Abstract
Objective: To assess arterial distensibility using pulse wave velocity (PWV) measurements and its relation with endothelium dependent vasodilatation (EDV) in patients with cardiological syndrome X.
Methods: The study group consisted of 92 patients: 52 with syndrome X (34 women, 18 men, mean (SD) age 45 (3) years) and 40 healthy volunteer controls (27 women, 13 men, mean (SD) age 41 (2) years) without risk factors of atherosclerosis and with negative ECG exercise test and normal proximal coronaries on transoesophageal echocardiography. Patients with arterial hypertension, diabetes mellitus, valvar disease, or cardiomyopathy were excluded. PWV measured by a Complior Colson device was calculated for each patient. EDV was assessed from two dimensional Doppler measurement using an Acuson Sequoia with 8 MHz linear transducer at rest, during postischaemic reactive hyperaemia, and after an oral dose of 400 μg of glyceryl trinitrate.
Results: PWV was significantly higher in patients with syndrome X than in healthy subjects (9.3 (0.7) m/s v 8.2 (0.9) m/s, respectively, p < 0.001). Baseline brachial artery diameter was similar in the syndrome X and control groups (4.0 (0.6) mm v 4.08 (0.64) mm, NS). EDV was impaired in patients with syndrome X compared with controls (6.6 (3.0)% v 11.1 (3.9)%, p < 0.001). Endothelium independent vasodilatation was similar in both groups. In patients with syndrome X there was a positive correlation between PWV and the degree of EDV (r = 0.864, p < 0.001). The cut off value for PWV was 8.5 m/s, with a sensitivity of 62% and a specificity of 91%.
Conclusions: EDV but not glyceryl trinitrate induced vasodilatation is decreased in patients with syndrome X. There is a strong correlation between PWV and the degree of endothelial dysfunction of peripheral arteries in patients with syndrome X. PWV assessment may be useful to identify abnormal vascular physiology in these patients.
Keywords: endothelial function, pulse wave velocity, atherosclerosis, syndrome X
Approximately 10% of coronary arteriograms performed for the assessment of patients with chest pain show normal coronary anatomy.1–3 Chest pain is the hallmark of syndrome X, but its aetiology in this group of patients appears to be heterogeneous and remains unclear. Patients with syndrome X are characterised by the presence of chest pain, ST segment depression, and a positive ECG exercise test but normal coronary angiogram.4 The underlying mechanism of ischaemia in these patients has not been described. An abnormal coronary flow reserve may indicate that the observed symptoms in syndrome X have a microvascular origin.5 Invasive methods of assessing endothelium dependent and independent vasomotor responses of coronary arteries have shown that vasodilation in response to pharmacological or metabolical agents is impaired.6 Abnormal findings of myocardial perfusion in syndrome X can be explained by an impaired vasodilation capacity of coronary arteries.7 A common finding in the majority of patients with syndrome X is endothelial dysfunction of the coronary arteries.8 After supplementation with l-arginine, improvement in endothelium dependent vasodilation (EDV) was observed, which suggests that impaired nitric oxide synthesis may have a key role in the development of symptoms in syndrome X. However, some studies failed to show such a relation, indicating that there are defects in adenosine metabolism.9 The correlation between EDV of coronary and brachial artery vasomotor responses, especially in patients with coronary artery disease, has been described.10,11 Findings of Teragawa and colleagues12 suggest that flow mediated dilatation of the brachial artery may be used to detect the presence of coronary artery disease in patients suspected of having it. Abnormal brachial dilatation has also been observed in patients with microvascular angina or with coronary spastic angina.13,14
The speed of pulse wave propagation throughout the arterial tree depends on the elastic and geometric properties of the arterial wall. Since blood is an incompressible fluid enclosed in the system of elastic arteries, the energy released by ventricular ejection is propagated mainly along the arterial wall. An increase in pulse wave velocity (PWV) may be used as an early indicator of arterial stiffening, characteristic of the development of atherosclerosis.15 The aim of this study was, by assessing endothelial function of the brachial artery, to investigate whether endothelial dysfunction in patients with syndrome X is a generalised process. To examine the systemic vascular bed we also measured pulse wave propagation as a marker of arterial distensibility. Our secondary objective was to investigate a possible correlation between endothelial function and PWV measurements in patients with syndrome X.
