Abstract
A suite of protein and nonprotein amino acids were detected with high-performance liquid chromatography in the water- and acid-soluble components of an interior fragment of the Martian meteorite Nakhla, which fell in Egypt in 1911. Aspartic and glutamic acids, glycine, alanine, β-alanine, and γ-amino-n-butyric acid (γ-ABA) were the most abundant amino acids detected and were found primarily in the 6 M HCl-hydrolyzed, hot water extract. The concentrations ranged from 20 to 330 parts per billion of bulk meteorite. The amino acid distribution in Nakhla, including the d/l ratios (values range from <0.1 to 0.5), is similar to what is found in bacterially degraded organic matter. The amino acids in Nakhla appear to be derived from terrestrial organic matter that infiltrated the meteorite soon after its fall to Earth, although it is possible that some of the amino acids are endogenous to the meteorite. The rapid amino acid contamination of Martian meteorites after direct exposure to the terrestrial environment has important implications for Mars sample-return missions and the curation of the samples from the time of their delivery to Earth.
Since the report by McKay et al. (1) that the Martian meteorite ALH84001 contained possible evidence for life on Mars, essentially all of the ensuing controversy has focused on Martian meteorites collected in Antarctica. Given the intense interest in the question of life on Mars, it is surprising that little attention has been directed to the Shergotty, Nakhla, and Chassigny (SNC) class of meteorites, many of which did not fall in Antarctica. Of particular interest is Nakhla, the first Martian meteorite found to contain carbonates and hydrous minerals apparently associated with aqueous alteration processes on Mars (2, 3). Carbonates are present in both Nakhla and ALH84001, and they are enriched in 13C (δ13C = +30‰ to +55‰), compared with carbonates on Earth, indicating that these minerals are Martian in origin (4). Nakhla has a crystallization age of only 1.3 × 109 years (5, 6) in contrast to ∼4.5 × 109 years for ALH84001 (7). Based on secondary mineral assemblages, the Nakhla parent rock was apparently exposed to aqueous solutions after it was formed (8), which raises interesting questions: How long was the era that aqueous processes may have persisted on Mars; and does Nakhla carry evidence of organic compounds that were present on Mars during that period.
In contrast to ALH84001, which has a terrestrial age of 13,000 years (1), Nakhla was an observed fall that occurred about 40 km east of Alexandria, Egypt (31°19′N, 30°21′E) near the oasis of El Nakhla el Baharia at approximately 9 a.m. on June 28, 1911 (9). The Nakhla stones were apparently derived from the explosion of a larger meteorite, which left a trail of white smoke inclined 30° to the horizontal. The area in which the meteorite fragments were found was personally investigated by W. F. Hume of the Geological Survey of Egypt within 20 days after the fall (10). About 40 stones of various sizes, some of which had been retrieved by local eye-witnesses within a day of the fall, were collected; they had a combined mass of approximately 10 kg (9, 10). Approximately half of the recovered stones were covered by a shiny, black fusion crust; one of these, acquired by the Natural History Museum (London) in 1913, was recently made available to researchers. We report here the results of amino acid analyses on an interior piece of this meteorite stone, 2 to 3 cm from the fusion crust. The Nakhla specimen contains a unique set of amino acids that are distinctly different from the Antarctic Martian meteorites EETA79001 and ALH84001, which contain amino acids apparently derived from Antarctic ice meltwater (11, 12).
MATERIALS AND METHODS
The Nakhla Meteorite and Control Samples.
The single piece of Nakhla (split 7, parent 0) weighing approximately 2.2 g, was selected for this study from the interior of the [Natural History Museum (London)] Nakhla specimen by the National Aeronautics and Space Administration (NASA) Johnson Space Center meteorite curator (M. Lindstrom). Although the early curatorial history of the stone is unclear, over the last approximately 30 years the meteorite has been stored in a desiccator (M. Lindstrom, personal communication). The sample was inspected at the Scripps Institution of Oceanography (SIO) under a microscope; many amber-colored globules were noted. These globules are similar to the “iddingsite” aqueous alteration features reported previously in Nakhla (2, 3).
