardiac abnormality was diagnosed prenatally in two fetuses who had CCTGA diagnosed as part of the complex abnormality postnatally. These two fetuses were excluded from the study.
Echocardiographic features
The fetal echocardiograms were recorded by a Toshiba SSA 270A ultrasound system (Toshiba Medical Systems, Crawley, UK) and Hewlett Packard 1000, 2000, and 5500 ultrasound systems (Philips Inc, Andover, Massachusetts, USA). The videotape recordings of all fetal echocardiograms were analysed retrospectively to document the features of CCTGA that were identifiable on the scan images and that had helped to make the original diagnosis. These features were sought in both the four chamber and great artery views. In the four chamber view this included reversed differential insertion of the atrioventricular valves and identification of the moderator band of the right ventricle to help recognition of this chamber. The atrioventricular valves are differentially inserted because the setting of the atrioventricular valves is more apical in the right ventricle. The phrase “reversed differential insertion” is used here to indicate that the more apically positioned atrioventricular valve, although still a marker of the right ventricle, is not in the correct position. The origin and orientation of the great arteries were also documented.
The cardiothoracic ratio was measured retrospectively by on-screen calibration of a still frame of the video record. The angle between the cardiac axis and the midline was also measured to assess the cardiac position. The cardiothoracic ratio usually increases slightly with advancing gestation, with the 95th centile increasing from 0.5 at 18 weeks to 0.55 at term.5 The normal axis lies at a 45° angle (range 22–75°) to the left of an anteroposterior line drawn from the spine to the anterior chest wall.6
The situs had been determined at the time of the original scan by establishing left and right in the fetus, noting the position of the heart and the arrangement of the aorta and inferior vena cava in the abdomen.
Other abnormalities
Additional cardiac abnormalities and any associated extracardiac abnormalities were documented.
Outcome
The outcome data were derived from our extensive departmental database supplemented with information from postnatal echocardiograms and retrospective examination of postmortem reports.
Statistical analysis
The statistical analysis in this study was limited to descriptive statistics.
RESULTS
Tables 1–4 summarise the results.
Table 1.
Gestational age, referral reason, cardiothoracic ratio (CTR), and cardiac position in 34 fetuses
| Patient | Gestational age (weeks) | Referral reason | CTR | Heart angle (in degrees left or right midline) |
| 1 | 22 | ?CHD | 0.49 | 40° left |
| 2 | 23 | ?CHD | 0.47 | 53° left |
| 3 | 19 | ?CHD | 0.49 | 25° right |
| 4 | 19 | ?CHD | 0.4 | 60° left |
| 5 | 20 | ?CHD | 0.49 | 40° right |
| 6 | 17 | ?CHD | 0.49 | 0 |
| 7 | 20 | ?CHD | 0.5 | 55° left |
| 8 | 22 | ?CHD | 0.46 | 40° left |
| 9 | 19 | ?CHD | 0.49 | 50° left |
| 10 | 21 | ?CHD | 0.48 | 45° right |
| 11 | 20 | ?CHD | 0.51 | 50° left |
| 12 | 18 | ?CHD | 0.44 | 30° right |
| 13 | 21 | ?CHD | 0.45 | 60° left |
| 14 | 17 | ?CHD | 0.48 | 40° right |
| 15 | 22 | ?CHD | 0.51 | 24° left |
| 16 | 26 | ?CHD | 0.53 | 50° left |
| 17 | 31 | ?CHD | 0.6 | 55° left |
| 18 | 20 | ?CHD | 0.58 | 42° left |
| 19 | 24 | ?CHD | 0.58 | 40° left |
| 20 | 20 | ?CHD | 0.45 | 36° right |
| 21 | 23 | ?CHD | 0.45 | 65° left |
| 22 | 15 | FH | 0.51 | 60° left |
| 23 | 18 | ?CHD | 0.51 | 35° left |
| 24 | 20 | ?CHD | 0.49 | 50° left |
| 25 | 21 | ?CHD | 0.43 | 50° left |
| 26 | 21 | ?CHD | 0.44 | 60° left |
| 27 | 21 | ?CHD | 0.47 | 40° left |
| 28 | 20 | ?CHD | 0.47 | 30° left |
| 29 | 20 | ?CHD | 0.70 | 50° left |
| 30 | 21 | ?CHD | 0.62 | 50° left |
| 31 | 22 | ?CHD | 0.51 | 48° left |
| 32 | 20 | Diabetes | 0.62 | 48° left |
| 33 | 22 | ?CHD | 0.55 | 50° left |
| 34 | 15 | Nuchal oedema | 0.5 | 36° left |
?CHD, suspected congenital heart disease during obstetric ultrasound scanning; FH, family history.
