Abstract
Background
Internal auditory artery (IAA) spasm is thought to be one of the causes of postoperative sensory hearing loss after attempted hearing preservation removal of an acoustic neuroma. The use of topical papaverine, a nonspecific vasodilator, to prevent vascular insufficiency to the inner ear and to improve hearing outcomes has been suggested but not proven.
Materials and Methods
Vasospasm was mechanically induced by compressing the IAA in the control ears of six rabbits after application of topical saline. The subsequent reduction of cochlear blood flow (CBF) was measured using a laser-Doppler (LD) flow-monitoring technique. Functional loss of cochlear activity was verified with distortion product otoacoustic emissions (DPOAE). The contralateral experimental ears were treated with the topical application of papaverine directly to the IAA and cochleovestibular nerve complex. CBF and DPOAE were compared between the control and papaverine treated ears for 3-minute and 5-minute IAA compressions.
Results
Every control ear demonstrated some degree of post-compression IAA vasospasm (i.e., reduced CBF) and reduction of DPOAE. Nearly complete recovery of CBF and DPOAE to baseline was observed in all of the papaverine treated ears.
Conclusions
An animal model of IAA vasospasm was described. Mechanically induced vasospasm of the IAA was prevented by the topical application of papaverine. These findings have clinical implications for surgical procedures involving the internal auditory canal/cerebellopontine angle such as acoustic neuroma removal.
Keywords: Vasospasm, Internal auditory artery, Acoustic neuroma, Papaverine, Rabbit, Distortion product otoacoustic emissions
Advances in magnetic resonance imaging (MRI) techniques have resulted in earlier diagnosis of acoustic neuromas (AN) and other internal auditory canal (IAC) and cerebellopontine angle (CPA) tumors (1–3). Many of these patients retain useful hearing in the ear with tumor (1–5). Attempts at hearing preservation tumor removal can be unsuccessful, even when the cochlear nerve is anatomically intact (6–8). In these cases, one possibility is that the blood supply to the cochlea, via the internal auditory artery (IAA), has been compromised. In addition to transection and thrombosis of the nutrient vessels, interruption of the cochlear circulation can result from vasospasm of the IAA caused by physical manipulation during IAC dissection (9,10). Topical application of vasodilators, such as papaverine, has been shown to prevent and reverse intracranial arterial vasospasm (11,12).
To date, no consistently effective technique for intra-operative monitoring of cochlear blood flow (CBF) has been described for human use during AN tumor removal surgery. However, a number of investigators have reported on the use of laser Doppler (LD) to measure CBF in a variety animal models (13–16). The current study used CBF measures based on the LD to monitor the effects of topical papaverine in an animal model of IAA vasospasm.
SUBJECTS AND METHODS
Subjects
Six young (younger than age 3 mo) albino rabbits weighing between 2 and 2.5 kg were used for this study. The protocol for the care and use of rabbits was approved by the Institutional Animal Care and Use Committee. Several days before experimentation, DPOAE at 2f1-f2 were obtained from each ear to assess adequate cochlear function using standard techniques (15,16). Briefly, two equilevel (L1 = L2) primary tones at an optimal f2-to-f1 ratio of 1.25 were acoustically mixed and presented to the ear via a custom-designed probe sealed tightly into the external auditory canal. DPOAE levels as a function of frequency, i.e., DP grams, were plotted from 1 to 12 kHz for equilevel primary tones from 45 to 75 dB sound pressure level (SPL) at 5-dB intervals. During the actual experimental manipulations of CBF, DP-tracking functions at 4, 8, and 12 kHz were obtained by monitoring DPOAE levels in response to L1 = L2 = 60-dB SPL primary tones. For both DP grams and the DP-tracking functions, DPOAE frequencies were converted to the geometric mean frequency (GMF) to adequately describe the generator site in rabbits for 2f1-f2 DPOAE at the stimulus levels used here. These steps were repeated just before surgery and also after the opening of the middle ear cavity to ensure continued normal cochlear function.
The animals were anesthetized with an intramuscular injection of ketamine hydrochloride (50 mg/kg) mixed with xylazine hydrochloride (10 mg/kg). The anesthetized animals were placed on a feedback-controlled hearing blanket set to maintain a core body temperature of 38°C within a standard double-walled, walk-in, sound-treated chamber (Industrial Acoustics, Bronx, NY). After otoscopic examination to confirm normal ears, a head stabilization device was placed according to a standard procedure previously described (17). Surgical anesthesia was maintained by administration of supplemental doses of ketamine/xylazine as needed according to regular monitoring for the elicitation of an eye-blink or pain reflex.
