Abstract
Objectives: To clarify the role of infarct and non-infarct sites on left ventricular (LV) remodelling after myocardial infarction by measuring brain natriuretic peptide (BNP) from each site.
Methods and results: BNP from the aorta and the anterior interventricular vein (AIV) was measured in 45 patients with first anterior myocardial infarction at one, six, and 18 months. The LV was significantly dilated (> 10 ml/m2 of end diastolic volume from one to 18 months) in 20 patients (remodelling (R) group) but not in 25 others (non-remodelling (NR) group). Patient characteristics and LV functions did not differ significantly at one month but plasma BNP concentration was higher in group R than in group NR (336 (288) v 116 (106) pg/ml, p < 0.01), predicting the degree of LV dilatation. The difference in BNP concentration between the aortic root and AIV (ΔBNP), reflecting BNP secreted from the infarct site, did not differ at one month. In both groups BNP and ΔBNP significantly decreased from one to six months (p < 0.05) and decreased from six months to 18 months, but the change was not significant. BNP and ΔBNP were significantly higher in group R than in group NR after six months, when LV dilatation was not evident in both groups.
Conclusion: Enhanced BNP secretion at one month in the non-infarct and infarct ventricular sites predicts subsequent LV dilatation (that is, remodelling). The slower process of LV remodelling decreased BNP secretion at both sites. Thus, BNP concentration should be useful for monitoring ventricular remodelling after infarction.
Keywords: brain natriuretic peptide, anterior myocardial infarction, left ventricular dilatation, remodelling, prediction
We1 and others2 previously reported that synthesis and secretion of brain natriuretic peptide (BNP) was augmented before progressive ventricular dilatation after myocardial infarction and that plasma BNP concentration predicted magnitude of subsequent left ventricular (LV) dilatation.3,4 Plasma BNP concentration thus reflects LV wall stress leading to dilatation. The difference in plasma BNP concentration between the aortic root and the anterior interventricular vein (AIV) also increases, indicating increased wall stretch at the infarct site.5
LV dilatation after myocardial infarction results from both regional wall expansions at the infarct site and volume overload-type hypertrophy in non-infarct myocardium in response to increased wall stress.6 Thus, simultaneous measurement of BNP secretion from the infarct site and from the entire ventricle would help to determine mechanisms of LV dilatation. Regional wall stress along with LV dilatation would decline after healing of the infarct. Sequential changes in plasma BNP concentration measured at infarct and non-infarct sites would clarify the role of each site in long term LV remodelling.
Over 18 months, we studied relations between concentrations of BNP, especially BNP at the infarct site, and LV dilatation in patients with their first anterior myocardial infarction.
PATIENTS AND METHODS
Patients
Of 102 patients admitted to our coronary care unit with their first anterior myocardial infarction who received successful reperfusion by primary coronary angioplasty with or without stenting, 45 fulfilled the study inclusion criteria: age below 80 years; absence of cardiomyopathy, severe valve disease, ventricular septal defect, atrial fibrillation, or cardiogenic shock (systolic pressure < 80 mm Hg); follow up cardiac catheterisation and measurement of plasma BNP at one, six, and 18 months after the onset; and no further cardiac events such as unstable angina, myocardial infarction, or silent occlusion of an infarct related artery during the study period. Anterior myocardial infarction was defined by ECGs showing ST segment elevation in at least two contiguous precordial leads and was confirmed by coronary angiograms depicting a total or subtotal proximal occlusion of the left anterior descending coronary artery and subsequent increase of creatine kinase activities more than twice normal range. Of these, we reported on 39 patients previously.1 The protocol was approved by the ethics committee of Osaka Police Hospital.