METHODS
Patient population
The study population consisted of 52 patients with angina, without prior myocardial infarction (mean (SD) age 45 (3) years, 34 women, 18 men) who underwent coronary angiography in our cardiology department during 1998 and 1999. They fulfilled the characteristic criteria for the diagnosis of syndrome X: angina-like chest pains, positive ECG exercise test, normal coronary arteries on angiography, and no metabolic disorders.
Exclusion criteria were a previous diagnosis of dilated or constrictive cardiomyopathy, a previous myocardial infarction, arterial hypertension, metabolic disorders such as diabetes mellitus, dyslipidaemia, and insulin resistance syndrome, ECG conductibility and rhythm disorders, valvar heart disease, Barlow’s syndrome, or any other known functional peripheral arterial disorders. None of our patients was treated at the time of examination with angiotensin converting enzyme inhibitors, long acting nitrates, statins or hormone replacement therapy.
Forty healthy volunteers (mean (SD) age 41 (2) years, 27 women, 13 men) without any symptoms or risk factors of coronary artery disease and with a normal ECG and echocardiogram were enrolled in the control group. Each patient from the control group underwent an ECG exercise test, which was negative. Coronary angiography was not performed in this group for ethical reasons. Before enrolment in the control group, each patient underwent transoesophageal echocardiographic examination of proximal parts of the coronary arteries. Those with abnormal findings in transoesophageal echocardiography or turbulent flow suggesting the presence of atherosclerosis in the coronary arteries were excluded from the study.
Study protocol
An exercise test (Bruce protocol) was said to be positive if there was at least 0.2 mV of horizontal ST segment depression or elevation in at least two leads. The ECG exercise test was aborted if the heart rate reached submaximal values, patients were complaining of chest pain, complex forms of cardiac arrhythmias were observed, or criteria for a positive ECG test were reached.
Blood samples were taken from each patient and analysed for serum urea, electrolyte, cholesterol, and triglyceride concentrations.
Echocardiographic assessment was performed in all patients according to the standards of the American Society of Echocardiography, with measurements of diastolic and systolic septal and posterior wall thickness, left ventricle diameters, and ejection fraction.
Coronary angiography was performed in patients with syndrome X using the standard Judkins technique on a General Electric Advantax LX system (General Electric Medical Systems, Milwaukee, Wisconsin, USA). All images were digitally stored in DICOM format on an Hewlett Packard Visualise workstation (Hewlett-Packard Co, Palo Alto, California, USA) for further analysis.
Endothelial function was assessed with an 8 MHz linear array transducer and Acuson Sequoia 256 echocardiographic system (Acuson, Mountain View, California, USA) according to the protocol described by Fathi and colleagues16 and Celermajer and associates.17 The right brachial artery was scanned at rest, during reactive hyperaemia, and after administration of sublingual glyceryl trinitrate (GTN). Before the first scan the patient rested in the supine position for at least 10 minutes. Then the brachial artery diameter and blood flow velocity were measured. Blood flow was measured from the pulsed Doppler signal at a maximum incidence angle of 60°, with the Doppler gate positioned in the middle of the arterial lumen. Reactive hyperaemia was induced with inflation of a pneumatic tourniquet to a pressure of 200 mm Hg for five minutes. The artery diameter was measured 45–60 seconds after cuff deflation and flow velocity recordings were started 15 seconds after cuff release. After 15 minutes an additional baseline scan was recorded and then four minutes after 400 μg sublingual GTN was given, the last images were collected. ECG monitoring was continuous during the procedure, and ECG R wave measurements were averaged from four consecutive beats. Arterial diameters were measured from the anterior to the posterior “m” line at the end of diastole. All measurements were taken by two observers who were unaware of clinical details. The previously estimated mean (SD) intersonographer variability of flow mediated dilatation measurement in our echocardiography laboratory was 1.2 (0.4)%.