Two mineral samples were used for evaluation of procedural blanks. Crushed serpentine (a hydrated magnesium silicate) and palagonite (an amorphous, weathered basaltic glass) were heated at 500°C for several hours and then processed at the same time as the Nakhla samples. For reference samples, an interior piece of the Murchison carbonaceous chondrite (13, 14), a powdered sample of the Antarctic Martian meteorite ALH84001 (12), and a sample sediment from the seafloor at the Nile Delta (15) were analyzed simultaneously with Nakhla. The Nile Delta sample was from a core collected in 1964 by the German research vessel Meteor at a water depth of 120 m, off the coast near the site of the Nakhla fall. The core has been stored at the GEOMAR Research Center for Marine Geosciences (Kiel, Germany) at a temperature of 10°C to 15°C over the last 10 years (J. Gruetzner, personal communication). We analyzed an oriented slice from the top 0 to 15 cm of the core. We assume this sediment is representative of the deltaic environment of the Nakhla landing site. To our knowledge, soil samples from the fall locality were not collected with the Nakhla meteorite stones in 1911. Samples collected from this now highly developed area would not be representative of the rural agricultural farmland region that existed there in 1911.
Processing Procedures.
Amino acids and their enantiomeric ratios were independently investigated in Nakhla, in the meteorite control samples, and in blanks by laboratories at SIO and at the NASA Jet Propulsion Laboratory (JPL), by using similar extraction procedures and analyses by HPLC, coupled with o-phthaldialdehyde/N-acetyl-l-cysteine (OPA/NAC) (Sigma Aldrich) derivatization (16, 17) and UV-fluorescence detection. Single chunks of the Nakhla and ALH84001 meteorite samples were crushed into a fine powder with an annealed (500°C overnight) mortar and pestle in a positive pressure (1-μm filtered air) clean room and then transferred to clean vials. The Murchison meteorite and the Nile Delta samples were both fine grained and did not require crushing prior to analysis.
A portion of each sample (∼200 mg) was sealed in a clean test tube with 1 ml of double-distilled water and boiled for 24 hr in a heating block set at 100°C. The tubes containing the samples were cooled to room temperature and the outside was rinsed with a 50:50 mixture of 95% ethanol and double-distilled water. The ethanol was found to contain traces of ethanolamine. The test tubes were then opened and centrifuged for 5 min to separate sample particulate from the H2O supernatant. Ten percent of the water supernatant from each tube was transferred to a new test tube, dried under vacuum and desalted by using cation exchange resin (AG50W-X8, Bio-Rad ) prior to HPLC analysis to determine free amino acids associated with the bulk sample (unhydrolyzed water-soluble fraction). The remaining water supernatant was transferred to a separate test tube, dried under vacuum, hydrolyzed in double-distilled liquid 6 M HCl at 100°C for 24 hr or under 6 M HCl vapor at 150°C for 3 hr (18, 19), desalted, and analyzed by using OPA/NAC derivatization and HPLC separation to determine bound amino acids in the bulk sample matrix (hydrolyzed water-soluble fraction). To investigate the free and bound amino acids associated exclusively with the carbonate component of these samples, separate Nakhla, Murchison, and Nile Delta samples were extracted by using both 1 M and 6 M HCl instead of hot water, following the procedure used in a previous analysis (12) of ALH84001.
RESULTS AND DISCUSSION
Meteorite Amino Acid Analyses.
