Abstract
Objective: To assess psychomotor development and neurological sequelae in infants after surgery for congenital heart defects.
Design and setting: Single institution prospective cohort study.
Patients: 90 of 112 consecutive surviving infants of less than 1 year of age, without brain anomalies, conditions, or syndromes associated with delayed mental development, who underwent cardiac surgery during an 18 month period; 20 control infants with minor or no congenital heart defects.
Main outcome measures: Griffiths developmental scales and standardised neurological examination at 1 year.
Results: Mean (SD) developmental quotient (DQ) in index infants was 99 (10.6), compared with 106.7 (6.6) in controls (p < 0.001). DQ was lower in infants after palliative surgery (n = 16; 88 (12.2)) than after corrective surgery (n = 74; 101.4 (8.6)) (p < 0.001). Of the 90 index infants, 24 (27%) had a DQ below 93.5 (more than 2 SD below the mean of controls). Developmental delay (DQ < 93.5) was more common after palliative surgery (10/16, 63%) than after corrective surgery (14/74, 19%) (p < 0.001). Of the 90 index infants, 29 (32%) had neurological abnormalities, compared with only one of the 20 controls (5%) (p = 0.013). Neurological abnormalities were more frequent after palliative surgery (11/16, 69%) than after corrective surgery (18/74, 24%) (p < 0.001).
Conclusions: There is a considerable rate of neurodevelopmental impairment at 1 year of age in infants after cardiac surgery. Psychomotor impairment and neurological sequelae are apparently more severe in infants in whom only palliative surgery is possible.
Keywords: congenital heart disease, neurodevelopmental outcome
Congenital heart defects (CHD) are among the most common birth defects, and nearly one third of them will require surgical intervention in infancy.1 Thanks to advances in cardiothoracic surgical methods, extracorporeal circulation techniques, and intensive care medicine, it is now possible to undertake definitive corrective surgery early in life. However, infants with complex CHD require a multistep approach and often undergo palliative surgery first to improve oxygen saturation and haemodynamic conditions. Increasing survival rates have been associated with a shift in focus from heart related morbidity and death to concern for brain integrity,2–5 and developmental and neurological outcome has come under increasing scrutiny. Several studies evaluating various surgical procedures in subsets of children with CHD have found evidence of developmental delay and neurological deficits in selected groups of infants.6–15 However, the extent of neurodevelopmental impairment in otherwise normal children with CHD who are subjected to cardiac surgery in early infancy still has to be defined.
To investigate this problem, we undertook prospective neurological and neurodevelopmental examinations before surgery (when possible) and at 1 year of age in a single institution cohort of consecutive infants with various types of CHD who had undergone corrective or palliative surgery within the first year of life. In contrast to most other studies, enrolment of infants was not limited to those with a specific diagnosis, such as transposition of the great arteries, but covered the whole spectrum of CHD, to reduce the chances of selection bias. Within the same tertiary care paediatric cardiology centre setting, a control group was recruited of infants not requiring any cardiac surgery or catheterisation during the first year of life, who were examined alongside the surgically treated infants with CHD at 1 year of age.
METHODS
Subjects
In the 18 month period between 15 April 1998 and 14 October 1999, 135 infants with CHD underwent cardiac surgery within their first 11 months at the German Heart Institute Berlin, and had none of the following exclusion criteria: previous cardiac surgery in another hospital; gestational age < 32 weeks; umbilical artery pH < 7.1; five minute Apgar score < 7; brain malformation as detected by ultrasound; or syndromes associated with neurodevelopmental disability such as Down’s syndrome or DiGeorge syndrome. One hundred and twelve of these infants were discharged alive after corrective or palliative surgery. Of those 112 eligible infants, 99 (88%) were enrolled in the neurodevelopmental follow up programme with informed parental consent. The parents of 13 infants decided against participating because they lived too far away (> 300 km, n = 8) or for other unspecified reasons (n = 5).
We also recruited 20 control outpatients with no or only minor cardiac defects without haemodynamic significance (for example, a small ventricular or atrial septal defect). Those infants were seen for echocardiography in our hospital clinics. The control infants were born within the same time period as the infants with CHD who had undergone open heart surgery. The control infants had never been admitted to hospital for heart disease, nor had received any specific drug treatment.
The study was approved by the institutional review board of the Charité Medical Centre.
Operative and perioperative data
Cranial ultrasound examinations were undertaken before and after surgery. The type of surgical procedure, the use of cardiopulmonary bypass and deep hypothermic circulatory arrest (DHCA), the number and duration of cardiopulmonary bypass and DHCA procedures, the lowest rectal temperature, age at operation, duration of sedation and mechanical ventilation, length of stay in the intensive care unit, and overall length of hospital stay during the first year of life were noted.