Cardiac catheterisation and analysis of left ventriculography
Cardiac catheterisation was performed at one month (25–36 days), six months (5–9 months), and 18 months (16–23 months) after onset, with written informed consent at the time of each procedure. Details of cardiac catheterisation were described previously.1 Briefly, haemodynamic parameters including mean right atrial pressure, LV end diastolic pressure, and systemic blood pressure were measured. Blood was collected simultaneously from the aortic root and AIV for measurement of plasma BNP. To collect blood from the AIV, a 5 French NIH catheter with end hole was placed in the coronary sinus through the brachial vein and then advanced to the AIV with a guidewire. Blood from the AIV and from the aortic root was sampled simultaneously. After completion of haemodynamic measurements and blood sampling, left ventriculography was performed. Lastly, coronary angiography was performed after intracoronary administration of glyceryl trinitrate (0.1–0.3 mg). Left ventriculography was performed in the 30° right anterior oblique position with a power injection and analysed as described previously.7 Briefly, endocardial contours of the LV at end diastole and end systole were traced. The LV volume at these times was measured by the area–length method8 and the ejection fraction was calculated.9 Regional LV function was assessed by centre line methods as described previously.10 Briefly, the centre line was defined as the point midway between the end diastolic and end systolic endocardial contours. Motion was measured along 100 chords within the LV shape constructed perpendicularly to the centre line and plotted in units of SD of normal data based on ventriculograms obtained in 100 normal subjects. Regional wall motion abnormality in the centre of the infarct region was calculated by averaging the motion of chords within the most hypokinetic half of the infarct related territory (chords 10 to 66) and expressed as SD per chord. A hypokinetic area was calculated where motion was depressed by more than 2 SD below the normal mean, expressed as the percentage of endocardial contour length.
Thallium-201 single photon emission computed tomography
Myocardial single photon emission computed tomography was performed at about one month (19–38 days) after onset of myocardial infarction with a large field of view rotating double crystal gamma camera (OPTIMA, GE Medical, Minneapolis, Minnesota, USA) equipped with a low energy general purpose collimator and a computer interface (Starcam 4000; GE Medical). Image acquisition commenced in the 30° right anterior oblique projection about three hours after the injection of 114 MBq 201Tl. Thirty two sequential images separated by 5.6° intervals were acquired over 180°. Data were stored in a 64 × 64 matrix for subsequent analysis.
Infarct size was assessed according to methods previously described.7 Briefly, mean defect severity was determined from the area under the circumferential curve more than 2 SD below normal at each slice. Curves were obtained by dividing the image of the LV into six layers at equidistant intervals perpendicular to its long axis. Defect volume was defined by summation of mean defect severity in each slice and was expressed in arbitrary units.
Measurement of BNP
All blood samples were collected in a chilled plastic syringe and transferred to chilled siliconised disposable tubes containing aprotinin and EDTA. The plasma fraction was immediately separated at 4°C and stored at −80°C until analysis. Plasma natriuretic peptide concentrations were measured by a radioimmunoassay kit specific for BNP (Shionoria, Osaka, Japan).11 The lower limit of sensitivity for BNP in this assay was 4.0 pg/ml. Intra-assay and interassay variances were 4.4% and 2.0%, respectively.
Statistical analysis
All data were analysed by SPSS version 11.0 (SPSS Inc, Chicago, Illinois, USA). Data are expressed as mean (SD). For continuous variables, differences between groups were assessed by Student’s t test; for categorical variables, Fisher’s exact test was used. One way analysis of variance was used to assess differences in variables between one, six, and 18 months and two way analysis of variance was used to compare the groups during the study period. For the value of BNP, a non-parametric test was applied. The Kruskal-Wallis test was used to compare more than two groups. Two way analysis was done according to the Friedman’s method. Multivariate linear regression analysis was used to identify the independent factors determining the degree of LV increment during the study period. Results were considered significant when the p < 0.05.
RESULTS
Patient characteristics
Among 45 patients enrolled in this study, 20 patients (remodelling (R) group) had significant LV dilatation, defined as an increase in end diastolic volume index (EDVI) of at least 10 ml/m2 between 1–18 months after the onset, and 25 patients did not (non-remodelling (NR) group). No significant differences were evident between these two groups in terms of age, sex distribution, presence of established risk factors (diabetes mellitus, hyperlipidaemia, and hypertension), time from onset to reperfusion, angiographic characteristics, or defect volume. Medications given from immediately after admission through to 18 months did not differ between the groups, except for angiotensin converting enzyme (ACE) inhibitor and diuretics, which were taken by significantly more patients in group R than in group NR (table 1). No patients had coronary intervention besides the acute phase during the study period.
Table 1.