Table 4.
Outcome and confirmation of diagnosis
| Patient | Prenatal diagnosis | Outcome | Postnatal or PM diagnosis |
| 1 | CCTGA, EA | Alive | CCTGA, EA |
| 2 | CCTGA, VSD, EA, Coarct | Alive | CCTGA, VSD EA, Coarct |
| 3 | CCTGA | Alive | CCTGA |
| 4 | CCTGA, VSD, Coarct | Alive | CCTGA, VSD, Coarct |
| 5 | CCTGA, VSD, PS | Alive | CCTGA, VSD, Coarct |
| 6 | CCTGA, VSD, CHB PS | Alive | CCTGA, VSD, CHB, late diagnosis PS |
| 7 | CCTGA, VSD | Alive | CCTGA, VSD |
| 8 | CCTGA | Alive | CCTGA |
| 9 | CCTGA | Alive | CCTGA |
| 10 | CCTGA, VSD, PS | Alive | CCTGA, VSD, PS |
| 11 | CCTGA, VSD | Alive | CCTGA, VSD, CHB |
| 12 | CCTGA, VSD, PS | Alive | CCTGA, VSD PAt |
| 13 | CCTGA, PS | Alive | CCTGA, PS, 2:1 AV block |
| 14 | CCTGA, VSD, PS | Alive | CCTGA, VSD PS |
| 15 | CCTGA, VSD, PS | Alive | CCTGA VSD PS |
| 16 | CCTGA, VSD | Alive | CCTGA, VSD PS |
| 17 | CCTGA, PS | Alive | CCTGA, PS |
| 18 | CCTGA | Alive | CCTGA, mild PS |
| 19 | CCTGA, VSD, hypoplastic RV | Alive | AV discord, DORV VSD, straddling RAVV |
| 20 | CCTGA, VSD, PS | INFD | CCTGA, VSD PS |
| 21 | CCTGA, VSD | INFD | CCTGA, VSD |
| 22 | CCTGA, EA, CHB | INFD | CCTGA, EA, CHB |
| 23 | CCTGA | NND | CCTGA |
| 24 | CCTGA, EA | TOP | CCTGA, EA |
| 25 | CCTGA, TAt, VSD, Coarct | TOP | CCTGA, TAt, VSD Coarct |
| 26 | CCTGA, VSD, PS | TOP | CCTGA, VSD PS/PAt |
| 27 | CCTGA, VSD, PS | TOP | Refused PM |
| 28 | CCTGA, TAt, VSD, Coarct | TOP | Refused PM |
| 29 | CCTGA, EA, Coarct | TOP | Refused PM |
| 30 | CCTGA, VSD, EA | TOP | Refused PM |
| 31 | CCTGA, VSD, PS | TOP | Refused PM |
| 32 | CCTGA, VSD, EA | TOP | AV disc, DORV, VSD EA |
| 33 | CCTGA, Coarct, hypoplastic RV | TOP | CCTGA, Coarct, hypoplastic RV |
| 34 | CCTGA, EA | TOP | Refused PM |
CCTGA, congenitally corrected transposition of the great arteries; DORV, double outlet right ventricle; INFD, infant death; NND, neonatal death; PM, postmortem; RAVV, right atrioventricular valve; TAt, tricuspid atresia; TOP, termination of pregnancy.
Echocardiographic features
Reversed differential insertion
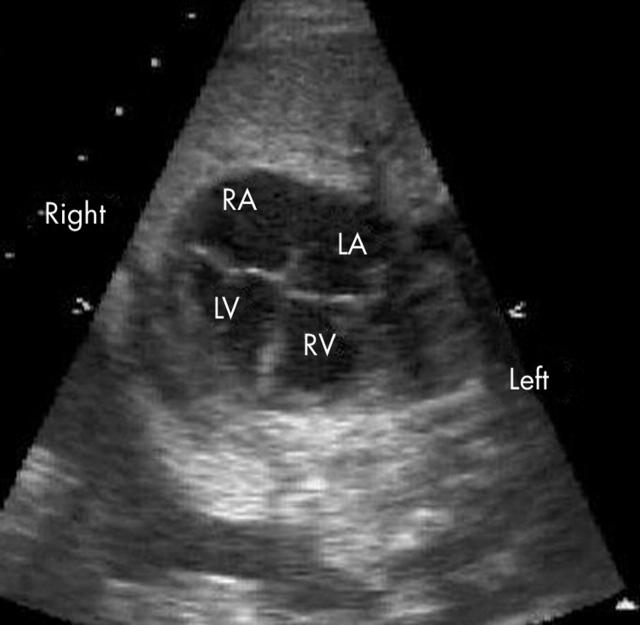
Reversed differential insertion of the atrioventricular valves was easily identified in 26 of 34 fetuses (76%) (fig 1). Of the fetuses where reversed differential insertion could not be seen, five had an associated ventricular septal defect, in two there was associated atrioventricular valve atresia, and in the remainder the image quality was poor.