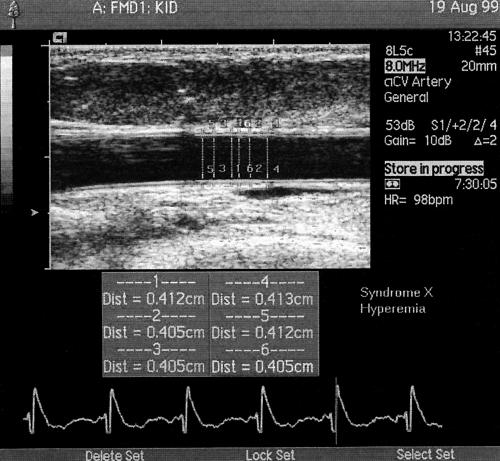
Vasodilatation was expressed as the percentage increase in artery diameter during reactive hyperaemia (EDV) and after GTN administration (endothelium independent vasodilatation). Reactive hyperaemia was calculated as the maximum flow over the 15 seconds after cuff release divided by the baseline flow values (fig 1).
Figure 1.
Brachial artery scans after reactive hyperaemia. During each examination the result was calculated as the mean of six measurements.
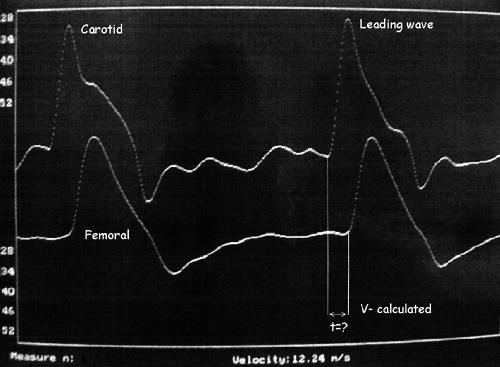
PWV was measured automatically with a Complior Colson device based on an IBM Aptiva 486 DX personal computer according to the methods described by Asmar and colleagues.18 PWV was calculated from measurements of the pulse transit time between two recording sites, the femoral and carotid external arteries, at a sampling frequency of 500 Hz. During preprocessing analysis the gain of each waveform was adjusted to obtain an equal signal for the two waveforms. During PWV measurements, after pulse waveforms of sufficient quality were recorded, the digitisation process was initiated by the operator and automatic calculation of the time delay between two upstrokes was started (fig 2). The interobserver repeatability (repeatability coefficient) for the automatic PWV calculation in our laboratory was 0.876 (95% confidence interval ± 1.95 repeatability coefficient).
Figure 2.
Pulse wave measurement in patients with syndrome X.
Statistical analysis was performed with the Statistica 5.0 data analysis program using non-parametrical U Mann-Whitney tests. Correlations were calculated with the Spearman test. All results are expressed as mean (SD).
The investigation conforms with the principles outlined in the Declaration of Helsinki.
RESULTS
Table 1 shows demographic and biochemical data.
Table 1.
Basic data in patients with syndrome X and the control group
| Syndrome X group | Control group | p Value | |
| Number of patients | 52 | 40 | NS |
| Women | 34 | 27 | NS |
| Average age (years) | 45 (3) | 41 (2) | 0.01 |
| Smokers | 3 | 0 | 0.01 |
| Positive family history for IHD | 5 | 0 | 0.01 |
| Systolic blood pressure (mm Hg) | 124.7 (7.9) | 124.8 (7.2) | NS |
| Diastolic blood pressure (mm Hg) | 77.1 (6.3) | 77.1 (6.2) | NS |
| Total cholesterol (mmol/l) | 4.72 (0.19) | 4.68 (0.22) | NS |
| LDL cholesterol (mmol/l) | 2.72 (0.32) | 2.71 (0.33) | NS |
| HDL cholesterol (mmol/l) | 1.52 (0.14) | 1.53 (0.15) | NS |
| Triglycerides (g/l) | 1.04 (0.12) | 1.01 (0.13) | NS |
| Resting heart rate (beats/min) | 75.6 (7.8) | 71.0 (9.7) | 0.01 |
| Maximum heart rate (beats/min) | 151 (10) | 164 (12) | 0.01 |
| Maximum ST depression (mm) | 2.4 (0.4) | 0.9 (0.4) | 0.01 |
| Exercise test duration (min) | 6.8 (1.8) | 9.6 (0.9) | 0.01 |
| Ischaemic pain present (number) | 29 | 0 | NA |
Values are mean (SD). HDL, high density lipoprotein; IHD, ischaemic heart disease; LDL, low density lipoprotein; NA, not applicable; NS, not significant.