Demographic and angiographic characteristics of patients in non-remodelling (NR) and remodelling (R) groups
| Variable | NR group (n = 25) | R group (n = 20) | p Value |
| Age (years) | 62 (9) | 63 (9) | NS |
| Men | 23 (92%) | 15 (75%) | NS |
| Time to reperfusion after onset of infarction (hours) | 12.6 (16.3) | 18.3 (23.3) | NS |
| Systemic hypertension | 5 (20%) | 5 (25%) | NS |
| Diabetes mellitus | 7 (28%) | 1 (5%) | NS |
| Hyperlipidaemia* | 14 (56%) | 14 (70%) | NS |
| Medications | |||
| ACE inhibitors | 12 (48%) | 17 (85%) | <0.05 |
| Nitrates | 20 (80%) | 12 (60%) | NS |
| Calcium channel antagonists | 6 (24%) | 6 (30%) | NS |
| Diuretics | 2 (8%) | 9 (45%) | <0.05 |
| β Blockers | 3 (12%) | 0 (0%) | NS |
| Antiplatelet agents | 25 (100%) | 20 (100%) | NS |
| Angiographic characteristics | |||
| Coronary arteries with >75% narrowing | NS | ||
| 0 | 7 (28%) | 6 (30%) | |
| 1 | 13 (52%) | 8 (40%) | |
| 2 | 4 (16%) | 5 (25%) | |
| 3 | 1 (4%) | 1 (5%) | |
| Defect volume (arbitrary units) | 938 (708) | 1385 (832) | NS |
Data are mean (SD) or number (%).
*Defined as serum total cholesterol concentration >5.69 mmol/l or triglycerides >1.95 mmol/l.
ACE, angiotensin converting enzyme; NS, not significant.
Changes in LV function over time
Table 2 presents data concerning LV function. No significant differences in LV end diastolic volume, end systolic volume, ejection fraction, regional wall motion abnormality, percentage hypokinetic area, or haemodynamic variables were evident between the two groups at one month. LV end diastolic and end systolic volumes increased significantly during the study period in group R. In group NR, no significant increment in end diastolic or end systolic volume was observed. Reflecting this pattern, end systolic and end diastolic LV volumes and ejection fraction differed significantly between the two groups during the study period. Regional wall motion abnormalities during the study period were worse in group R than in group NR but the differences were not significant. The percentage hypokinetic area in group NR at one month was somewhat smaller than that in group R and decreased after one month. The percentage hypokinetic area in group R, however, did not change; as a result, percentage hypokinetic area during the study period was significantly smaller than in group R. Haemodynamic variables including heart rate, systolic aortic pressure, LV end diastolic pressure, and mean atrial pressure were not significantly different between the two groups or at the follow up times.
Table 2.
Left ventricular function and haemodynamic variables in NR and R groups during the study period
| NR group | R group | |||||
| 1 month | 6 months | 18 months | 1 month | 6 months | 18 months | |
| Days after index event | 30 (7) | 201 (49) | 620 (115) | 32 (5) | 215 (35) | 550 (119) |
| Ejection fraction (%)* | 53 (15) | 52 (14) | 50 (14) | 44 (16) | 42 (14) | 44 (13) |
| EDVI (ml/m2)* | 63 (17) | 62 (21) | 58 (18) | 61 (16) | 77 (22)† | 84 (22)† |
| ESVI (ml/m2)* | 31 (15) | 31 (17) | 31 (15) | 35 (17) | 47 (22) | 49 (21)† |
| RWM abnormality (SD/chords)* | −2.98 (1.23) | −2.84 (1.28) | −2.93 (1.26) | −3.34 (1.09) | −3.40 (0.97) | −3.39 (0.79) |
| Hypokinetic area (%)* | 37 (19) | 34 (20) | 30 (19) | 48 (22) | 52 (19) | 49 (16) |
| Heart rate (beats/min) | 74 (14) | 72 (17) | 72 (15) | 82 (17) | 75 (14) | 70 (11)† |
| Systolic aortic pressure (mm Hg) | 137 (26) | 135 (29) | 144 (20) | 131 (35) | 134 (37) | 143 (35) |
| LVEDD (mm Hg) | 10 (4) | 11 (5) | 11 (5) | 14 (6) | 15 (8) | 12 (5) |
| MAP (mm Hg) | 4 (2) | 4 (3) | 4 (3) | 4 (1) | 4 (5) | 4 (6) |
*p<0.05 NR v R (two way analysis of variance); †p<0.05 v one month within each group.
EDVI, end diastolic volume index; ESVI, end systolic volume index; LVEDD, left ventricular end diastolic pressure; MAP, mean right atrial pressure; RWM, regional wall motion.