Figure 1.
Four chamber view of the heart illustrating reversed differential insertion. The left sided atrioventricular valve is more apically positioned and thus the right ventricle (RV) is left sided and connected to the left atrium (LA). LV, left ventricle; RA, right atrium.
Recognition of right ventricle by moderator band
The right ventricle could be identified by the moderator band (fig 2) in 30 of 34 fetuses (88%) and was left sided in all but one (case 12), where it was right sided in association with complete situs inversus.
Figure 2.
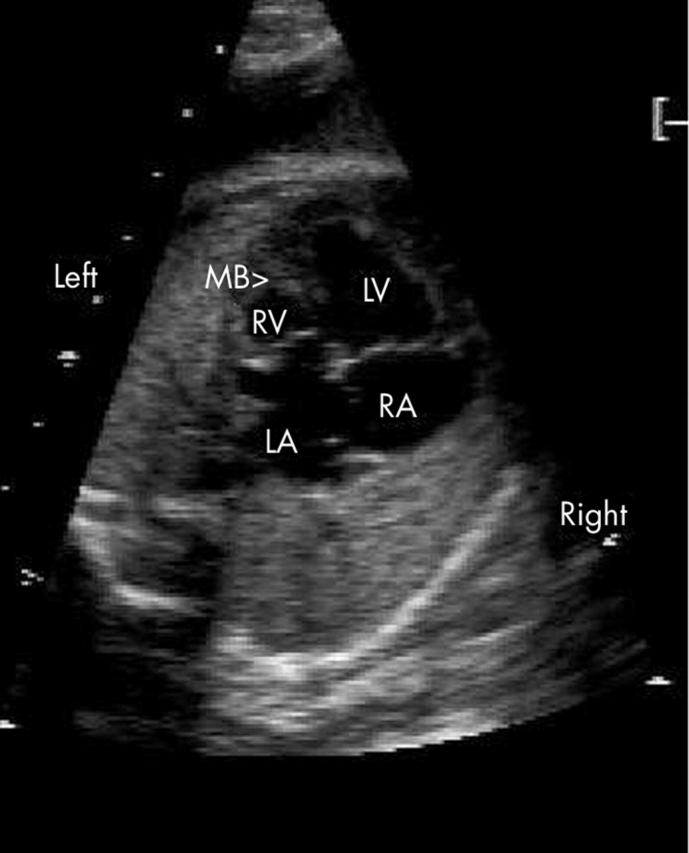
In this example, the right ventricle (RV) is also left sided but appears smaller than the left ventricle (LV). The moderator band (MB) and reversed differential insertion are both easily recognised.
Origin of the great arteries
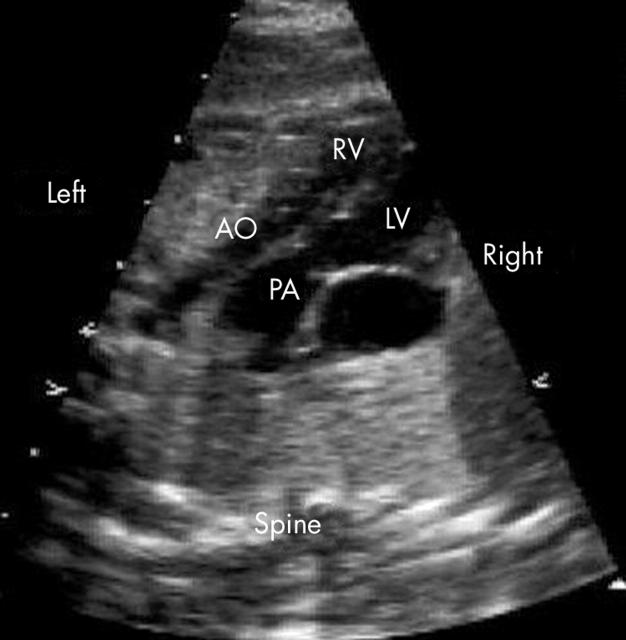
A parallel or abnormal orientation of the great arteries was seen in all fetuses. The pulmonary artery was the first vessel seen moving cranially from four chambers (figs 3 and 4). The aorta was anterior and to the left in all fetuses with the exception of the one fetus with situs inversus.