To measure CBF, a commercial device (LaserFlo BPM; Vasamedics, Eden Prarie, MN, U.S.A.), with a wavelength of 780 nm and an optical power of 2 mW at the probe tip was used. A rigid needle-sensor probe directed the laser beam. The sample rate for recording the CBF data was 2 points per second, with the online display consisting of a running average that was computed over a 10-second interval. CBF measurements are described as arbitrary units (AU), because the probe could not be specifically calibrated for measuring CBF from the cochlea. At the beginning of each experiment, the AU scale was arbitrarily set to 0 before the probe was placed in the RW niche of the cochlea.
Before performing an occlusion experiment, the timing clocks of the laser Doppler device and the microcomputer’s digital signal processing (DSP) board were synchronized. DPOAE levels and phases, as well as CBF, were then plotted as a function of time using customized software installed on a personal microcomputer (Macintosh Quadra 700; Apple Computers, Cupertino, CA, U.S.A.). The data were then transferred offline and analyzed using a commercially available spreadsheet (Microsoft Excel version 2000; Microsoft, Redmond, WA, U.S.A.).
Experimental Procedures
The subsequent surgical steps for exposing the IAA have been previously described in detail for a protocol developed in this laboratory (16,17). Briefly, after confirming that a particular rabbit’s DPOAE were consistent with the normal rabbit database for our laboratory, the first surgical step under visualization via an operating microscope was to expose the cochlea and round window (RW) through a postauricular approach for placement of the LD probe to measure CBF. To this end, by exposing the external surface of the auditory bulla with a curette and then drilling with a diamond burr, the middle was entered between the bulla’s thick and thin posterior walls. The cochlea was further exposed using either the diamond burr or the rongeurs until the RW was clearly visualized. After this procedure, the opening into the middle ear was closed using surgical gauze and a postsurgical DP gram at L1 = L2 = 60-dB SPL was obtained to confirm adequate cochlear function as compared with that before surgery. This procedure was then repeated for the contralateral, or control, ear.
After exposure of the middle ear, the area of the CPA was exposed via a suboccipital posterior craniotomy detailed previously (16,17). In brief, a posterior midline incision was made inferior to the occipital process. The dorsal neck muscles were then sectioned and retracted away from the midline. Using rongeurs to remove parts of the posterior calvarium, the cerebellum and brainstem were visualized through the operating microscope. Throughout this procedure, viability of the animal was monitored by observing changes in respiratory pattern, and additional doses of the ketamine/xylazine anesthetic regimen were administered as necessary. After exposing this area, the LD probe was placed into the RW niche on the side to be tested via the opening created by the previously described middle ear surgery. The probe was adjusted using a micromanipulator so that an optimal level of CBF was detected. Then, a small piece of surgical gauze was placed in the middle ear to ensure a dry area and to reduce the opening.
The first ear in each of the initial four animals to be investigated was tested with the administration of papaverine (American Regent Laboratories, Shirley, NY). After placing the LD probe, papaverine at a concentration of 30 mg/mL was applied to a piece of Gelfoam (Upjohn Company, Ontario, Canada). Under visualization with the operating microscope, the porus of the IAC was identified. The Gelfoam treated with papaverine was then placed directly on the eighth nerve complex as it entered the IAC for 20 minutes. After this procedure, to establish a baseline before compression, DPOAE tracking functions were obtained for each ear for GMFs at 4-kHz, 8-kHz, and 12-kHz GMF by measuring the emissions level as a function of the primary tones at 60 dB SPL for 60 to 90 seconds. Then, a glass microprobe was used to compress the exposed eighth nerve complex. The first compression was maintained for 3 minutes and then released. DPOAE levels at each of the 4-kHz, 8-kHz, and 12-kHz monitoring frequencies and CBF were recorded from the onset of ischemia through 20 minutes after release of the compression. Usually, 15-minute to 20-minute were needed to prepare and clear blood from the operating field between the end of the first monitoring session and the beginning of the second session. Papaverine was administered again for 20 minutes, and the compression was repeated, but this time held for 5 minutes. Again, DPOAE levels and CBF were measured from the onset of compression to 20 minutes after release.
The contralateral ear was then tested in a similar manner, using Gelfoam treated with saline instead of papaverine. As a 3-minute compression was performed, DPOAE levels and CBF were measured again from the onset of ischemia through 20 minutes after release. Because CBF and DPOAE never returned to near-baseline in the control ears, the subsequent 5-minute compression interval was omitted. For the latter two animals (of a total of six) used in this study, the procedure was reversed, i.e., the first (control) ear tested was treated with saline and the second (experimental) ear was treated with papaverine.
RESULTS
IAA compressions for 3 minutes in the young rabbits resulted in some degree of vasospasm in all control ears. However, in contrast, pretreatment with topical papaverine prevented vasospasm in all experimental ears, after both 3-minute and 5-minute compressions. Vasospasm was identified in the control ears as incomplete return of CBF to baseline, preblockage levels. In the experimental ears, CBF and DPOAE returned to normal or near-normal after both 3-minute and 5-minute compressions.