Clinical seizures after surgery were recorded by the nurses or the physicians caring for the infant. The criteria for a definite clinical seizure included the occurrence of a single or a recurrent motor event with tonic or clonic movements of an extremity or cranial muscles, associated with change in consciousness not responsive to manipulation.
Neurodevelopmental assessment
Neurological examinations were carried out before surgery except in critically ill infants who required sedation, mechanical ventilation, or emergency surgical intervention; and again in all infants at 1 year of age, at least six weeks after the last surgical procedure. Standardised neurological examinations16 included assessment of muscle tone, movement patterns, posturing, deep tendon reflexes, and special senses (hearing and vision). Activity level was taken into account. Findings were classified as normal or abnormal, and abnormalities were scored as mild, moderate, or severe. Children with definite cerebral palsy were excluded from classification into any other categories except that of special senses (hearing and vision).
The Griffiths developmental scales,17 translated and standardised for a German reference population,18 were used to assess the developmental status of the infants; they covered locomotor skills, personal/social skills, hearing/speech, eye–hand coordination, and cognitive performance. Total developmental quotients (DQ) were calculated by dividing the number of items successfully executed by the infant’s age (in months). Corrections were made for preterm birth and any period of time an infant had been sedated and mechanically ventilated, assuming that psychomotor development is minimal or non-existent under sedation/mechanical ventilation. This conservative approach tends therefore to underestimate neurodevelopmental delay, especially in infants with complex CHD requiring prolonged intensive care. At the time of assessment, investigators were blinded to the type of cardiac defect and to the medical history. Measuring of somatic data (weight, length, head circumference) was undertaken by the same investigator.
Statistical analysis
We used SPSS for Windows 9.0 (SPSS Inc, Chicago, Illinois, USA) for all calculations. Probability values of p < 0.05 were considered to be significant. Data are given as mean (SD) or median with minimum and maximum. Categorical variables between groups were analysed by χ2 contingency tables, with Fisher exact calculations if one expected cell value was below 5. Continuous variables were compared by the Mann–Whitney U test or the Kruskal–Wallis test.
RESULTS
Patient characteristics and surgical intervention
Of the 99 infants with CHD originally enrolled, 98 were alive at one year of age. One infant was excluded after suffering severe hypoxic brain injury during a surgical procedure unrelated to CHD at 8 months of age. The parents of seven infants did not show up for the 1 year examination. Of the 90 infants actually examined at 1 year of age, 61% were male, 39% female, and 82% were from German mother tongue households. In the study group of 90 CHD infants examined, there were 50 cyanotic and 40 acyanotic heart defects. Sixty eight of the 90 infants (76%) were examined neurologically before their first cardiac operation.
Seventy five infants underwent surgery during cardiopulmonary bypass, and two infants during deep hypothermic circulatory arrest. Fourteen infants underwent more than one cardiac operation. Seventy four (82%) underwent anatomical correction of CHD, and 16 (18%) underwent palliative procedures and remained cyanotic after surgery.
The diagnostic categories of CHD of the infants with corrected heart defects and the infants who underwent palliative surgery are shown in table 1.
Table 1.
Diagnostic categories of congenital heart defects
| Corrective surgery (n=74) | |
| Transposition of the great arteries | 17 |
| Transposition of the great arteries with aortic arch hypoplasia | 1 |
| Anomalous pulmonary venous connection | 3 |
| Coarctation of the aorta | 10 |
| Coarctation of the aorta with ventricular septal defect | 1 |
| Atrial or ventricular septal defect or combination of both | 21 |
| Atrioventricular septal defect | 3 |
| Anomalous origin of the left coronary artery | 1 |
| Tetralogy of Fallot | 11 |
| Pulmonary atresia with ventricular septal defect | 1 |
| Hypertrophic obstructive cardiomyopathy | 1 |
| Interrupted aortic arch | 1 |
| Patent ductus arteriosus Botalli | 2 |
| Double outlet right ventricle with transposition of the great arteries | 1 |
| Palliative surgery (n=16) | |
| Tetralogy of Fallot | 3 |
| Transposition of the great arteries with ventricular septal defect and pulmonary valve stenosis | 1 |
| Tricuspid atresia (including one with transposition of the great arteries and one with pulmonary valve stenosis) | 4 |
| Pulmonary atresia with: | |
| – ventricular septal defect | 3 |
| –combined atrioventricular septal defect | 2 |
| –double outlet right ventricle with transposition of the great arteries | 1 |
| Tricuspid hypoplasia and hypoplastic right ventricle | 2 |
The control group comprised the following cardiac diagnoses: ventricular septal defect (9), atrial septal defect (7), normal heart (2), aneurysm of the coronary arteries (1), patent ductus arteriosus Botalli (1); 45% were male, 55% female, and 75% were from German mother tongue households.