Plasma BNP concentration
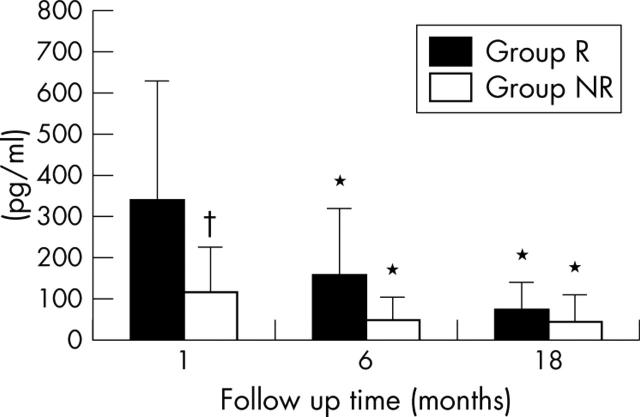
Plasma BNP concentration was significantly higher in group R (336 (288) pg/ml) than in group NR (116 (106) pg/ml) at one month (fig 1). Plasma BNP concentration in group R decreased significantly from one to six months (152 (162) pg/ml), then further decreased from six months to 18 months (71 (65) pg/ml), but the difference between six and 18 months did not reach significance. A similar change was observed in group R: plasma BNP concentration in group NR significantly decreased from one to six months (44 (58) pg/ml) and did not change from six months to 18 months (42 (66) pg/ml). Plasma BNP concentrations was significantly higher in group R than in group NR during the study period.
Figure 1.
Plasma brain natriuretic peptide (BNP) concentration at the aortic root shown at 1, 6, and 18 months after the onset of myocardial infarction. *p < 0.05 within group differences between 1 month and 6 or 18 months; †p < 0.05 remodelling (R) group versus non-remodelling (NR) group at 1 month (two way analysis of variance).
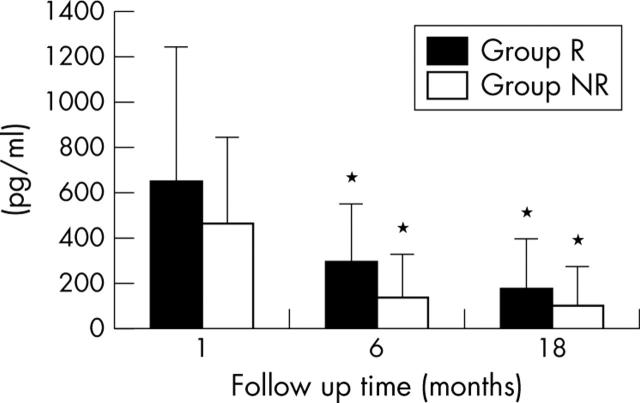
The difference in plasma BNP concentration between the aortic root and AIV (ΔBNP), representing the amount of BNP released from the infarct site, was slightly higher in group R (665 (583) pg/ml) than in group NR (483 (365) pg/ml) at one month, but this was not significant (fig 2). ΔBNP in group R and group NR decreased significantly from one to six months (297 (256) pg/ml for group R; 145 (189) pg/ml for group NR) and decreased from six months to 18 months (190 (207) pg/ml for group R, 110 (166) pg/ml for group NR) but the difference was not evident between six months and 18 months, similar to the changes in BNP concentration. ΔBNP was significantly higher in group R than in group NR at both six months and 18 months.
Figure 2.
Difference in plasma BNP concentration between the aortic root and the anterior intraventricular vein (ΔBNP) shown at 1, 6, and 18 months after the onset of myocardial infarction. *p < 0.05 within group differences between 1 month and 6 or 18 months (two way analysis of variance).
Relation between plasma BNP concentration and LV dilatation
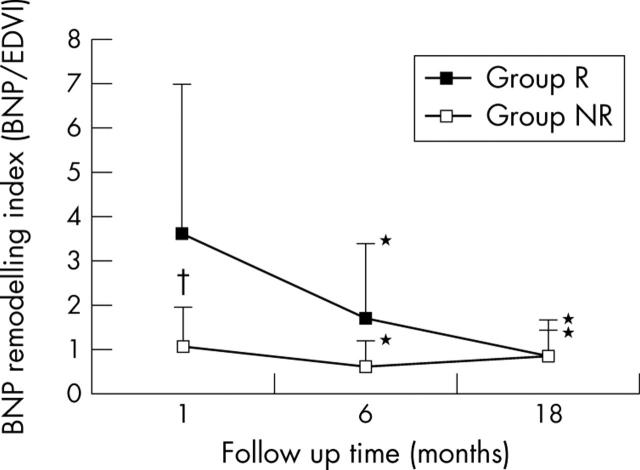
To summarise the changes in plasma BNP concentration and in end diastolic volume in both groups, fig 3 shows the BNP remodelling index expressed as BNP/EDVI. In both groups R and NR, the BNP remodelling index decreased significantly from one month to six months and decreased from six months to 18 months without significant difference. The BNP remodelling index was significantly higher in group R than in group NR during the entire study period.