Figure 3.
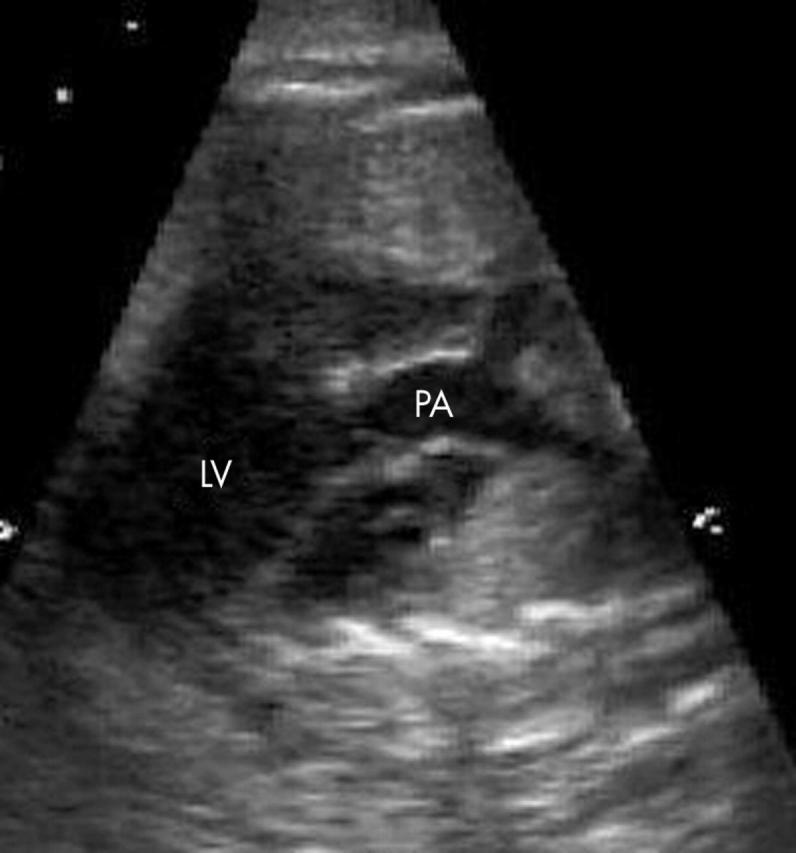
Moving cranially from the four chamber view seen in fig 1, the first vessel visualised is the pulmonary artery (PA).
Figure 4.
View of the great arteries of the example shown in fig 2. The great arteries arise in a parallel orientation and the aorta (AO) is anterior and to the left of the pulmonary artery (PA). The aorta is significantly smaller than the PA due to an associated coarctation of the aorta.
Combination of features
All the above features could be identified in 24 of 34 fetuses (71%).
Cardiac position and size
The cardiothoracic ratio was increased in four fetuses, of whom three had associated Ebstein’s anomaly. There was mediastinal shift towards the right in seven fetuses. Six of them had dextrocardia, one of whom also had complete situs inversus (case 12). In the seventh fetus the heart was in the midline (case 6).
Associated cardiac abnormalities
Table 3 shows the associated cardiac abnormalities, if any, in each fetus.
Table 3.