Figures 1 through 3 illustrate individual examples of the various effects of deliberate IAA compressions on CBF and DPOAE levels in untreated, control ears, whereas Figure 4 displays the average vasospasm-related effects on both of these measures. Clearly, control ears showed varying degrees of vasospasm, as indicated by the incomplete recovery of postcompression CBF and DPOAE levels. In general, in control ears, two major CBF patterns associated with vasospasm were observed after the release of the IAA compressions.
FIG. 1.
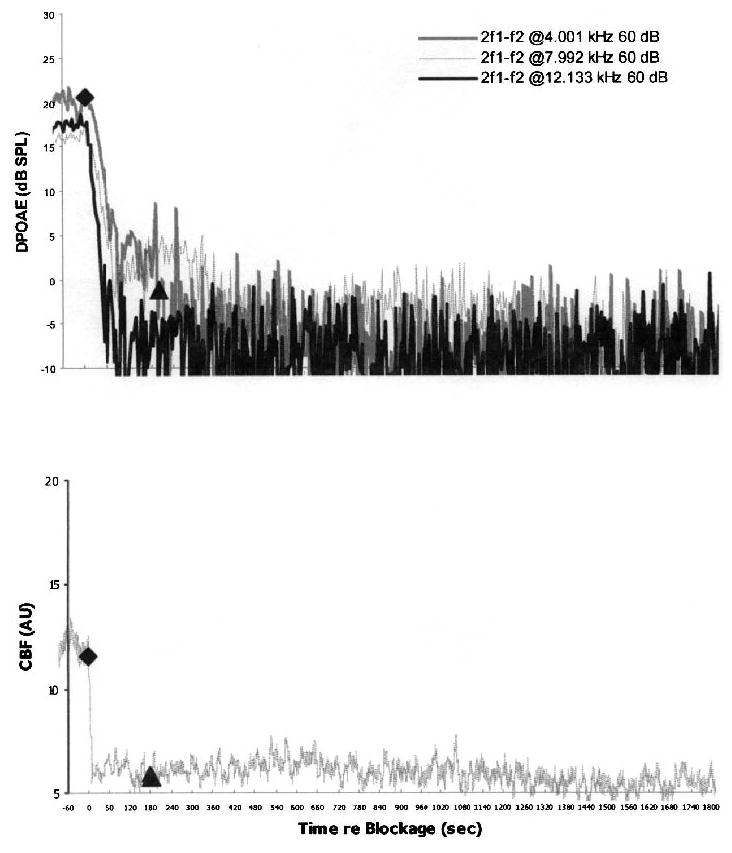
An example of vasospasm in a control ear. No recovery of CBF (lower plot) and the corresponding DPOAE levels in response to 60-dB SPL primaries (upper plot) was observed at any monitoring frequency after a 3-minute IAA compression. For this and subsequent CBF plots: diamond, onset of IAA compression; triangle, release of IAA compression; dB SPL, decibels sound pressure level. See text for explanation of AU measures of CBF.
FIG. 3.
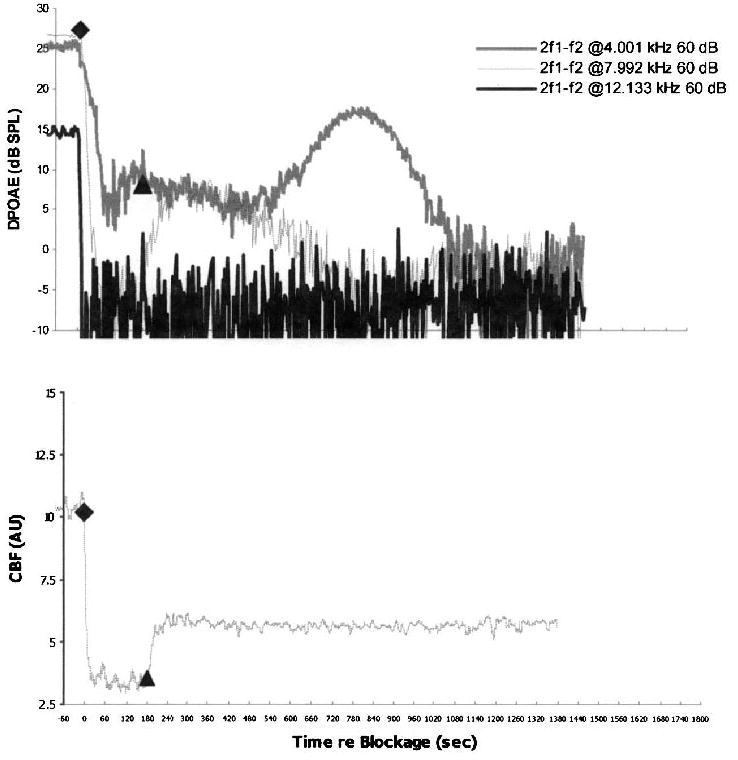
An example of vasospasm in a control ear with a rapid partial recovery of CBF after a 3-minute IAA compression. In the lower plot, postrelease CBF quickly stabilized to approximately 65 percent below its precompression baseline levels. In the upper plot, DPOAE levels at 4 kHz and 8 kHz partially recovered in the immediate postrelease period, only to decrease into the noise–floor by 8 minutes after release; 12-kHz DPOAE never recovered.