Surgical interventions in the infants with CHD are given in table 2, and their characteristics are shown in table 3. The three groups (corrective surgery, palliative surgery, and controls) did not differ with respect to maternal age, maternal education, marital status, family income, birth data (weight, lengths, head circumference), gestational age, Apgar scores, and age at neurodevelopmental examination. Infants who underwent corrective surgery did not differ from the palliative surgery group with respect to age at first surgery or use and duration of cardiopulmonary bypass; infants with corrected heart defects scored lower for rectal temperature during surgery than infants who underwent palliative surgery (p = 0.001). Infants in the corrective surgery group had a shorter duration of hospital stay than those in the palliative surgery group (p = 0.001). Also, the percentage of infants with repeated cardiac operations was smaller in the corrective group than in the palliative surgery group (p = 0.016). At the time of follow up, the percentage of children with microcephaly (head circumference < 3rd centile) and low body weight (weight < 3rd centile) was smaller in the corrective group than in the palliative surgery group (p = 0.053 and p = 0.003, respectively).
Table 2.
Surgical interventions of infants with congenital heart disease
| Variable | Corrective surgery (n=74) | Palliative surgery (n=16) |
| Infants with two surgical procedures | 8 | 4 |
| Infants with three surgical procedures | 0 | 2 |
| Number of operations with CBP | 69 | 17 (central aorto-pulmonary shunt, n=6; central aorto-pulmonary shunt + outflow tract reconstruction, n=7; Glenn op, n=4) |
| Number of operations without CPB | 13 (repair of coarctation of the aorta, n=11; ligation of patent ductus arteriosus Botalli, n=2) | 7 (central aorto-pulmonary shunt, n=7) |
| Number of operations with deep hypothermic circulatory arrest | 2 (repair of interrupted aortic arch, n=1; repair of transposition of the great arteries with aortic arch hypoplasia, n=1) | 0 |
CBP, cardiopulmonary bypass.
Table 3.
Characteristics of infants with congenital heart disease
| Variable | Corrective surgery (n=74) | Palliative surgery (n=16) | Controls (n=20) |
| Preoperative characteristics | |||
| Birth data | |||
| Body weight <3rd centile | – | 1 (6%) | – |
| Body length <3rd centile | 1(1%) | – | – |
| Head circumference <3rd centile | – | 1 (7%)* | – |
| Gestational age (weeks) | 40 (32 to 42) | 40 (35 to 40) | 40 (35 to 41) |
| Apgar score at 5 minutes | 10 (7 to 10) | 9 (7 to 10) | 10 (8 to 10) |
| Age at first surgery (months) | 3 (0 to 11) | 3 (0 to 10) | – |
| Heart defect | |||
| Cyanotic | 34 (46%) | 16 (100%) | – |
| Oxygen saturation before surgery (%) | 96 (33 to 99) | 77 (40 to 95) | – |
| Oxygen saturation at time of neurodevelopmental testing (%) | 99 (98 to 100) | 85 (78 to 93) | – |
| Surgical data | |||
| CPB | 62 (84%) | 13 (81%) | – |
| Circulatory arrest | 2 (3%) | – | – |
| Lowest rectal temperature (°C) | 30 (15 to 36) | 36 (30 to 36.5) | – |
| Repeated cardiac surgery† | 8 (11%) | 6 (38%) | – |
| Perioperative data | |||
| Length of stay on intensive care unit (days)‡ | 4 (1 to 22) | 6 (2 to 52) | – |
| Duration of hospital stay (days)§ | 22 (7 to 166) | 62 (19 to 315) | 0 (0 to 3) |
| Sociodemographic factors | |||
| Maternal age at birth (years) | 30 (26 to 33) | 28 (17 to 43) | 30 (20 to 39) |
| Maternal education | |||
| Completed high school or more | 66 (89%) | 14 (88%) | 19 (95%) |
| Marital status | |||
| Married or living with partner | 65 (88%) | 14 (88%) | 18 (90%) |
| Family income per year (euros) | |||
| <15 000 | 21 (28%) | 4 (25%) | 5 (25%) |
| 15 000 to 30 000 | 40 (54%) | 12 (75%) | 10 (50%) |
| >30 000 | 13 (18%) | – | 5 (25%) |
| Follow up data at 1 year | |||
| Age at examination (months) | 12.2 (10.8 to 14.4) | 12.1 (10 to 14.1) | 12.4 (10.6 to 14) |
| Body weight <3rd centile¶ | 15 (20%) | 7 (44%) | – |
| Body length <3rd centile | 8 (11%) | 3 (19%) | – |
| Head circumference <3rd centile** | 7 (10%) | 7 (44%) | – |
Data are numbers (percentages) for categorical, or median (range) for numerical variables.