Associated cardiac lesions
| Patient | Isolated | VSD | PS | PAt | EA | TR | Coarct | CHB | Other |
| 1 | No | No | No | No | Yes | Yes | No | No | None |
| 2 | No | Yes | No | No | Yes | Yes | Yes | No | None |
| 3 | Yes | No | No | No | No | No | No | No | None |
| 4 | No | Yes | No | No | No | No | Yes | No | None |
| 5 | No | Yes | Yes | No | No | No | No | No | None |
| 6 | No | Yes | Yes | No | No | No | No | Prenatal | None |
| 7 | No | Yes | No | No | No | No | No | No | None |
| 8 | Yes | No | No | No | No | No | No | No | None |
| 9 | Yes | No | No | No | No | No | No | No | None |
| 10 | No | Yes | Yes | No | No | No | No | No | None |
| 11 | No | Yes | No | No | No | No | No | Postnatal | None |
| 12 | No | Yes | Yes | Postnatal | No | No | No | No | None |
| 13 | No | No | Yes | No | No | No | No | Postnatal 2:1 block | None |
| 14 | No | Yes | Yes | No | No | No | No | No | None |
| 15 | No | Yes | Yes | No | No | No | No | No | None |
| 16 | No | Yes | Mild postnatal | No | No | No | No | No | None |
| 17 | No | No | Yes | No | No | No | No | No | None |
| 18 | Yes | No | Mild postnatal | No | No | No | No | No | None |
| 19 | No | Yes | No | No | No | No | No | No | None |
| 20 | No | Yes | Yes | No | No | No | No | No | None |
| 21 | No | Yes | No | No | No | No | No | No | None |
| 22 | No | No | No | No | Yes | No | No | Prenatal | None |
| 23 | Yes | No | No | No | No | No | No | No | None |
| 24 | No | No | No | No | Yes | No | No | No | None |
| 25 | No | Yes | No | Yes | No | No | No | No | AV valve atresia |
| 26 | No | Yes | Yes | No | No | No | No | No | None |
| 27 | No | Yes | Yes | Yes | No | No | No | No | None |
| 28 | No | Yes | No | No | No | No | Yes | No | AV valve atresia |
| 29 | No | No | No | No | Yes | Yes | No | No | None |
| 30 | No | Yes | No | No | Yes | Yes | No | No | None |
| 31 | No | Yes | Yes | No | No | No | No | No | None |
| 32 | No | Yes | No | No | Yes | No | No | No | None |
| 33 | No | No | No | No | No | No | Yes | No | None |
| 34 | No | No | No | No | Yes | Yes | No | No | None |
CHB, complete heart block; Coarct, coarctation of the aorta; EA, Ebstein’s anomaly; PAt, pulmonary atresia; PS, pulmonary stenosis; TR, tricuspid regurgitation; VSD, ventricular septal defect.
Isolated cases
Isolated CCTGA was present in only five of 34 fetuses (14.7%).
Ventricular septal defect
A ventricular septal defect was present in 21 fetuses (62%), of whom two had associated left sided atrioventricular valve atresia (tricuspid atresia).
Pulmonary stenosis
Twelve fetuses were thought to have pulmonary stenosis on the antenatal scan. This was confirmed in nine fetuses, one fetus had pulmonary atresia at postmortem, and in two fetuses no postmortem had been performed for confirmation. In a further two fetuses, pulmonary stenosis had not been suspected on the antenatal scan but mild pulmonary stenosis was documented postnatally.
Ebstein’s anomaly
Ebstein’s anomaly was diagnosed prenatally in eight babies (fig 5) and this was confirmed in five, with permission for postmortem being refused for three.
Figure 5.
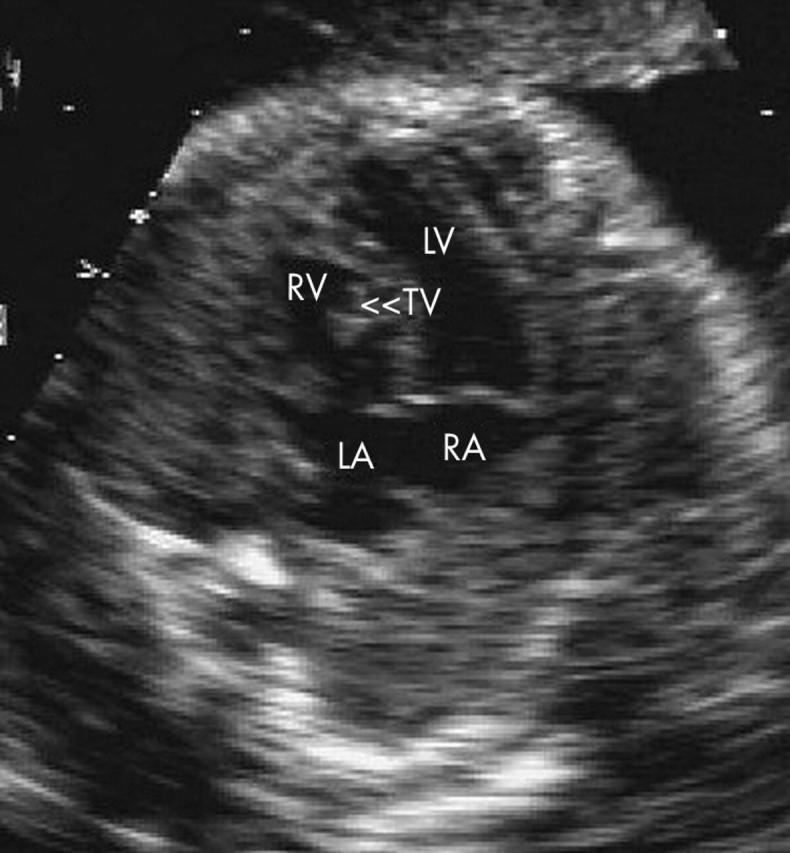
Example of atrioventricular discordance associated with Ebstein’s anomaly of the tricuspid valve (TV).