The analyses of Nakhla revealed that amino acids were present mainly in the 6 M HCl-hydrolyzed, hot water extract (Fig. 1). The predominant amino acids detected were glutamic acid and glycine, with lesser amounts of aspartic acid, serine, alanine, β-alanine, and γ-amino-n-butyric acid (γ-ABA). A similar distribution of amino acids was also found in hot 6 M HCl extracts of Nakhla. However, we found that after the Nakhla sample was treated directly with hot 6 M HCl, a much lower yield of amino acids was recovered (4 to 186 ppb) than in the acid-hydrolyzed water extract (18 to 327 ppb). This finding indicates that some of the amino acids were destroyed during direct 6 M HCl extraction. In contrast, only trace levels of serine, glycine, and alanine were detected in the 6 M HCl hydrolyzed, hot water extract of ALH84001, as previously reported (12). A much more diverse set of amino acids was found in the hot water extract of Murchison, which is consistent with the results of previous investigations of this meteorite (13). The amino acids α-aminoisobutyric acid (AIB) and isovaline, which are two of the most abundant amino acids detected in Murchison and which are characteristic of amino acids of apparently extraterrestrial origin (14), were not detectable in any of the extracts of Nakhla or ALH84001.
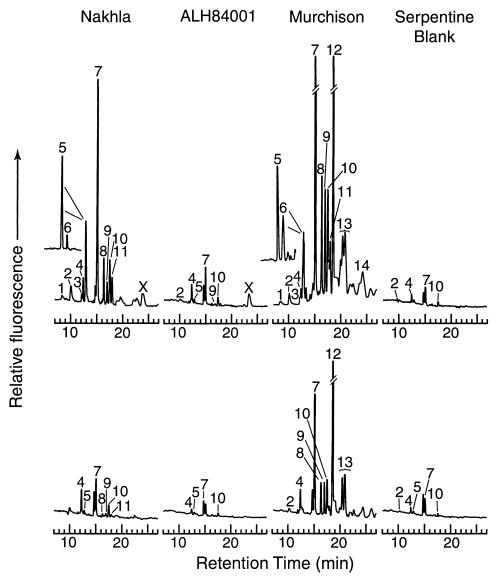
Figure 1.
The 7 to 27 min region of the HPLC chromatograms from the SIO analyses (similar chromatograms were obtained from JPL and are available on request). OPA/NAC derivatization (15 min) of amino acids in the 6 M HCl-hydrolyzed (Upper) and unhydrolyzed (Lower) hot water extracts from the Martian meteorite Nakhla, the Antarctic Martian meteorite ALH84001, the Murchison carbonaceous chondrite, and the serpentine blank. All procedures were performed at 25°C, and flow rates were 1 ml/min. With the exception of glutamic acid, conditions for amino acids enantiomeric separations for the mobile phase were as follows: A (50 mM sodium acetate) at pH 5.6, B (methanol); gradient, 0 to 4 min, 0% B; 4 to 14 min, 0% to 37% B; 14 to 24 min, 37% to 42% B; 24 to 29 min, 42% to 60% B. For glutamic acid separation (Inset) conditions for the mobile phase were A (50 mM sodium acetate) at pH 8.0, B (methanol); gradient, 0 to 1 min, 0% B; 1 to 16 min, 0% to 10% B; 16 to 22 min, 10% B; 22 to 29 min, 10% to 60% B. Peaks were identified by comparison of the retention times with those of an amino acid standard run in parallel, as follows: 1, d-aspartic acid; 2, l-aspartic acid; 3, d-serine; 4, l-serine; 5, l-glutamic acid; 6, d-glutamic acid; 7, glycine; 8, β-alanine; 9, d-alanine; 10, l-alanine; 11, γ-amino-n-butyric acid (γ-ABA); 12, α-aminoisobutyric acid (AIB); 13, d/l-isovaline; 14, methylamine; and X, unknown.
One peak observed only in the hot water extracts of both Nakhla and ALH84001 (marked “X” in Fig. 1), had a retention time (∼23 min) similar to methylamine. A similar peak had also been found in a previous examination of ALH84001 (12); however, after spiking the sample with authentic methylamine, two peaks were observed, indicating that the original peak was not methylamine (12). The mass of the unknown peak in Nakhla and ALH84001 could not be determined by liquid chromatography mass spectroscopy (LCMS) because of the very low concentration of this compound in these meteorites (<10 ppb). The peak remains unidentified in both of the Martian meteorite samples. The presence of methylamine in the hydrolyzed-water extract of the Murchison meteorite was confirmed and has also been detected in previous analyses of this meteorite (20, 21).