Figure 3.
Changes in BNP remodelling index, expressed as BNP/end diastolic volume index (EDVI). *p < 0.05 within group differences between 1 month and 6 or 18 months; †p < 0.05 R group versus NR group at 1 month (two way analysis of variance).
Factors predictive of degree of LV dilatation
An increase in end diastolic volume (ΔEDVI) from one to six months was significantly correlated with LV end diastolic pressure (r = 0.422, p < 0.01), ejection fraction (r = −0.322, p < 0.05), defect volume (r = 0.391, p < 0.01), and plasma BNP concentration at one month (r = 0.532, p < 0.001). ΔEDVI from one to 18 months was significantly correlated with ejection fraction (r = −0.399, p < 0.01), regional wall motion abnormality (r = −0.313, p < 0.05), percentage hypokinetic area (r = 0.378, p < 0.05), LV end diastolic pressure (r = 0.369, p < 0.05), defect volume (r = 0.460, p < 0.01), and plasma BNP concentration at one month (r = 0.436, p < 0.01). No significant correlation was noted between ΔBNP and ΔEDVI either from one to six months or from one to 18 months. To elucidate the factors that predict the degree of LV dilatation, multivariate linear regression analysis with incremental method was adopted. In this method, the number of variables in the regression model increases step by step until residual variables are all insignificant. As a result, plasma BNP at one month and diuretics were the variables that correlated significantly with ΔEDVI from one to six months or from one to 18 months among the factors LV function, haemodynamic variables, age, sex, and medications (ACE inhibitor and diuretics).
DISCUSSION
Our study shows that secretion of BNP at the infarct segment was augmented after myocardial infarction and gradually decreased independently of subsequent LV dilatation one month after the index event. We also showed that LV dilatation enhanced BNP synthesis at the infarct and non-infarct segments, leading to a significantly high plasma BNP concentration in patients with LV dilatation up to six months; after that, plasma BNP concentration reached a steady state reflecting the decreased rate in the progression of LV dilatation at 18 months. Furthermore, we confirmed that plasma BNP concentration is a powerful short term predictor of LV dilatation as shown in previous reports,1,2 in addition to long term prediction.
BNP concentration at one month and LV remodelling
Increased wall stress after myocardial infarction mostly derives from stretch in the border zone between infarct and non-infarct sites. Here BNP synthesis is presumably enhanced, as shown in experimental animals12 and in humans.13 BNP synthesis in the infarct area including the border zone, defined as ΔBNP, should be augmented in response to increased regional wall stress.5 ΔBNP was slightly higher in patients with than in patients without LV dilatation, but the difference did not attain significance at one month. This may be explained as the balance of the amount of residual viable myocardium synthesising BNP and the wall stress regulating BNP synthesis at the infarct site. The increased wall stress in group R compared with group NR augments BNP synthesis of a residual viable myocytes. On the other hand, residual myocytes at the infarct site, producing a small amount of BNP reflecting less wall stress, may be slightly greater than in group R, although in this study the difference in infarct size was not significant. As a result, the amount of BNP released from the infarct site was essentially similar in the two groups. As over 99% of circulating BNP was synthesised in the LV after myocardial infarction,5 plasma BNP concentration reflects the BNP synthesised at both infarct and non-infarct sites. The significant difference in plasma BNP concentration was evident at that point without a clear cut difference in ΔBNP, suggesting that higher regional wall stress in the non-infarct sites in patients with LV dilatation enhances BNP synthesis at non-infarct sites.