FIG. 4.
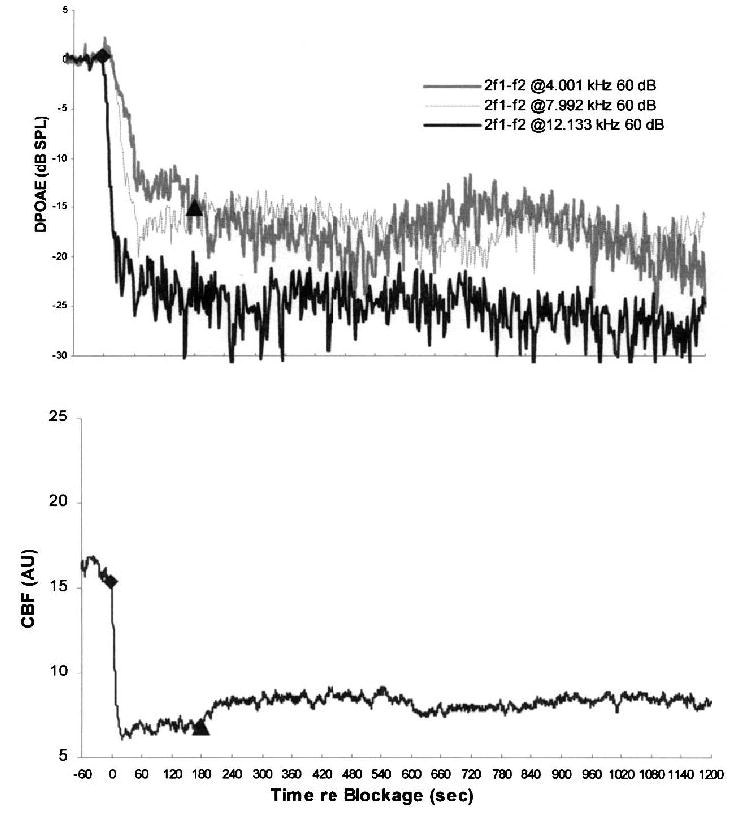
Mean time courses for DPOAE levels and CBF during and after 3-minute IAA compressions in six control ears that were not pre-treated with papaverine showing average vasospasm response. The lower plot shows mean values of CBF characterized by a slow, initial recovery of the cochlear blood supply after release of the IAA and stabilization after 40 seconds at a level of approximately 15 to 20 percent above the initial baseline compression values. The upper plot demonstrates corresponding average values for DPOAE levels recorded at the three GMF of 4 kHz, 8 kHz, and 12 kHz. DPOAE levels did not recover to precompression baseline levels; however, for 4 kHz and 8 kHz, DPOAE partially recovered. At 12 kHz, DPOAE levels after release remained at background noise–floor levels.
As detailed in Figure 1, one pattern consisted of no recovery of either CBF or DPOAE in that these responses remained at noise–floor levels for 4-, 8-, and 12-kHz GMF DPOAE stabilized at 0, 5, and 10 dB SPL, respectively. The second common postcompression CBF pattern consisted of a delayed, but partial, recovery of blood flow, as illustrated in Figures 2 and 3. In both of these cases, the rate and amount of recovery for DPOAE generated over high-frequency regions of the cochlea consistently lagged behind the corresponding recovery for lower-frequency DPOAE.
FIG. 2.