In the unhydrolyzed, hot water extract of Nakhla (Table 1), low levels (2 to 43 ppb above background) of serine, glycine, l-alanine, β-alanine, and γ-ABA were detected. However, much higher concentrations of these amino acids (18 to 327 ppb), along with several others, including d- and l-aspartic and glutamic acids, were found after acid hydrolysis of the hot water extract. Accordingly, the amino acids in the hot water extract derive almost entirely from some bound, acid-labile precursor, possibly a macromolecular material similar to humic acid. The large increase in amino acid yield after 6 M HCl hydrolysis was not observed in the hot water extract of ALH84001, which indicates that amino acids in that stone are predominantly in the free form.
Table 1.
Summary of the average blank corrected amino acid concentrations in the hot water extracts of the Martian meteorite Nakhla and of the Nile Delta sediment as measured by two separate analyses, one at SIO and the other at JPL
| Water extract* | d-Asp | l-Asp | d-Ser | l-Ser | d-Glu | l-Glu | Gly | β-Ala | d-Ala | l-Ala | γ-ABA | AIB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nakhla | ||||||||||||
| Free | <2 ± 1 | <2 ± 1 | 2 ± 1 | 16 ± 11 | <2 ± 1 | 1 ± 1 | 43 ± 16 | 14 ± 2 | <1 ± 1 | 33 ± 19 | 4 ± 3 | <1 ± 1† |
| Total | 23 ± 7 | 63 ± 12 | 21 ± 13 | 81 ± 35 | 44 ± 9 | 278 ± 7 | 327 ± 1 | 100 ± 15 | 18 ± 12 | 55 ± 11 | 99 ± 11 | <1 ± 1† |
| Nile Delta | ||||||||||||
| Free | 30 ± 21 | 293 ± 114 | 23 ± 15 | 123 ± 1 | 26 ± 18 | 83 ± 58 | 488 ± 84 | 187 ± 3 | <1 ± 1 | 4 ± 1 | 8 ± 5 | <1 ± 1† |
| Total | 337 ± 43 | 781 ± 60 | 174 ± 50 | 430 ± 141 | 298 ± 2 | 1,013 ± 143 | 2,168 ± 73 | 598 ± 142 | 256 ± 22 | 782 ± 59 | 205 ± 10 | <1 ± 1† |
All values are reported in parts per billion (ppb) on a bulk sample basis. The uncertainties are based on the SD of the average value of the two separate measurements.
Free indicates amino acids in an unhydrolyzed sample. Total (free + bound) indicates amino acids in a sample hydrolyzed by 6 M HCl.
Derivatization for 15 min.
d-Amino Acids in Nakhla.
One of the most interesting aspects of the Nakhla analyses is the presence of significant amounts of the d-enantiomers of aspartic and glutamic acids, as well as possibly of alanine, in the 6 M HCl-hydrolyzed, hot water extracts (the presence of d-alanine in Nakhla could not be confirmed by the JPL analyses). In contrast, no d-amino acids were detected in the Antarctic Martian meteorites EETA79001 and ALH84001 (11, 12) with the possible exception of minute amounts of d-alanine (<2 ppb) in the 6 M HCl acid-hydrolyzed, hot water extract of ALH84001. The calculated d/l ratios in the hydrolyzed, water extracts of Nakhla were 0.37 ± 0.19 for aspartic acid, 0.16 ± 0.05 for glutamic acid, and 0.33 ± 0.23 for alanine. The precise determination of these ratios is difficult, given the low concentrations of these amino acids in Nakhla.