*Data available from 14 children.
†Corrective v palliative surgery; p=0.016.
‡Corrective v palliative surgery; p=0.050.
§Corrective v palliative surgery; p=0.001.
¶Corrective v palliative surgery; p=0.053.
**Corrective v palliative surgery; p=0.003.
Neurodevelopmental examination before surgery
Of the 68 infants who underwent neurodevelopmental examination before first cardiac surgery, 25 (37%) had neurological abnormalities that were judged to be mild in 23 (92%) and moderate in two (8%). Neurological abnormalities were seen in 19 of 58 infants (33%) before corrective surgery, compared with six of 10 infants (60%) before palliative surgery (p = 0.1) (table 4).
Table 4.
Neurological findings in infants before corrective or palliative cardiac surgery (some infants had multiple neurological abnormalities)
| Corrective surgery (n=58) | Palliative surgery (n=10) | |
| Overall abnormalities | 19 (33%) | 6 (60%) |
| Hypotonia | 10 (17%) | 2 (20%) |
| Hypertonia | 7 (12%) | 3 (30%) |
| Asymmetria | 5 (9%) | 2 (20%) |
| Strabism | 3 (5%) | 2 (20%) |
| Cerebral palsy | 0 | 0 |
Early clinical course after surgery
All infants had normal cranial ultrasound scans before surgery. Two infants had intracranial haemorrhage after surgery, one with interrupted aortic arch who underwent corrective surgery, and one with tetralogy of Fallot who received a central aortopulmonary shunt. Both suffered clinical seizures. Four further infants developed seizure activity during the early postoperative course without abnormal findings in the cranial ultrasound scans. Thus, six of the 90 infants (7%) had seizures during the postoperative course (3/74 (4%) after corrective surgery compared with 3/16 (19%) after palliative surgery; p = 0.066). None of these infants had seizures after discharge.
Both infants with intracranial haemorrhage showed developmental delay at 1 year of age (more than 2 SD below the mean of controls, see below). One suffered from cerebral palsy and the other had muscular hypotonia. All four infants with postoperative seizures but normal ultrasound scans had a normal developmental outcome at 1 year of age (developmental quotient within the mean ± 2 SD range of controls), with a normal neurological examination in three and muscular hypotonia in one.
Developmental testing
The time elapsed between the last date of cardiac surgery and the date of the neurodevelopmental examination was 9.6 months (range 1.8–13.7 months) in the corrective surgery group, and 9.8 months (range 2.1–13.2 months) in the palliative surgery group. Seventy nine per cent (71/90) were examined at least six months after the last cardiac surgical procedure. Only three infants (5%) underwent cardiac surgery during the two months before follow up, with a duration of hospital admission not exceeding three weeks.
The mean (SD) DQ in children who underwent cardiac surgery was 99 (10.6), and in the controls it was 106.7 (6.6) (p < 0.001). DQ values were lower in infants after palliative surgery (88 (12.2)) than after corrective surgical procedures (101.4 (8.6); p < 0.001). The DQ of the children who underwent only one surgical intervention was slightly higher than in children with more than one cardiac operation (p = 0.022). However, DQ did not differ between children with one surgical intervention and those with repeated operative procedures in either the corrective surgery group (101.8 (8.4) v 98.5 (10.6); p = 0.38) or the palliative surgery group (89.7 (17.3) v 85.1 (9.7); p = 0.33).