Because of the enrolment criteria, patients in the control group were younger than those in the syndrome X group (mean (SD) 41 (2) years v 45 (3) years, respectively) to minimise the risk for silent coronary disease.
Blood analysis showed significantly higher plasma concentrations of total cholesterol, low density lipoprotein fraction, and triglycerides in the syndrome X population than in controls. High density lipoprotein concentrations were much lower in the syndrome X group. However, all of these values were within the normal ranges (table 1).
ECG exercise tests were positive only in patients with syndrome X. In the syndrome X group, baseline heart rate was significantly higher and total exercise phase was significantly shorter than in the controls (table 1). In 29 patients with syndrome X, chest pain occurred during the test at a mean (SD) exercise time of 4.9 (1) minutes.
Echocardiographic examination confirmed that both groups had normal cardiac size and function with clinically insignificant differences between the groups. The ejection fraction was slightly lower in patients with syndrome X than in the control group.
Arterial vasodilator tests
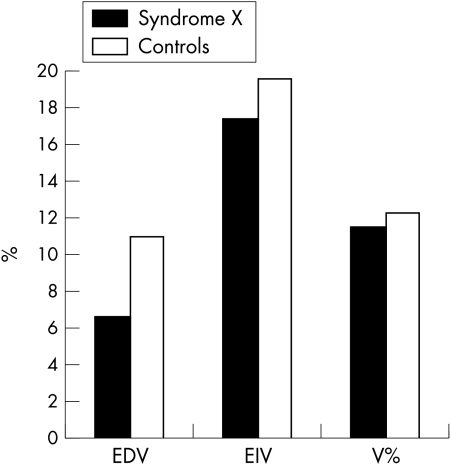
Under baseline conditions there were no significant differences in measurements of brachial artery diameter (4.0 (0.6) mm v 4.08 (0.64) mm, NS) and baseline blood flow velocities (74.1 (14.2) cm/s v 73 (13.9) cm/s, NS) between the syndrome X and control groups, respectively. After oral administration of GTN the mean increase in blood flow velocity and in arterial diameter was similar in both groups and there was no significant difference in endothelium independent vasodilatation (mean (SD) percentage arterial diameter increase 18.1 (5.1)% v 19.6 (5.1)%, NS).
After the induction of reactive hyperaemia the mean (SD) increase in arterial diameter was significantly lower in patients with syndrome X than in controls (0.27 (0.12) mm v 0.44 (0.16) mm, respectively), which showed that EDV was significantly impaired in patients with syndrome X (6.6 (3.0)% v 11.1 (3.9)% in controls, p < 0.001) (fig 3).
Figure 3.
Comparison of mean increase in brachial artery diameter after reactive hyperaemia between the syndrome X and control groups.
During hyperaemia the increase in blood flow velocity was similar in the syndrome X and control groups.
PWV measurements
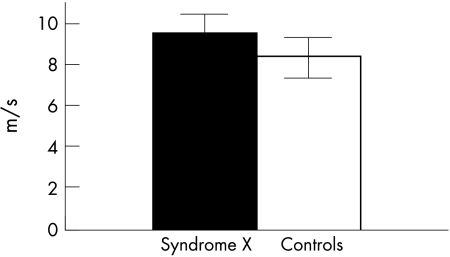
In the syndrome X group pulse wave propagation time between measurement sites was significantly shorter than in the control group (65.1 (6.2) ms v 76.1 (8.2) ms, respectively, p < 0.001).
Comparing with the syndrome X group, PWV values were significantly lower in controls (9.3 (0.7) m/s v 8.2 (0.9) m/s, p < 0.001), as fig 4 shows.
Figure 4.
Mean (SD) pulse wave velocity (m/s) measurements in the study groups.
A PWV of 8.5 m/s allows for differentiation between groups with a sensitivity of 62% and a specificity of 91%.