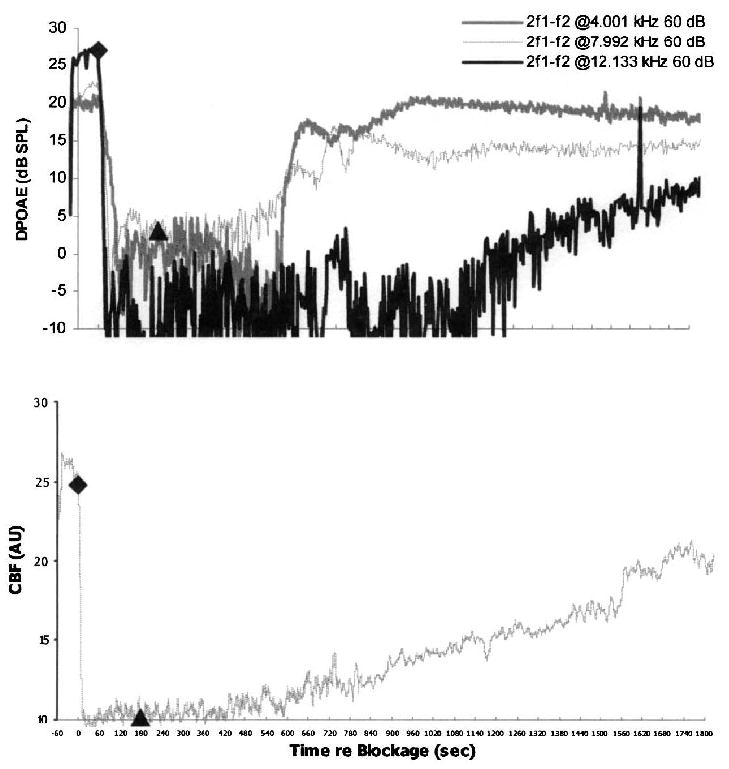
An example of vasospasm in a control ear with delayed partial recovery of CBF and DPOAE after release of a 3-minute IAA compression. The lower plot shows an approximate 6-minute postrelease delay in recovery of the CBF. The upper plot depicts the corresponding DPOAE recorded at 4 kHz, 8 kHz, and 12 kHz. Note that 4 kHz DPOAE recovered relatively quickly to precompression baseline values. At 8 kHz and, more dramatically, at 12 kHz, significant delays in recovery occurred with DPOAE stabilization below precompression baseline values.
Figure 4 shows the average DPOAE and CBF values computed for all six control ears in which different degrees of vasospasm were detected in response to the 3-minute occlusions. On average, CBF recovered to only approximately 43 percent of its baseline rate. Similarly, whereas 12-kHz DPOAE essentially did not recover above noise–floor levels, DPOAE at 4-kHz and 8-kHz returned to approximately 30 percent of their precompression magnitudes.
In contrast, CBF in ears pretreated with papaverine consistently returned to baseline levels within 5 to 20 seconds of compression release, thus exhibiting the typical rapid recoveries observed in previous compression experiments in older rabbits (16,17). In fact, in the papaverine ears, CBF demonstrated a transient overshoot to supra-baseline rates before returning to values that were at, or slightly below, control flow rates. Conversely, as expected, postcompression delays in CBF recovery were generally longer for the 5-minute compressions than for the more rapid recoveries described for the shorter 3-minute occlusions.
Figure 5 shows the average effects of the 3-minute compressions on DPOAE and CBF. In these cases, whereas CBF recovered to control values within a few seconds of release of the blockage, emissions returned within approximately 2 to 3 dB of baseline for 4-kHz, 8-kHz, and 12-kHz DPOAE within 48 (range: 34–166 s), 50 (range: 19–508 s), and 240 seconds (range: 115–180 s), respectively.
FIG. 5.
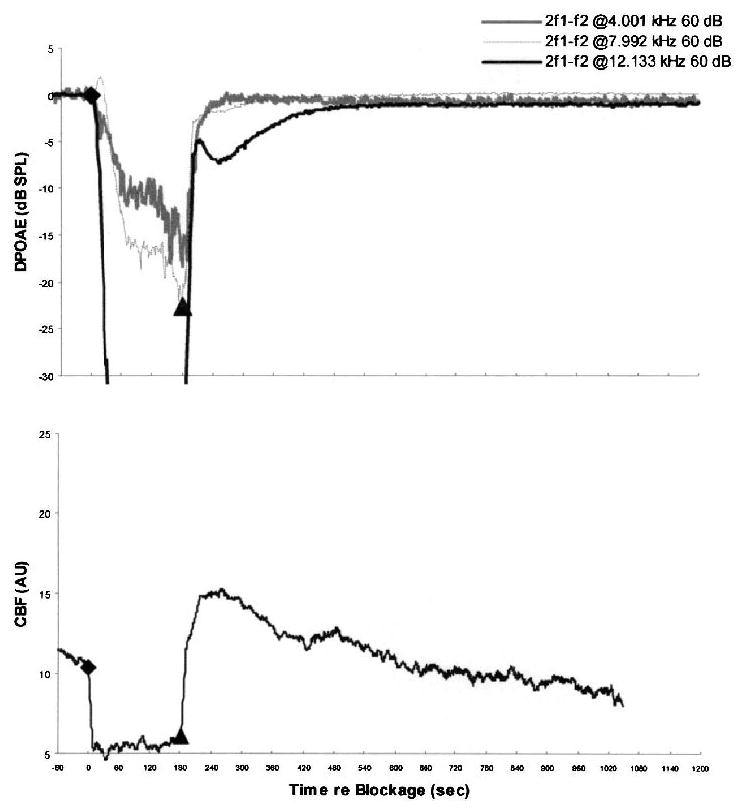
The time courses of average values of DPOAE and CBF during and after 3-minute IAA compressions in six experimental ears pretreated with papaverine. The lower plot shows that CBF recovered quickly after release of IAA compression and even transiently overshot the precompression baseline values. The upper plot shows DPOAE levels at the three test frequencies, which recovered quickly to baseline or near-baseline values.