Change in plasma BNP concentration after one month and LV remodelling
From one to six months, the concentration of plasma BNP and of BNP released from the infarct site (ΔBNP) decreased significantly in both groups; however, the concentration of plasma BNP and ΔBNP were significantly higher in patients with LV dilatation than in patients without LV dilatation at six months, suggesting that the ventricular wall stress after myocardial infarction is still evident at six months after the onset during LV dilatation. In patients without LV dilatation, functional recovery after fibrotic scar formation may decrease LV wall stress during the first six months resulting in the termination of LV dilatation. On the other hand, in patients with LV dilatation, poor recovery of regional wall motion at the infarct site results in a sustained excess of ventricular wall stress leading to LV dilatation.14,15 Plasma BNP concentration and ΔBNP in patients with LV dilatation decreased from six to 18 months but was significantly higher than in patients without LV dilatation. LV dilatation was not evident from six months to 18 months even in patients with LV dilatation. These findings suggest that the slower process of LV remodelling decreased BNP secretion and resulted in insignificant changes in BNP concentration, although further studies with more patients will be necessary.
Prediction of long term LV dilatation
As previously reported, plasma BNP concentration at one week or one month after the onset of myocardial infarction correlated significantly with the degree of LV dilatation at six months.1,2 In the present study, plasma BNP at one month was significantly correlated with severity of LV dilatation both from one to six months and from one to 18 months. Multiple regression analysis identified plasma BNP concentration as a determinant of LV dilatation. Besides BNP concentration, the use of diuretics was identified as a factor determining LV dilatation. Although all medications were started immediately after admission, diuretics were mainly given to patients with clinical heart failure. The selection bias may be closely related to the identification of factors by multivariate linear regression analysis. These findings indicate that increased wall stress reflected by plasma BNP concentration is the key factor determining future LV function.
Study limitations
Although this study was limited to patients who were successfully reperfused during the acute phase without new cardiac events including silent restenosis, ischaemia, or reocclusion of the infarct related artery, over an 18 month period from the index event16,17 not all LV remodelling could be predicted.
We assumed that the difference in BNP concentration between the aorta and the AIV reflects infarct site BNP production. However, some blood may be drained from the infarct site independently from the AIV through minimal veins draining directly into the cardiac cavities, as well as into the rest of the coronary sinus venous system. When this drainage occurs, it may be underestimated by our calculation of BNP production in the infarct site. Nevertheless, the contribution of this error would not be large, and the conclusion is still applicable. Circulating BNP concentration was not affected by the drainage of blood from the infarct site through the AIV because circulating BNP was assumed to be produced in the infarct and the non-infarct LV. BNP can be produced in various parts of the circulation system including the right ventricle, atria, and pulmonary vein in addition to the LV. The amount of BNP produced in these other areas is very low as previously reported 5,18,19 and did not affect our conclusion.
Our patients were not given medications that are usually administered in western countries; despite the high use of antiplatelets, in this study β blocking agents were rarely used. The high incidence of vasospasm after myocardial infarction20 or of side effects (for example, bradycardia and hypotension) in Japanese compared with white patients may be why β blockers are rarely given. The significantly higher use of ACE inhibitor might have affected the production of BNP in group R, but multivariate linear regression analysis showed that ACE inhibitor was not an independent predictor of LV remodelling. Furthermore, ACE inhibitor has been shown to have little preventive effect on LV dilatation in patients without poor LV function such as patients in group R.21 Even if medications used in this study did not relate to the major conclusion, the effect of medication must be considered. However, the number of patients is too small to assess the effects of pharmacological interventions.22 Further, a large scale study will be necessary to assess relations between plasma BNP concentration and LV remodelling under a variety of conditions likely to affect the process.
In conclusion, the present study of plasma BNP and ΔBNP clarifies the important role of the non-infarct segment in LV remodelling. Furthermore, BNP may prove to be a useful clinical tool to predict remodelling and assess the timing of the process, but this study did not provide this proof.