Coarctation of the aorta
An associated coarctation of the aorta was diagnosed in six fetuses prenatally (fig 4). This was confirmed in four babies: two postnatally, both of whom had a coarctation repair, and two at postmortem examination. For the other two, permission for postmortem was declined.
Tricuspid regurgitation
Tricuspid regurgitation was noted in five fetuses on the prenatal scan, all of whom had associated Ebstein’s anomaly.
Heart block
Two babies had associated complete heart block during fetal life and one developed this postnatally. A further baby developed 2:1 atrioventricular block postnatally.
Extracardiac abnormalities
One baby had cataracts diagnosed postnatally and died at the time of cataract surgery. No identified chromosomal abnormalities were identified in any of the babies in this study.
Outcome
Table 4 shows the outcome of the 34 fetuses. This table also allows comparison of the prenatal and postnatal diagnoses. There are 19 survivors, one baby died in the neonatal period, and three died in infancy. Parents elected to terminate 11 pregnancies (32%). However, for six of these fetuses the parents refused permission for necropsy so that the diagnosis could not be confirmed.
DISCUSSION
This diverse category of cardiac abnormality can be diagnosed with a high degree of accuracy in specialised centres, but may be confusing for sonographers and those inexperienced at examining the fetal heart. Thus, CCTGA may not easily be identified during routine obstetric ultrasound investigation, unless there are associated malformations such as Ebstein’s anomaly or an absent atrioventricular connection. The fetal spectrum is thus biased towards these cases. However, prenatal detection during obstetric screening is clearly possible, as the majority of fetuses (91%) in this series were referred because of a suspected problem during routine obstetric screening.
The following echocardiographic features should alert the sonographer to this condition.
Firstly is reversed differential insertion of the atrioventricular valves. This was identified echocardiographically in 76% of our fetuses. Although expected in this condition, it is not consistently seen, particularly if there are associated lesions, such as a ventricular septal defect, or if image quality is suboptimal. In this situation the moderator band may be more helpful to identify the right ventricle.
Secondly is the moderator band in the left sided or posterior ventricle. In 88% of our fetuses the moderator band was visualised and helped to identify the right ventricle.
Thirdly is abnormal orientation of the great arteries. In the normal heart the first vessel viewed moving cranially from four chambers is the aorta. Loss of the normal crossover of the great arteries and the first vessel viewed when moving cranially from the four chamber view being the pulmonary artery should alert the sonographer. However, this finding may also be seen in ventriculoarterial discordance with atrioventricular concordance. The first two features also need to be sought to make a correct diagnosis.
All the above features were identified in 71% of fetuses in this study.
Isolated cases of CCTGA without other cardiac malformations are rare. In one postnatal series only 9% of CCTGAs were isolated.7 In our series five of 34 (14.7%) were isolated. The most commonly seen associated malformations are ventricular septal defects, pulmonary stenosis or atresia, and tricuspid valve malformations.1,8,9,10,11 Conduction abnormalities are also common postnatally in association with CCTGA.1,10,12,13 Prenatally the diagnosis of CCTGA and conduction disturbances is much less common, although this has been reported.14,15
The natural history and clinical presentation are variable and depend on the presence and severity of associated cardiac lesions. Long term survival in this condition is possible, particularly if there are no associated abnormalities.16,17 Huhta et al18 described the overall survival of 70% at five years and 64% at 10 years. More recently, Rutledge et al19 reported a survival of 92% at five years, 91% at 10 years, and 75% at 20 years. In this latter study, as in others, risk factors for mortality were identified as tricuspid regurgitation, right ventricular dysfunction, and complete atrioventricular block. In our fetal series, the patients are still relatively young and we cannot comment on long term survival, as regurgitation of the systemic atrioventricular valve, right ventricular dysfunction, and atrioventricular block can all be progressive.19,20 However, in the shorter term, only five babies in our series were noted to have tricuspid regurgitation prenatally, all of whom had associated Ebstein’s anomaly. Of these, three pregnancies were terminated and two survived. A further 17 babies are still alive, none of whom had tricuspid regurgitation on the prenatal echocardiogram. Congenital heart block was present at diagnosis or developed in three babies, of whom two are alive and one died in infancy.