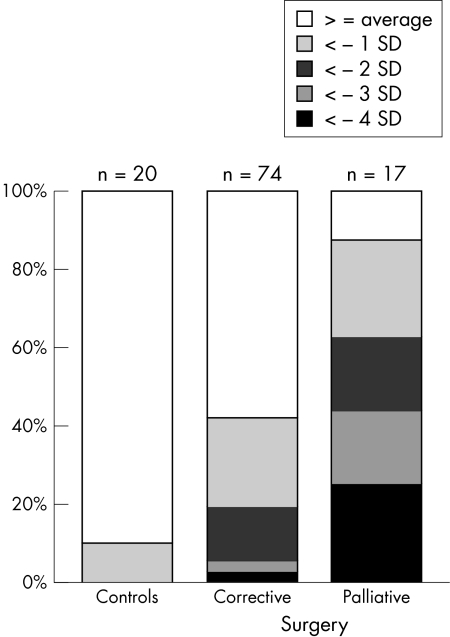
Twenty four of the 90 infants (27%) who underwent cardiac surgery and none of the controls had a DQ below 93.5, equivalent to more than 2 SD below the mean of controls. Thus defined, developmental delay (DQ < 93.5) was more common among infants who had undergone palliative surgery (10/16, 63%) than in the group who had undergone corrective surgery (14/74, 19%) (p < 0.001). The relative percentage of infants with more severe developmental impairment was consistently higher in the palliative surgery group than in the corrective surgery group (fig 1). The differences in psychomotor development between controls and infants who had undergone cardiac surgery, as well as those between infants who had undergone corrective or palliative surgery, were evident throughout all five subscales (locomotor skills, personal and social skills, hearing and speech, eye–hand coordination, and cognitive performance), as shown in table 5. In all subscales, differences were significant for the comparison of cardiac surgery versus controls, with p values ranging from 0.001 to 0.046, and for the comparison of palliative versus corrective surgery, with p values ranging from < 0.001 to 0.015.
Figure 1.
Developmental quotients at 1 year of age in infants with congenital heart disease who had undergone corrective or palliative surgery, respectively, compared with controls. Data of controls were used to calculate mean and standard deviation, and infants were then grouped accordingly.
Table 5.
Scores of developmental subscales in infants who had undergone corrective or palliative surgery, respectively, compared with controls
| Scores | Corrective surgery (n=74) | Palliative surgery (n=16) | Controls (n=20) |
| Locomotor skills | 99.4 (20.1) | 73.5 (16.4) | 108.2 (19.7) |
| Personal and social skills | 103.0 (9.1) | 91.3 (14.0) | 107.7 (8.9) |
| Hearing and speech | 94.9 (11.4) | 85.1 (14.6) | 98.6 (8.5) |
| Eye and hand coordination | 101.7 (9.6) | 91.6 (14.6) | 109.8 (9.8) |
| Cognitive performance | 102.5 (13.6) | 92.5 (12.3) | 109.7 (10.3) |
The main categories of CHD that were surgically corrected showed comparable psychomotor developmental scores, with no differences noted in the statistical tests: transposition of the great arteries (n = 17), DQ 103.9 (6.5); coarctation of the aorta (n = 10), DQ 103.2 (8.9); atrial or ventricular septal defect or combination of both (n = 21), DQ 101.7 (7.3); tetralogy of Fallot (n = 11), DQ 101.2 (7.8). All groups scored higher than the infants with palliative cardiac surgery, with p values ranging from < 0.001 to 0.005.
Neurological examination
Of the 90 infants who underwent cardiac surgery, 29 (32%) had neurological abnormalities—judged to be mild in 22 (76%) or moderate in seven (24%)—compared with one of the 20 control infants (5%) (p = 0.013) (table 6). Neurological abnormalities were seen in 18 of 74 infants (24%) after corrective surgery, compared with 11 of 16 infants (69%) after palliative surgery (p = 0.0005).
Table 6.
Neurological findings at 1 year of age in infants who had undergone corrective or palliative cardiac surgery or controls (some infants had multiple neurological abnormalities)
| Corrective surgery (n=74) | Palliative surgery (n=16) | Controls (n=20) | |
| Overall abnormalities | 18 (24%) | 11 (69%) | 1 (5%) |
| Hypotonia | 5 (7%) | 7 (44%) | 0 |
| Hypertonia | 6 (8%) | 0 | 0 |
| Asymmetry | 8 (11%) | 2 (13%) | 1 (5%) |
| Strabismus | 6 (8%) | 2 (13%) | 0 |
| Cerebral palsy | 0 | 1 (6%) | 0 |
Of the 68 children who had neurological examination before surgery, 25 (37%) had neurological abnormalities. Nine (36%) also showed neurological abnormalities at 1 year (six children with corrected heart defects and three who underwent palliative surgery).
Of the 22 children who could not be examined neurologically before surgery, 10 (45%) had abnormal neurological status at 1 year (four of six children with palliative surgery and six of 16 children with corrected heart defects).
Infants not followed up
In all, 13 infants were lost to follow up, either because they lived too far away or because the parents were worried about subjecting the child to stress. With one exception, those infants had undergone corrective surgery. Otherwise, the 13 infants lost to follow up and the 90 infants who actually underwent neurodevelopmental assessment at 1 year of age did not differ significantly with respect to any of the variables in table 3.