If the amino acids detected in Nakhla had been formed by abiotic processes on Mars, they would originally have been racemic (d/l ∼1). Contamination of the meteorite by terrestrial l-amino acids after it fell to Earth could have significantly lowered the initial d/l ratios of any endogenous amino acids, perhaps to values close to those we measured. We have observed a similar lowering of d/l ratios in an interior sample of Murchison during its residence on Earth. In measurements carried out in 1970, soon after Murchison fell (13), the d/l ratios for alanine, aspartic acid, and glutamic acid were close to 1.0. Analyses of the same Murchison sample today (Fig. 1) yielded d/l ratios in the range of 0.3 to 0.5 for aspartic acid, 0.7 to 0.9 for glutamic acid, and 0.7 to 1.0 for alanine. Concentrations of the abiotic nonprotein amino acids in Murchison (14), such as AIB and racemic isovaline, were found to be similar in both the 1970 and 1998 analyses, indicating that these amino acids have not been affected by terrestrial contamination. The lack of AIB and racemic isovaline in Nakhla, and the presence of γ-ABA but not other 4-carbon amino acids such as α-ABA or β-ABA (14), suggests that the d-amino acids in Nakhla are probably biotic in origin.
Origin of d-Amino Acids, β-Alanine, and γ-ABA in Nakhla.
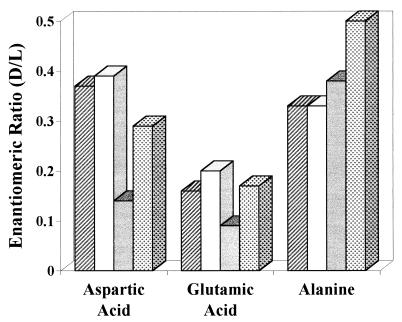
A more likely explanation for the presence of d-amino acids in Nakhla is contamination of the meteorite by bacterially derived amino acids associated with the environment of the landing site of the meteorite. The d/l ratios of aspartic acid, glutamic acid, and alanine that were found in Nakhla (Fig. 2) are similar to those found in a common marine cyanobacterium, Synechococcus bacillaris (22). Similar ratios have been found in ultrafiltered (<100-nm-pore size), oceanic dissolved organic matter in which the d-amino acids were attributed to peptidoglycan remnants derived from bacterial cell walls (22). In analyses of a Nile Delta sediment sample collected roughly 60 km offshore from the landing site, we found d/l ratios for aspartic acid, glutamic acid, and alanine that were similar to those found in Nakhla (Table 1 and Fig. 2).
Figure 2.
Enantiomeric ratios for aspartic acid, glutamic acid, and alanine in acid-hydrolyzed hot water extracts from the Martian meteorite Nakhla (column 1) and from the Nile Delta sediment (column 2). Also shown are enantiomeric ratios (from ref. 24) in a common marine bacterium, Synechococcus bacillaris (column 3), and in average ultrafiltered, dissolved, oceanic organic matter (column 4).
In addition to the presence of d-amino acids, the relatively high concentrations (∼100 ppb) of the nonprotein amino acids β-alanine and γ-ABA in Nakhla provide further evidence that the amino acids in this meteorite are bacterial in origin. β-Alanine and γ-ABA have been detected in near-shore marine sediments (23–25) and in deep ocean pelagic clay sediments, where these two amino acids are predominant (26, 27). We also detected high levels (190 to 800 ppb) of bound β-alanine and γ-ABA in the Nile Delta sediment (Table 1). The presence of β-alanine and γ-ABA in marine sediments is generally attributed to the bacterially mediated α-decarboxylation of aspartic and glutamic acids, which takes place during the diagenesis of sedimentary organic matter (28). β-Alanine can also be produced from the anaerobic decomposition of uracil (25). The ratio of β-Ala to γ-ABA in the Nile Delta sediment (∼4) is considerably higher than that in Nakhla (∼1), which could indicate other sources of these amino acids in Nakhla. However, the β-Ala/γ-ABA ratio in marine sediments is highly variable (values range from 0.5 to 4); thus the ratio in Nakhla is not unusual for sedimentary deposits.
Rapid Terrestrial Amino Acid Contamination of Meteorites.