The ability of the right ventricle to sustain the systemic circulation over time is of major concern in the long term for patients with CCTGA. Conventional repair leaves the right ventricle as the systemic ventricle but patients undergoing a biventricular repair are more likely to develop right ventricular dysfunction than are patients whose symptoms are palliated or who do not undergo surgery.19,21 Right ventricular function can also deteriorate with time.20 Thus, the double switch procedure has been advocated as a treatment option for this group of patients, as this restores the morphological left ventricle as the systemic ventricle. However, although favourable early and midterm results have been reported for this procedure, long term follow up is still needed to evaluate the overall benefits of this option.21–24 None of the patients in this fetal series have progressed to having a double switch and at present either are receiving palliative treatment or have not undergone surgery.
Conclusions
CCTGA is a well defined and specific cardiac abnormality that may be associated with a heterogeneous spectrum of other cardiac abnormalities and that can be accurately identified prenatally. Specific echocardiographic features can be recognised by obstetric sonographers during obstetric ultrasound screening, which may improve the prenatal detection of this type of malformation. The short term outlook for babies with a prenatal diagnosis is good but longer term follow up is needed. With improving surgical techniques the longer term outlook may become more favourable and this will affect the counselling of parents prenatally.
Table 2.
Echocardiographic features identified
| Patient | Differential insertion | Moderator band seen | First vessel from 4 chambers | Parallel or abnormal orientation of great arteries |
| 1 | Reversed | Yes | PA | Yes |
| 2 | Reversed | Yes | PA | Yes |
| 3 | Reversed | Yes | PA | Yes |
| 4 | Reversed | Yes | PA | Yes |
| 5 | Reversed | Yes | PA | Yes |
| 6 | Not seen | Yes | PA | Yes |
| 7 | Reversed | Yes | PA | Yes |
| 8 | Reversed | Yes | PA | Yes |
| 9 | Reversed | Yes | PA | Yes |
| 10 | Reversed | Yes | PA, but difficult | Yes |
| 11 | Reversed | Yes | PA | Yes |
| 12 | Reversed | Yes | PA | Yes |
| 13 | Not seen | Yes | PA | Yes |
| 14 | Reversed | Yes | PA | Yes |
| 15 | Reversed | Yes | PA | Yes |
| 16 | Reversed | Yes | PA | Yes |
| 17 | Reversed | Yes | PA | Yes |
| 18 | Reversed | Yes | PA | Yes |
| 19 | Reversed | Yes | PA | Yes |
| 20 | Not seen | Yes | PA, but difficult | Yes |
| 21 | Not seen | Yes | PA | Yes |
| 22 | Reversed | Yes | PA | Yes |
| 23 | Reversed | Yes | PA | Yes |
| 24 | Reversed | No | PA | Yes |
| 25 | AV valve atresia | No, RV hypoplasia | PA | Yes |
| 26 | Not seen | Yes | PA | Yes |
| 27 | Reversed | Yes | PA | Yes |
| 28 | AV valve atresia | No, RV hypoplasia | PA | Yes |
| 29 | Reversed | No | PA | Yes |
| 30 | Reversed | Yes | PA, but difficult | Yes |
| 31 | Not seen | Yes | PA | Yes |
| 32 | Reversed | Yes | PA | Yes |
| 33 | Reversed | Yes | PA | Yes |
| 34 | Reversed | Yes | PA | Yes |
AV, atrioventricular; PA, pulmonary artery; RV, right ventricle.
We declare that there are no competing interests.
REFERENCES
- 1.Bjarke BB, Kidd BSL. Congenitally corrected transposition of the great arteries: a clinical study of 101 cases. Acta Paediatr Scand 1976;65:153–60. [DOI] [PubMed] [Google Scholar]
- 2.Fyler DC, Buckley LP, Hellenbrand WE, et al. Report of the New England regional infant cardiac program. Pediatrics 1980;65:377–461. [PubMed] [Google Scholar]
- 3.Scott DJ, Rigby ML, Miller GAH, et al. The presentation of symptomatic heart disease in infancy based on ten years experience (1973–82). Br Heart J 1984;52:248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Rokitansky K. Die Defecte der Scheidewande des Herzens. Vienna: W Braumuller, 1875:83–6.