DISCUSSION
Although the majority of children who underwent cardiac surgery in the first year of life had neurodevelopmental scores within the normal range at 1 year of age, we found an association between cardiac surgery in the first year and a significantly poorer performance on tests of developmental and neurological function compared with a control population tested at the same time. The magnitude of the effect was considerable, as the difference between the neurodevelopmental scores of the two groups was approximately 1.1 SD, and more than one quarter of all children studied after cardiac surgery were considered to be developmentally delayed. In addition, mild and moderate neurological deficits became apparent in nearly a third of the infants at the age of 1 year. More severe heart defects not amenable to surgical correction in the first year of life were accompanied by an increased risk of developmental and neurological abnormalities at 1 year. Nearly two thirds of the infants with complex cardiovascular malformations not amenable to corrective surgery showed developmental delay, and a similar number of infants displayed neurological deficits.
Our study group included infants with various types of CHD presenting the whole spectrum of congenital heart defects, with a variety of haemodynamic situations before and after surgery. These infants also differed with respect to the mode of surgical approach, the number of surgical interventions, and the chronological age at which surgery was performed. Therefore a multitude of risk factors may have contributed to the low developmental scores and the high neurological morbidity at 1 year in our cohort.
A subset of the children with complex CHD may have subtle congenital cerebral dysgenesis3,19,20 not detected by cranial ultrasound before surgery, as well as submicroscopic chromosomal aberrations, both of which may influence neurodevelopmental outcome. The haemodynamic and homeostatic implications of CHD carry a significant risk for brain injury before cardiac surgery. Damage may be caused by cerebral venous thrombosis, thromboembolism, infarction, abscess formation, mycotic aneurysms, and hypoperfusion.6,21–24 In this cohort of patients, minor neurological abnormalities were found before surgery in more than a third of the infants examined, which is in agreement with findings from other centres.4,25–27 However, only a third of infants with neurological abnormalities before surgery still had these at 1 year of age. In contrast to a recent study,27 microcephaly in our cohort was a rare finding at birth. However, it was present in a quarter of the children at 1 year, the majority of whom had undergone palliative cardiac surgery. These findings underline the heterogeneous factors that may have an adverse influence on the developmental outcome of infants with CHD before and after surgery.
During surgery with cardiopulmonary bypass, the infant’s brain may be subjected to global or focal ischaemia caused by gaseous and particulate microemboli and hypoperfusion.28–32 Haemodynamic instability and medical complications during postoperative intensive care may then have additional adverse effects on the central nervous system.5,33 The developing brain shows great vulnerability during synaptogenesis, also known as the brain growth spurt, which in humans extends from the sixth month of gestation into the first year of life.34 During this time, exposure of the brain to barbiturates, benzodiazepines, and several other neurotropic drugs commonly used in paediatric anaesthesia and intensive care medicine may cause widespread apoptotic neurodegeneration, deleting millions of neurones.35 Excitotoxic sequelae of ischaemic events may be aggravated in the developing neonatal brain, showing patterns that are quite different from those observed later in life.36
In calculating the age at evaluation we subtracted any time during which an infant was sedated or mechanically ventilated, and this led to a conservative estimate of the infant’s psychomotor retardation, using mean and SD of the population standard (105.6 and 8.0) dating from the 1970s18 instead of those of the control group (106.7 and 6.6). This could have reduced the number of infants with CHD who were considered to have developmental delay. Higher scores in an actual control group, as compared with scores that would be expected from the standardised norms of the Griffiths test, have also been observed recently in England.7 While these considerations may be important for the interpretation of the data gathered, they do not change the pattern of the results. Interestingly, the overall magnitude of neurological deficits and psychomotor retardation of the children with corrected heart defect in this investigation was similar to that in a study of 1 year old infants with transposition of the great arteries who had undergone open heart surgery using cardiopulmonary bypass with or without circulatory arrest.9
In addition to conditions causing frank or subtle brain damage, developmental delay in children with severe CHD has been attributed to four further factors7:
children with cyanotic CHD are often physically less able to interact with their environment, limiting their exploratory behaviour
maternal overprotectiveness may limit the child’s social interaction
the inconsistent human and physical environment encountered during prolonged and repeated hospital admissions may compromise psychomotor development
feeding difficulties may make parents feel inadequate, causing them to withdraw emotional support from their infant.
These factors are apparently related to illness severity, and the CHD infants having palliative surgery not only comprised those with the most severe illness, as measured by prolonged and repeated hospital admissions, but they also had the worst neurodevelopmental outcome. However, the data from this investigation are not suitable for identifying the relative contributions of medical and psychosocial factors.