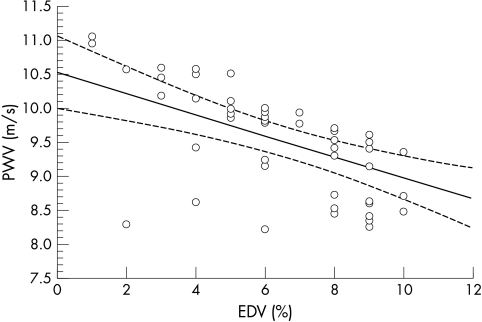
Further analysis showed the presence of a strong inverse correlation between PWV and EDV (fig 5). The regression equation is PWV = 15.07 − 0.75 × EDV, Pearson’s r = −0.864, p < 0.01.
Figure 5.
Correlation between pulse wave velocity (PWV) and endothelium dependent vasodilatation (EDV), where PWV = 15.07 − 0.75 × EDV, Pearson’s r = −0.864, p<0.01.
In our study we did not find any significant correlation between PWV and age, sex, or mean blood pressure in the studied groups.
DISCUSSION
Our study showed two main abnormalities in peripheral arterial function in patients with syndrome X: impaired EDV and increased PWV. The response to nitrates was not altered, indicating that endothelium independent vasodilatation is not impaired in patients with syndrome X.
In previous studies reduced acetylcholine induced vasodilation of coronary arteries in syndrome X was reported.19 Endothelial dysfunction in coronary arteries may lead to reduction in arterial flow reserve and induce ischaemic pain in syndrome X. This theory may by supported by the results of endothelium vasodilator tests performed with intracoronary acetylcholine infusions, which showed an abnormal constrictive reaction especially in sites with increased risk for development of atherosclerosis such as proximal parts or bifurcation of the arteries in patients with syndrome X.6–8,19
Several conditions such as hypercholesterolaemia, hyperglycaemia, low high density lipoprotein cholesterol, acute postprandial hypertriglyceridaemia, active and passive cigarette smoking, susceptibility of low density lipoprotein to oxidation, hypertension, and early stages of atherosclerosis are associated with impaired EDV.20,21 In studies performed by Celermajer and colleagues,22,23 they observed a strong correlation between atherosclerotic risk factors and the degree of endothelial dysfunction. Anderson and colleagues24 showed that brachial artery vasodilator tests correlated with measures of coronary endothelial function.
Results of our study indicate that impaired endothelial function in syndrome X is likely to be a generalised process involving the peripheral conduit arteries and may be similar to that observed in atherosclerotic heart disease.11 Evidence of generalised endothelial dysfunction in syndrome X was also described in other studies.25 In some patients with impaired flow mediated vasodilation defects of thallium 201 distribution were also described; moreover, endothelial function in these patients was restored after administration of l-arginine, indicative of nitric oxide synthesis defects in syndrome X.7
Pathogenesis of chest pain in syndrome X is not well explained. The absence of atherosclerotic changes in coronary angiography cannot exclude the possibility of intramural plaques. As described in an intravascular ultrasonography study by Erbel and colleagues,26 despite the lack of stenoses in coronary angiography, abnormal changes in coronary artery walls were common, usually increased intima thickness, although small intramural atherosclerotic plaques were also found.
Our second finding in syndrome X was an increased PWV, reflecting increased arterial stiffness. A close relation between increased PWV and atherosclerosis development has been reported.27,28 Measurements in patients with coronary artery disease showed decreased compliance of large arteries. To the best of our knowledge, PWV has not been assessed before in patients with syndrome X. In our study we found a significant difference in PWV between patients with syndrome X and healthy controls, and we established a cut off value of PWV that is useful in differentiating these patients with satisfactory specificity and sensitivity. PWV measurements provide highly reproducible estimates of arterial distensibility, particularly with the automated method proposed by Asmar and colleagues.18 Some studies suggest that the PWV increase may be used as an early indicator of atherosclerosis development, and a significant decrease in compliance in large arteries was found in patients with atherosclerosis risk factors such as heterozygous familial hypercholesterolaemia.29
A significant correlation between increased arterial stiffness and atherosclerosis formation was described. In some studies, however, hypertension was shown to influence arterial rigidity more than arteriosclerotic risk factors.30 All patients selected for our study were normotensive so as to minimise the effect of arterial remodelling caused by increased arterial pressure.