- 5.Paladini D, Chita SK, Allan LD. Prenatal measurement of cardiothoracic ration in evaluation of congenital heart disease. Arch Dis Child 1990;65:20–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comstock C. Normal fetal heart axis and position. Obstet Gynecol 1987;70:255–9. [PubMed] [Google Scholar]
- 7.Lundstrom U, Bull C, Wyse RKH, et al. The natural and ‘unnatural’ history of congenitally corrected transposition. Am J Cardiol 1990;65:1222–9. [DOI] [PubMed] [Google Scholar]
- 8.Schiebler GL, Edwards JE, Burchell HB, et al. Congenital corrected transposition of the great vessels: a study of 33 cases. Pediatrics 1961;27 (Suppl) :849–88. [PubMed] [Google Scholar]
- 9.Ellis K, Morgan BC, Blumenthal S, et al. Congenitally corrected transposition of the great vessels. Radiology 1962;79:35–50. [DOI] [PubMed] [Google Scholar]
- 10.Friedberg DZ, Nadas AS. Clinical profile of patients with congenitally corrected transposition of the great arteries. N Engl J Med 1970;282:1053–9. [DOI] [PubMed] [Google Scholar]
- 11.Allwork SP, Bentall HH, Becker AE, et al. Congenitally corrected transposition of the great arteries: morphologic study of 32 cases. Am J Cardiol 1976;38:911–23. [DOI] [PubMed] [Google Scholar]
- 12.Huhta J, Maloney JD, Ritter D, et al. Complete atrioventricular block in patients with atrioventricular discordance. Circulation 1983;67:1374–7. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RH, Becker AE, Arnold R, et al. The conducting tissue in congenitally corrected transposition. Circulation 1974;50:911–23. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt KG, Ulmer HE, Silverman NH, et al. Perinatal outcome of fetal complete atrioventricular block: a multicentre experience. J Am Coll Cardiol 1991;17:1360–6. [DOI] [PubMed] [Google Scholar]
- 15.Davison MB, Radford DJ. Fetal and neonatal congenital complete heart block. Med J Aust 1989;150:192–8. [DOI] [PubMed] [Google Scholar]
- 16.Presbitero P, Somerville J, Rabajoli F, et al. Corrected transposition of the great arteries without associated defects in adult patients: clinical profile and follow-up. Br Heart J 1995;74:57–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda U, Furuse M, Suzuki O, et al. Longterm survival in aged patients with corrected transposition of the great arteries. Chest 1992;101:1382–5. [DOI] [PubMed] [Google Scholar]
- 18.Huhta JC, Danielson GK, Ritter DG, et al. Survival in atrioventricular discordance. Pediatr Cardiol 1985;6:57–60. [DOI] [PubMed] [Google Scholar]
- 19.Rutledge JM, Nihill MR, Fraser CD, et al. Outcome of 121 patients with congenitally corrected transposition of the great arteries. Pediatr Cardiol 2002;23:137–45. [DOI] [PubMed] [Google Scholar]
- 20.Graham TP, Bernard YD, Mellen BG, et al. Long term outcome in congenitally corrected transposition of the great arteries: a multi-institutional study. J Am Coll Cardiol 2000;36:255–61. [DOI] [PubMed] [Google Scholar]
- 21.Voskuil M, Hazekamp MG, Kroft LJ, et al. Post surgical course of patients with congenitally corrected transposition of the great arteries. Am J Cardiol 1999;83:558–62. [DOI] [PubMed] [Google Scholar]
- 22.Langley SM, Winlaw DS, Stumper O, et al. Midterm results after restoration of the morphologically left ventricle to the systemic circulation in patients with congenitally corrected transposition of the great arteries. J Thorac Cardiovasc Surg 2003;125:1229–41. [DOI] [PubMed] [Google Scholar]
- 23.Karl TR, Weintraub RG, Brizard CP, et al. Senning plus arterial switch operation for discordant (congenitally corrected) transposition. Ann Thorac Surg 1997;64:495–502. [DOI] [PubMed] [Google Scholar]
- 24.Reddy VM, McElhinney DB, Silverman NH, et al. The double switch procedure for anatomical repair of congenitally corrected transposition of the great arteries in infants and children. Eur Heart J 1997;18:1470–7. [DOI] [PubMed] [Google Scholar]