Although developmental tests such as the Griffiths developmental scales have concurrent validity, there are several limitations inherent in testing at the age of 1 year. The scores of 12 month old children have limited predictive validity.37,38 Domains such as language or visual–motor integration are not easily tested in very young children,39 and the abilities of these children will become more evident in the ensuing years.
Conclusions
Infants undergoing cardiac surgery have an increased risk for poor neurodevelopmental outcome at 1 year of age. Neurodevelopmental deficits are especially prominent in infants with complex cardiac malformations in whom only palliative surgery is possible in the first year of life. As the majority of these infants are candidates for additional surgical procedures (for example, a Fontan operation), their overall risk for neurodevelopmental deficits later in life may increase even further. We conclude that neurodevelopmental monitoring is highly desirable for infants and children with complex cardiac malformations, to provide more information about the risk factors, about when to institute preventive measures, and about when to start early intervention programmes. It is important that all children needing surgical intervention be monitored neurodevelopmentally, starting in early infancy, and that those with developmental deficits be identified. Early intervention programmes may have a positive influence on these children’s development.40 Assessments of the children during the ensuing years of life will clarify the degree to which they can compensate for their deficits, and whether our findings have clinical importance for later academic achievement.
Acknowledgments
This study was supported in part by the German Foundation for Cardiac Research (Deutsche Stiftung für Herzforschung).
REFERENCES
- 1.Gillum RF. Epidemiology of congenital heart disease in the United States. Am Heart J 1994;127:919–27. [DOI] [PubMed] [Google Scholar]
- 2.Ferry PC. Neurologic sequelae of cardiac surgery in children. Am J Dis Child 1987;141:309–12. [DOI] [PubMed] [Google Scholar]
- 3.Ferry PC. Neurologic sequelae of open-heart surgery in children. An “irritating question”. Am J Dis Child 1990;144:369–73. [DOI] [PubMed] [Google Scholar]
- 4.Newburger JW, Jonas RA, Wernovsky G, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med 1993;329:1057–64. [DOI] [PubMed] [Google Scholar]
- 5.Bloom AA, Wright JA, Morris RD, et al. Additive impact of in-hospital cardiac arrest on the functioning of children with heart disease. Pediatrics 1997;99:390–8. [DOI] [PubMed] [Google Scholar]
- 6.Bellinger DC, Wernovsky G, Rappaport LA, et al. Cognitive development of children following early repair of transposition of the great arteries using deep hypothermic circulatory arrest. Pediatrics 1991;87:701–7. [PubMed] [Google Scholar]
- 7.Wray J, Sensky T. Controlled study of preschool development after surgery for congenital heart disease. Arch Dis Child 1999;80:511–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haneda K, Itoh T, Togo T, et al. Effects of cardiac surgery on intellectual function in infants and children. Cardiovasc Surg 1996;4:303–7. [DOI] [PubMed] [Google Scholar]
- 9.Bellinger DC, Jonas RA, Rappaport LA, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med 1995;332:549–55. [DOI] [PubMed] [Google Scholar]
- 10.Oates RK, Simpson JM, Cartmill TB, et al. Intellectual function and age of repair in cyanotic congenital heart disease. Arch Dis Child 1995;72:298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller G, Tesman JR, Ramer JC, et al. Outcome after open-heart surgery in infants and children. J Child Neurol 1996;11:49–53. [DOI] [PubMed] [Google Scholar]
- 12.Sharma R, Choudhary SK, Mohan MR, et al. Neurological evaluation and intelligence testing in the child with operated congenital heart disease. Ann Thorac Surg 2000;70:575–81. [DOI] [PubMed] [Google Scholar]
- 13.Wernovsky G, Stiles KM, Gauvreau K, et al. Cognitive development after the Fontan operation. Circulation 2000;102:883–9. [DOI] [PubMed] [Google Scholar]
- 14.Mahle WT, Clancy RR, Moss EM, et al. Neurodevelopmental outcome and lifestyle assessment in school-aged and adolescent children with hypoplastic left heart syndrome. Pediatrics 2000;105:1082–9. [DOI] [PubMed] [Google Scholar]
- 15.Wray J, Sensky T. Congenital heart disease and cardiac surgery in childhood: effects on cognitive function and academic ability. Heart 2001;85:687–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thun-Hohenstein L, Largo RH, Molinari L, et al. Early fine motor and adaptive development in high-risk appropriate for gestational age preterm and healthy term children. Eur J Pediatr 1991;150:562–9. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths R. Abilities of young children. A comprehensive system of mental measurement for the first eight years of life. London: Young and Son, 1970.