Nakhla landed in an agricultural farmland region of the Nile River Delta that had experienced annual floods until the construction of the Aswan High Dam in Egypt was completed in 1970. Some of the stones apparently suffered extensive heating and fracturing during atmospheric entry and impact, exposing light green, diopside-olivine grains in the interior of the rock (10). The larger fragments (each ∼1.5 kg) were found buried to depths of 10 to 30 cm (9), and one landed in an estuarine environment near the shore of Lake Edku (10). Because we found that the Nile River Delta sediment contains a distribution of amino acids similar to those present in Nakhla, it is possible that bacterially derived amino acids present in the sediment porewaters penetrated the meteorite. According to our estimates, if the average concentration of dissolved organic carbon in surficial (0-cm to 30-cm) estuarine sediment porewater is 1 mM (29), and we assume that roughly 10% of the total dissolved organic carbon consists of amino acids, then only 65 ml of porewater fluid infiltrating the original ∼600-g Nakhla stone used in this study would be necessary to yield the levels of amino acids that we observe in Nakhla (∼1100 ppb).
Terrestrial contamination of the outer part of a meteorite at the time of its fall is inevitable, and in some cases this contamination has been observed to extend below the surficial fusion crust. For example, in the Allende meteorite that fell in Mexico in 1969, amino acid contaminants were found to extend from the surface to a depth of a few millimeters (30). If the amino acids in the Nakhla stone are terrestrial in origin, then amino acid contamination has apparently penetrated much farther into the rock (2 to 3 cm) than into the Allende meteorite.
The presence in Nakhla of the nonprotein amino acids β-alanine and γ-ABA, with the d-enantiomers of aspartic acid, glutamic acid, and alanine, is strikingly similar to patterns found in bacterially degraded sediments on Earth. This similarity strongly suggests that the amino acids in Nakhla are terrestrial in origin; however, we cannot completely rule out that some of the amino acids in Nakhla were present in the meteorite when it fell to Earth. Bacterially derived amino acids probably infected Nakhla soon after it arrived on Earth, even though the meteorite was observed as it fell and its exposure to the environment was limited to not more than a day to a few weeks. It is also possible that bacterial contamination took place some time during the nearly 90-year residence of the meteorite on Earth.
The contamination of meteorites by terrestrial amino acids leaves a distinct amino acid fingerprint on the meteorite of its landing-site environment and is an extremely rapid process. Unfortunately, this greatly compromises the possibility that Martian meteorites can be used to assess whether organic compounds important in biology as we know it are present on Mars. NASA is planning a series of missions that will collect and return Martian surface samples to the Earth in the year 2008. It is imperative that these pristine Martian samples be kept isolated upon arrival at Earth to minimize terrestrial exposure and contamination.
Acknowledgments
We thank the Meteorite Steering Working Group, the Natural History Museum (London), and M. Lindstrom at the NASA Johnson Space Center, for providing this sample; J. Gruetzner with GEOMAR for providing the Nile Delta core sample; K. Kvenvolden for providing the Murchison sample; G. Kminek and M. Schubert for helpful discussions, and M. Clark for assistance with the figures. This research was supported by grants from the NASA Ancient Martian Meteorite Research Program and the NASA Specialized Center for Research and Training in Exobiology at the University of California at San Diego.