For 5-minute compressions, as shown in Figure 6, at the time when CBF returned to baseline flow rates, 4-kHz DPOAE recovered to within 2 dB of the control levels by an average of 154 seconds (range: 26–340 s). However, such DPOAE recovery at 4 kHz was observed for only five of the six ears investigated, with one ear showing no recovery whatsoever. For both 8-kHz and 12-kHz DPOAE, only two of the six ears had recovered to within 2 dB of their baseline values by approximately 372 seconds after compressions release. Again, high-frequency DPOAE recovered, in general, to lower levels than did lower-frequency emissions after the 5-minute compressions.
FIG. 6.
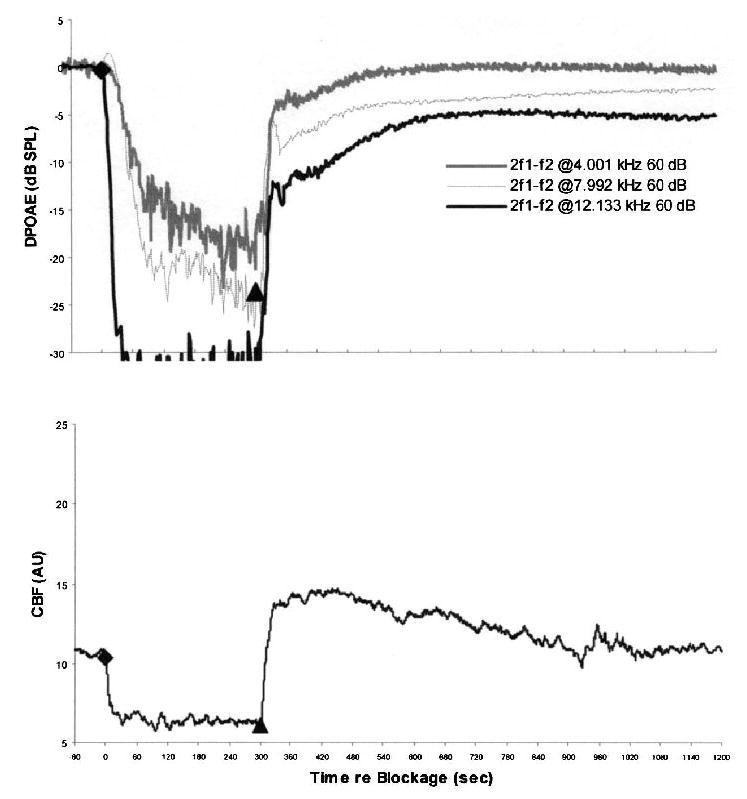
Time courses of average DPOAE levels and CBF during and after 5-minute IAA compressions in six ears pretreated with papaverine. The lower plot shows the rapid return of CBF after release of IAA compression with the characteristic overshoot of blood flow and the gradual return to precompression baseline values. In the upper plot, DPOAE levels recover rapidly, but never completely, to precompression baseline values. The level of DPOAE recovery was inversely related to emission frequency (i.e., 4-kHz DPOAE recovered to within 0.5 dB of precompression baseline, 8-kHz DPOAE to within 2.5–3 dB, and 12-kHz DPOAE to within 5–5.5 dB).
DISCUSSION
An animal model of IAA vasospasm was developed, which simulated one aspect of the pressure trauma on the cochlear–vestibular nerve complex during IAC/CPA surgery in humans. In all experimental ears, topically applied papaverine prevented vasospasm of the blood supply to the inner ear after direct physical compression of the IAA. In contrast, all control ears exhibited variably reduced LD-measured CBF and DPOAE, which suggested that variable degrees of compression-induced vasospasm occurred. That all the ears pretreated topically with papaverine showed recovery of CBF and DPOAE demonstrates that vasospasm, rather than thrombosis, of the IAA caused the compression-related reductions in these measures. It has been theorized that vascular compromise of the inner ear, i.e., transection, thrombosis, or vasospasm of the IAA, is one cause of hearing loss after attempted hearing preservation AN resection when the cochlear nerve has been clearly preserved (9,10). The data from the current study strongly suggest that topical application of papaverine before dissection in the IAC/CPA may prevent reduction of CBF and resulting cochlear outer hair cell (OHC) function by eliminating vasospasm of the IAA during human surgery. Papaverine has been shown to be one of the strongest nonspecific vasodilators. It has been applied intracisternally (18–20) and intraarterially/intravenously (21–23) to prevent and to treat vasospasm of cerebral arteries. To our knowledge, this is the first animal study to demonstrate the efficacy of topically applied papaverine to prevent vasospasm of the IAA.