EDV impairment in our patients with syndrome X was closely correlated with PWV values. Our results indicating impairment of endothelial function and decreased arterial distensibility may support the theory that the vascular abnormalities in syndrome X are a generalised process similar to those observed in the early stages of atherosclerosis.
Study limitations
We did not undertake an intravascular ultrasound examination, the ultimate means of visualising coronary vascular anatomy. However, our intention was to test non-invasive techniques for analysing the function of systemic arteries in syndrome X. Even with intravascular ultrasound technology, subtle anatomical abnormalities in distal parts of the coronary arteries may be undetectable. The age difference between groups may also have influenced our results, but we did not find a correlation between PWV and age in the studied patient populations, nor any correlation between endothelial function and age.
The results of our study do not provide firm evidence that there is a direct link between increased PWV and myocardial perfusion impairment in syndrome X, although the presence of a correlation between PWV and the degree of endothelial dysfunction may indicate such a relation. The cut off value of PWV was measured retrospectively and further prospective studies should be performed to investigate the usefulness of this parameter in identifying patients with syndrome X.
Conclusions
EDV is impaired in peripheral arteries of patients with syndrome X, and a significant rise in PWV reflects the increased arterial wall stiffness in such patients. A strong inverse correlation between PWV and EDV may indicate generalised arterial dysfunction in these patients. PWV measurements may be useful in differentiating patients with syndrome X from healthy subjects.
Abbreviations
EDV, endothelium dependent vasodilatation
GTN, glyceryl trinitrate
PWV, pulse wave velocity
REFERENCES
- 1.Camici PG, Gistri R, Lorenzoni R. Coronary reserve end exercise ECG in patients with chest pain and normal coronary angiograms. Circulation 1992;86:179–86. [DOI] [PubMed] [Google Scholar]
- 2.Cannon RO, Camici PG, Epstein SE. Pathophysiological dilemma of syndrome X. Circulation 1992;85:883–92. [DOI] [PubMed] [Google Scholar]
- 3.Cannon RO, Epstein SE.Microvascular angina as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol 1986;16:1338–43. [DOI] [PubMed] [Google Scholar]
- 4.Favararo L, Masimi F, Maffei M. Syndrome X recenti. Prog Med 1989;80:281–5. [PubMed] [Google Scholar]
- 5.Cianflone D, Lanza GA, Maseri A. Microvascular angina in patients with normal coronary arteries and with other ischaemic syndromes. Eur Heart J 1995;16(suppl I):96–103. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan A, Mullins PA, Taylor G, et al. Both endothelium-dependent and endothelium-independent function is impaired in patients with angina pectoris and normal coronary angiograms. Eur Heart J 1997;18:60–8. [DOI] [PubMed] [Google Scholar]
- 7.Fragasso G, Rossetti E, Dosio F, et al. High prevalence of the thallium-201 reverse redistribution phenomenon in patients with syndrome X. Eur Heart J 1996;17:1482–7. [DOI] [PubMed] [Google Scholar]
- 8.Motz W, Vogt M, Rebenau O, et al. Evidence of endothelial dysfunction in coronary resistance vessels in patients with angina pectoris and normal coronary angiograms. Am J Cardiol 1991;68:996–1003. [DOI] [PubMed] [Google Scholar]
- 9.Bottcher M, Botker HE, Sonne H, et al. Endothelium-dependent and -independent perfusion reserve and the effect of L-arginine on myocardial perfusion in patients with syndrome X. Circulation 1999;99:1795–801. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman EH, Gerhard MD Uehata A, et al. Flow-induced vasodilatation of human brachial artery is impaired in patients in patients >40 years of of age with coronary artery disease. Am J Cardiol 1996;78:1210–4. [DOI] [PubMed] [Google Scholar]