ABBREVIATIONS
- ABA
amino-n-butyric acid
- AIB
α-aminoisobutyric acid
- JPL
Jet Propulsion Laboratory
- OPA/NAC
o-phthaldialdehyde/N-acetyl-l-cysteine
- SIO
Scripps Institution of Oceanography
References
- 1.McKay D S, Gibson E K, Jr, Thomas-Keprta K L, Vali H, Romanek C S, Clemett S, Chillier X D F, Maechling C R, Zare R N. Science. 1996;273:924–930. doi: 10.1126/science.273.5277.924. [DOI] [PubMed] [Google Scholar]
- 2.Bunch T E, Reid A M. Meteoritics. 1975;10:303–315. [Google Scholar]
- 3.Reid A M, Bunch T E. Meteoritics. 1975;10:317–324. [Google Scholar]
- 4.Jull A J T, Eastoe C J, Xue S, Herzog G F. Meteoritics. 1995;30:311–318. [Google Scholar]
- 5.Gale N H, Arden J W, Hutchison R. Earth Planet Sci Lett. 1975;26:195–206. [Google Scholar]
- 6.Nakamura N, Unruh D M, Tatsumoto M, Hutchison R. Geochim Cosmochim Acta. 1982;46:1555–1573. [Google Scholar]
- 7.Jagoutz E, Sorowka A, Vogel J D, Wanke H. Meteoritics. 1994;29:478–479. [Google Scholar]
- 8.Gooding J L, Wentworth S J, Zolensky M E. Meteoritics. 1991;26:135–143. [Google Scholar]
- 9.Prior G T. Mineral Mag. 1912;16:274–281. [Google Scholar]
- 10.Hume W F. Cairo Sci J. 1911;5:212–215. [Google Scholar]
- 11.McDonald G D, Bada J L. Geochim Cosmochim Acta. 1995;59:1179–1184. doi: 10.1016/0016-7037(95)00033-v. [DOI] [PubMed] [Google Scholar]
- 12.Bada J L, Glavin D P, McDonald G D, Becker L. Science. 1998;279:362–365. doi: 10.1126/science.279.5349.362. [DOI] [PubMed] [Google Scholar]
- 13.Kvenvolden K A, Lawless J G, Pering K, Peterson E, Flores J, Ponnamperuma C, Kaplan I R, Moore C. Nature (London) 1970;228:923–926. doi: 10.1038/228923a0. [DOI] [PubMed] [Google Scholar]
- 14.Kvenvolden K A, Lawless J G, Ponnamperuma C. Proc Natl Acad Sci. USA. 1971;68:486–490. doi: 10.1073/pnas.68.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Einsele G, Werner F German Science Foundation, editors. Meteor Scientific Research. C. Gebrueder Borntraeger (Berlin; 1968. pp. 21–42. [Google Scholar]
- 16.Zhao M, Bada J L. Nature (London) 1989;339:463–465. doi: 10.1038/339463a0. [DOI] [PubMed] [Google Scholar]
- 17.Zhao M, Bada J L. J Chromatogr A. 1995;690:55–63. doi: 10.1016/0021-9673(94)00927-2. [DOI] [PubMed] [Google Scholar]
- 18.Tsugita A, Uchida T, Mewes H W, Ataka T. J Biochem (Tokyo) 1987;102:1593–1597. doi: 10.1093/oxfordjournals.jbchem.a122209. [DOI] [PubMed] [Google Scholar]
- 19.Keil R G, Kirchman D L. Mar Chem. 1991;33:243–259. [Google Scholar]
- 20.Jungclaus G, Cronin J R, Moore C B, Yuen G U. Nature (London) 1976;264:126–128. [Google Scholar]
- 21.Pizzarello S, Feng X, Epstein S, Cronin J R. Geochim Cosmochim Acta. 1994;58:5579–5587. doi: 10.1016/0016-7037(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy M D, Hedges J I, Benner R. Science. 1998;281:231–234. doi: 10.1126/science.281.5374.231. [DOI] [PubMed] [Google Scholar]
- 23.Cole J J, Lee C. Biogeochemistry. 1986;2:299–312. [Google Scholar]
- 24.Cowie G L, Hedges J I. Nature (London) 1994;369:304–307. [Google Scholar]
- 25.Keil R G, Tsamakis E, Giddings J C, Hedges J I. Geochim Cosmochim Acta. 1998;62:1347–1364. [Google Scholar]
- 26.Schroeder R A, Bada J L. Earth-Sci Rev. 1976;12:347–391. [Google Scholar]
- 27.Wehlan J K. Geochim Cosmochim Acta. 1977;41:803–810. [Google Scholar]
- 28.Schroeder R A. Earth Planet Sci Lett. 1975;25:274–278. [Google Scholar]
- 29.Burdige D J, Zheng S. Limnol Oceanogr. 1998;43:1796–1813. [Google Scholar]
- 30.Oró J, Nakaparksin S, Lichtenstein H, Gil-Av E. Nature (London) 1971;230:107–108. doi: 10.1038/230107a0. [DOI] [PubMed] [Google Scholar]