The young age of the rabbits and the relatively long duration of vessel compression appeared to be significant factors in the development of this animal model of IAA vasospasm. All of the animals used in the current study were age 3 months or younger. Previous CBF/DPOAE experiments using older, larger rabbits did not demonstrate evidence of a vasospasm-based release of IAA compressions (16,17,24). In the previous experiments, evidence of IAA vasospasm was not observed, even after multiple (up to 10) 1-minute or longer single compressions. Mom et al. (9), using somewhat older, larger rabbits, reported full recovery of CBF and DPOAE after IAA compressions of up to 7 minutes. They noted reduced CBF after compression release, consistent with vasospasm only in those ears subjected to a procedure permitting a partial release of the blood flow obstruction during the IAA compression period. These authors theorized that metabolic factors, perhaps related to toxic oxygen radicals produced during the partial reperfusion process, contributed to the observed IAA vasospasm. Perhaps the combination of both young animals (younger than age 3 mo) and 3-minute compression times led to the consistently reproducible LD-measured CBF and DPOAE findings reported here that were consistent with IAA vasospasms.
The mechanism by which mechanical manipulations induced IAA vasospasms may involve arterial neuro-regulation. There is evidence that CBF is at least partially under sympathetic nervous system control. Histochemical techniques showing sympathetic fibers surrounding cochlear arteries (25–27), as well as pharmacological studies applying adrenergic receptor antagonists to these vessels (28,29), suggest that sympathetic innervation regulates the vascular tone of the cochlea. Sympathetic innervation of the cochlear arteries appears to come from the stellate ganglia bilaterally and the superior cervical ganglion ipsilaterally. In vessels found in the subarachnoid space, external mechanical compression of arterial walls induced vasoconstriction by exciting sympathetic nerve fibers found within the surrounding arachnoid strands (30–32).
Whatever factors contribute to trigger vasospasm in the IAA, persistent vascular smooth muscle contraction remains the final pathway of constriction of the lumen of the vessel. Calcium activity in the smooth muscle cells regulates contractility via calmodulin-activating myosin light-chain kinase, which in turn leads to phosphorylation of the myosin light-chain interacting with actin filaments to cause contraction. Cyclic guanosine monophosphate (cGMP) regulates sequestration of intracellular calcium via activation of the sarcoplasm membrane-bound calcium pump, which removes calcium and relaxes the smooth muscle cells (33–35).
Cyclic adenosine monophosphate (cAMP) may also cause vasorelaxation by decreasing activity of myosin light-chain kinase via inactivation of protein kinase A. Papaverine induces vasorelaxation by inhibiting phosphodiesterases, which are responsible for the breakdown of the cyclic nucleotides, resulting in an increase of cAMP and cGMP in the cytosol of the arterial smooth muscle (36,37). Among the routes of administration—intravenous, intraarterial, subarachnoid (i.e., topical)—of papaverine for preventing and treating vasospasm in central nervous system arteries, the subarachnoid route has been shown to be the most effective and intravenous the least effective for preventing vasospasm of cerebral arteries (21). Furthermore, RW application of papaverine appears to have no effect on CBF (38).
Interestingly, in the papaverine-treated ears, essentially total cochlear ischemia of 3-minute duration did not result in significant reduction in cochlear function as measured by DPOAE. After 5-minute IAA compressions, however, even with CBF recovery to baseline or near-baseline values, DPOAE did not fully recover. The degree of return of cochlear function after the more prolonged 5-minute ischemia was directly related to the location of the test frequency along the cochlear duct. After surgical manipulation, arterial vasospasm can endure an indeterminate length of time. Tumor removal surgery taking place within the IAC/CPA typically lasts more than several hours. It has long been known that interruption of the cochlear arterial supply causes physical degeneration or OHC within 30 minutes and inner hair cells (IHC) by 2 to 3 hours (39). Morphologic changes within the cochlea detected during transient (<15 min) hypoxia or anoxia, includes swelling of the OHC and afferent nerve endings beneath the IHC (40,41). These ultrastructural alterations, coupled with electrophysiological measurements (42–44), consistently appear in the basal turn region and demonstrate the sensitivity of the cochlea to ischemia. In the current study, the recovery of DPOAE after IAA occlusion was directly related to frequency. After both 3-minute occlusions in the control ears and 5-minute occlusions in the papaverine-treated ears, DPOAE levels were, in general, highest at 4 kHz and lowest at 12 kHz. These results suggest that reducing the time or even preventing vasospasm of the IAA and related arterial vessels may offer protection to cochlear function against ischemia, particularly in the organ of Corti regions serving the higher frequencies.