Abbreviations
ACE, angiotensin converting enzyme
AIV, anterior interventricular vein
BNP, brain natriuretic peptide
EDVI, end diastolic volume index
LV, left ventricular
Published Online First 17 March 2005
REFERENCES
- 1.Hirayama A, Yamamoto H, Sakata Y, et al. Usefulness of plasma brain natriuretic peptide after acute myocardial infarction in predicting left ventricular dilatation six months later. Am J Cardiol 2001;88:890–3. [DOI] [PubMed] [Google Scholar]
- 2.Nagaya N, Nishikimi T, Goto Y, et al. Plasma brain natriuretic peptide is a biochemical marker for the prediction of progressive ventricular remodeling after acute myocardial infarction. Am Heart J 1998;135:21–8. [DOI] [PubMed] [Google Scholar]
- 3.Pouleur H, Rousseau MF, van Eyll C, et al. Assessment of regional left ventricular relaxation in patients with coronary artery disease: importance of geometric factors and changes in wall thickness. Circulation 1984;69:696–702. [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer MA, Pfeffer JM. Ventricular enlargement and reduced survival after myocardial infarction. Circulation 1987;75:IV93–7. [PubMed] [Google Scholar]
- 5.Sumida H, Yasue H, Yoshimura M, et al. Comparison of secretion pattern between A-type and B-type natriuretic peptides in patients with old myocardial infarction. J Am Coll Cardiol 1995;25:1105–10. [DOI] [PubMed] [Google Scholar]
- 6.McKey R, Pfeffer M, Pasternark R, et al. Left ventricular remodeling after myocardial infarction: a corollary to infarct expansion. Circulation 1986;74:693–702. [DOI] [PubMed] [Google Scholar]
- 7.Hirayama A, Adachi T, Asada S, et al. Late reperfusion for acute myocardial infarction limits the dilatation of left ventricle without the reduction of infarct size. Circulation 1993;88:2565–74. [DOI] [PubMed] [Google Scholar]
- 8.Sandler H, Dodge H. The use of single plane angiography for the measurement of left ventricular volume in man. Am Heart J 1968;75:325–34. [DOI] [PubMed] [Google Scholar]
- 9.Dodge H, Sandler H, Baxley W, et al. Usefulness and limitation of radiographic methods for determining left ventricular volume. Am J Cardiol 1966;18:10–24. [DOI] [PubMed] [Google Scholar]
- 10.Sheehan F, Bolson E, Dodge H, et al. Advantages and applications of the centerline method for characterizing regional ventricular function. Circulation 1986;74:293–305. [DOI] [PubMed] [Google Scholar]
- 11.Kono M, Yamauchi M, Tsuji T, et al. An immunoradiometric assay for brain natriuretic peptide in human plasma. Kaku Igaku Gijutsu 1993;13:2–7. [Google Scholar]
- 12.Hama N, Itoh H, Shirakami G, et al. Rapid ventricular induction of brain natriuretic peptide gene expression in experimental acute myocardial infarction. Circulation 1995;92:1558–64. [DOI] [PubMed] [Google Scholar]
- 13.Jougasaki M, Yasue H, Mukoyama M, et al. Appearance of atrial natriuretic peptide in the ventricles in patients with myocardial infarction. Am Heart J 1990;119:92–6. [DOI] [PubMed] [Google Scholar]
- 14.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 1975;56:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossman W. Cardiac hypertrophy: useful adaptation or pathologic process? Am J Med 1980;69:576–84. [DOI] [PubMed] [Google Scholar]
- 16.White H, Cross D, Elliot J, et al. Long-term prognostic importance of patency of the infarct-related coronary artery after thrombolytic therapy for acute myocardial infarction. Circulation 1994;89:61–7. [DOI] [PubMed] [Google Scholar]
- 17.Brodie B, Stuckey T, Kissling G, et al. Importance of infarct-related artery patency for recovery of left ventricular function and late survival after primary angioplasty for acute myocardial infarction. J Am Coll Cardiol 1996;28:319–25. [DOI] [PubMed] [Google Scholar]
- 18.Murakami Y, Shimada T, Inoue S, et al. New insights into the mechanism of the elevation of plasma brain natriuretic polypeptide levels in patients with left ventricular hypertrophy. Can J Cardiol 2002;18:1294–300. [PubMed] [Google Scholar]
- 19.Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation 1994;90:195–203. [DOI] [PubMed] [Google Scholar]
- 20.Pristipino C, Beltrame JF, Finocchiaro ML, et al. Major racial differences in coronary constrictor response between Japanese and Caucasians with recent myocardial infarction. Circulation 2000;101:1102–8. [DOI] [PubMed] [Google Scholar]
- 21.Latini R, Maggioni AP, Flather M, et al. ACE inhibitor use in patients with myocardial infarction: summary of evidence from clinical trials. Circulation 1995;92:3132–7. [DOI] [PubMed] [Google Scholar]
- 22.Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. N Engl J Med 1992;327:669–77. [DOI] [PubMed] [Google Scholar]