- 11.Neunteufl T, Katzenschlager R, Hassan A, et al. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis 1997;129:111–8. [DOI] [PubMed] [Google Scholar]
- 12.Teragawa H, Kato M, Kurokawa J, et al. Usefulness of flow-mediated dilation of the brachial artery and/or the intima-media thickness of the carotid artery in predicting coronary narrowing in patients suspected of having coronary artery disease. Am J Cardiol 2001;88:1147–51. [DOI] [PubMed] [Google Scholar]
- 13.Bellamy MF, Goodfellow J, Tweddel AC, et al. Syndrome X and endothelial dysfunction.Cardiovasc Res 1998;40:410–7. [DOI] [PubMed] [Google Scholar]
- 14.Motoyama T, Kawano H, Kugiyama K, et al. Flow-mediated, endothelium-dependent dilatation of the brachial arteries is impaired in patients with coronary spastic angina. Am Heart J 1997;133:263–7. [DOI] [PubMed] [Google Scholar]
- 15.Blacher J, Asmar R, Djane S, et al. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 1999;33:1111–7. [DOI] [PubMed] [Google Scholar]
- 16.Fathi R, Marwick T. Noninvasive tests of vascular function and structure: why and how to perform them. Am Heart J 2001;141:694–703. [DOI] [PubMed] [Google Scholar]
- 17.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992;340:1111. [DOI] [PubMed] [Google Scholar]
- 18.Asmar RG, Benetos A, Topouchian J, et al. Assessement of arterial distensibility by automatic pulse wave velocity measurement. Hypertension 1995;26:485–90. [DOI] [PubMed] [Google Scholar]
- 19.Egashira K, Inou T, Hirooka Y, et al. Evidence of impaired endothelium dependent coronary vasodilatation in patients with angina pectoris and normal coronary angiograms. N Engl J Med 1993;328:1059–64. [DOI] [PubMed] [Google Scholar]
- 20.Piatti PM, Fragasso G, Monti LD, et al. Endothelial and metabolic characteristics of patients with angina and angiographically normal coronary arteries: comparison with subjects with insulin resistance syndrome and normal controls. J Am Coll Cardiol 1999;34:1452–60. [DOI] [PubMed] [Google Scholar]
- 21.Lambert J, van den Berg M, Steyn M, et al. Familial hyperhomocysteinaemia and endothelium-dependent vasodilatation and arterial distensibility of large arteries. Cardiovasc Res 1999;42:743–51. [DOI] [PubMed] [Google Scholar]
- 22.Celermajer DS, Sorensen KE, Bull C, et al. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol 1994;24:1468–74. [DOI] [PubMed] [Google Scholar]
- 23.Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 1993;88:2149–55. [DOI] [PubMed] [Google Scholar]
- 24.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 1995;26:1235–41. [DOI] [PubMed] [Google Scholar]
- 25.Lekakis JP, Papamichael CM, Vemmos CN, et al. Peripheral vascular endothelial dysfunction in patients with angina pectoris and normal coronary arteriograms. J Am Coll Cardiol 1998;31:541–6. [DOI] [PubMed] [Google Scholar]
- 26.Erbel R, Ge J, Bockisch A, et al. Value of intracoronary ultrasound and Doppler in the differentiation of angiographically normal coronary arteries: a prospective study in patients with angina pectoris. Eur Heart J 1996;17:880. [DOI] [PubMed] [Google Scholar]
- 27.Taquet A, Bonithon-Kopp C, Simon A, et al. Relations of cardiovascular risk factors to aortic pulse wave velocity in asymptomatic middle-aged women. Eur J Epidemiol 1993;9:298–306. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson IB, Webb DJ, Cockroft JR. Aortic pulse-wave velocity. Lancet 1999;354:1996–7. [DOI] [PubMed] [Google Scholar]
- 29.Pitsavos C, Toutouzas K, Dernellis J, et al. Aortic stiffness in young patients with heterozygous familial hypercholesterolemia. Am Heart J 1998;135:604–8. [DOI] [PubMed] [Google Scholar]
- 30.Bortolotto LA, Blacher J, Kondo T, et al. Assessment of vascular aging and atherosclerosis in hypertensive subjects, second derivative of photoplethysmogram versus pulse wave velocity. Am J Hypertens 2000;13:165–71. [DOI] [PubMed] [Google Scholar]