- 18.Brandt I. Griffiths Entwicklungsskalen (GES) zur Beurteilung der Entwicklung in den ersten beiden Lebensjahren. Basel: Beltz Verlag, 1983.
- 19.McConnell JR, Fleming WH, Chu WK, et al. Magnetic resonance imaging of the brain in infants and children before and after cardiac surgery. A prospective study. Am J Dis Child 1990;144:374–8. [DOI] [PubMed] [Google Scholar]
- 20.Miller G, Vogel H. Structural evidence of injury or malformation in the brains of children with congenital heart disease. Semin Pediatr Neurol 1999;6:20–6. [DOI] [PubMed] [Google Scholar]
- 21.Newburger JW, Silbert AR, Buckley LP, et al. Cognitive function and age at repair of transposition of the great arteries in children. N Engl J Med 1984;310:1495–9. [DOI] [PubMed] [Google Scholar]
- 22.O’Dougherty M, Wright FS, Loewenson RB, et al. Cerebral dysfunction after chronic hypoxia in children. Neurology 1985;35:42–6. [DOI] [PubMed] [Google Scholar]
- 23.Mayer JE. Development, growth, and cardiac surgery. Am J Dis Child 1991;145:33–4. [DOI] [PubMed] [Google Scholar]
- 24.du Plessis AJ, Chang AC, Wessel DL, et al. Cerebrovascular accidents following the Fontan operation. Pediatr Neurol 1995;12:230–6. [DOI] [PubMed] [Google Scholar]
- 25.Brunberg JA, Reilly EL, Doty DB. Central nervous system consequences in infants of cardiac surgery using deep hypothermia and circulatory arrest. Circulation 1974;50:II60–8. [PubMed] [Google Scholar]
- 26.Limperopoulos C, Majnemer A, Shevell MI, et al. Neurologic status of newborns with congenital heart defects before open heart surgery. Pediatrics 1999;103:402–8. [DOI] [PubMed] [Google Scholar]
- 27.Limperopoulos C, Majnemer A, Shevell MI, et al. Neurodevelopmental status of newborns and infants with congenital heart defects before and after open heart surgery. J Pediatr 2000;137:638–45. [DOI] [PubMed] [Google Scholar]
- 28.Moody DM, Bell MA, Challa VR, et al. Brain microemboli during cardiac surgery or aortography. Ann Neurol 1990;28:477–86. [DOI] [PubMed] [Google Scholar]
- 29.Greeley WJ, Ungerleider RM. Assessing the effect of cardiopulmonary bypass on the brain. Ann Thorac Surg 1991;52:417–19. [DOI] [PubMed] [Google Scholar]
- 30.Aoki M, Jonas RA, Nomura F, et al. Anti-CD18 attenuates deleterious effects of cardiopulmonary bypass and hypothermic circulatory arrest in piglets. J Card Surg 1995;10:407–17. [DOI] [PubMed] [Google Scholar]
- 31.Jonas RA. Neurological protection during cardiopulmonary bypass/deep hypothermia. Pediatr Cardiol 1998;19:321–30. [DOI] [PubMed] [Google Scholar]
- 32.du Plessis AJ. Neurologic complications of cardiac disease in the newborn. Clin Perinatol 1997;24:807–26. [PubMed] [Google Scholar]
- 33.Park SC, Neches WH. The neurologic complications of congenital heart disease. Neurol Clin 1993;11:441–62. [PubMed] [Google Scholar]
- 34.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev 1979;3:79–83. [DOI] [PubMed] [Google Scholar]
- 35.Ikonomidou C, Bittigau P, Ishimaru MJ, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science 2000;287:1056–60. [DOI] [PubMed] [Google Scholar]
- 36.Felderhoff-Mueser U, Ikonomidou C. Mechanisms of neurodegeneration after paediatric brain injury. Curr Opin Neurol 2000;13:141–5. [DOI] [PubMed] [Google Scholar]
- 37.Ross G. Some thoughts on the value of infant tests for assessing and predicting mental ability. J Dev Behav Pediatr 1989;10:44–7. [PubMed] [Google Scholar]
- 38.Ulvund SE. Predictive validity of assessments of early cognitive competence in light of some current issues in development psychology. Hum Dev 1984;27:76–83. [DOI] [PubMed] [Google Scholar]
- 39.Ross G, Lipper EG, Auld PA. Consistency and change in the development of premature infants weighing less than 1,501 grams at birth. Pediatrics 1985;76:885–91. [PubMed] [Google Scholar]
- 40.Majnemer A. Benefits of early intervention for children with developmental disabilities. Semin Pediatr Neurol 1998;5:62–9. [DOI] [PubMed] [Google Scholar]