Considering different factors that contribute to hearing loss in subjects operated within the IAC/CPA region, various manipulations, i.e., eighth nerve compression or stretching, should be taken into account. Monitoring auditory status intraoperatively by electrophysiological techniques, Colletti et al. demonstrated that such manipulations during tumor removal played an important role in hearing impairment, accounting for approximately 30 percent of postoperative hearing losses (45). Cauterization close to the eighth nerve, IAC drilling, and tumor removal in the IAC were found to be other dangerous factors associated with retrocochlear type hearing loss (46,47).
CONCLUSIONS
An animal model of IAA vasospasm was described. IAA constriction was induced by mechanical compression of the eighth nerve complex at the porus acusticus. Mechanically induced vasospasm of the IAA was prevented by topical application of papaverine. These findings have clinical implications for human surgery, such as procedures to remove acoustic neuromas and other tumors from the IAC/CPA.
Footnotes
This study was supported by the National Institute on Deafness and Other Communicative Disorders, Fullbright Commission, and the University of Miami Ear Institute.
References
- 1.Gordon ML, Cohen NL. Efficacy of auditory brainstem response as a screening test for small acoustic neuromas. Am J Otol. 1995;16:136–9. [PubMed] [Google Scholar]
- 2.Ruckenstein MJ, Cueva RA, Morrison DH, et al. A prospective study of ABR and MRI in the screening for vestibular schwannomas. Am J Otol. 1996;17:317–20. [PubMed] [Google Scholar]
- 3.Wilson FW, Talbot M, Mills L. A critical appraisal of the role of auditory brain stem response and magnetic resonance imaging in acoustic neuroma diagnosis. Am J Otol. 1997;18:673–81. [PubMed] [Google Scholar]
- 4.Telischi F. An objective method of analyzing cochlear vs noncochlear patterns of distortion-product otoacoustic emissions in patients with acoustic neuromas. Laryngoscope. 2000;110:553–62. doi: 10.1097/00005537-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Mobley SR, Odabasi O, Ahsan S, et al. Distortion-product otoacoustic emissions in nonacoustic tumors of the cerebellopontine angle. Otolaryngol Head Neck Surg. 2002;126:115–20. doi: 10.1067/mhn.2002.122264. [DOI] [PubMed] [Google Scholar]
- 6.Aoyagi M, Yokota M, Nakamura T, et al. Hearing preservation and improvement of auditory brainstem response findings after acoustic neuroma surgery. Acta Otolaryngol (Stockh) 1994;511S:40–6. doi: 10.3109/00016489409128299. [DOI] [PubMed] [Google Scholar]
- 7.Tucci DL, Telian SA, Kileny PR, et al. Stability of hearing preservation following acoustic neuroma surgery. Am J Otol. 1994;15:183–8. [PubMed] [Google Scholar]
- 8.Filipo R, Delfini R, Fabiani M, et al. Role of transient-evoked otoacoustic emisiions for hearing preservation in acoustic neuroma surgery. Am J Otol. 1997;18:746–9. [PubMed] [Google Scholar]
- 9.Mom T, Telischi FF, Martin GK, et al. Vasospasm of the internal auditory artery: Significance in cerebellopontine angle surgery. Am J Otol. 2000;21:735–42. [PubMed] [Google Scholar]
- 10.Morawski K, Namyslowski G, Lisowska G, et al. Intraoperative monitoring of the cochlear function using distortion product otoacoustic emissions in patients with cerebello-pontine angle tumors. Abstr Assoc Res Otolaryngol. 2002;25:204. doi: 10.1097/00129492-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 11.Kassell NF, Helm GH, Simmons N, et al. Treatment of cerebral vasospasm with intra-arterial papaverine. J Neurosurg. 1992;77:848–52. doi: 10.3171/jns.1992.77.6.0848. [DOI] [PubMed] [Google Scholar]
- 12.Shiokawa K, Kasuya H, Miyajima M, et al. Prophylactic effect of papaverine prolonged-release pellets on cerebral vasospasm in dog. Neurosurgery. 1998;42:109–16. doi: 10.1097/00006123-199801000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Levine RA, Bu-Saba N, Brown MC. Laser-Doppler measurements and electrocochleography during ischemia of the guinea pig cochlea: implication for hearing preservation in acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1993;102:127–36. doi: 10.1177/000348949310200210. [DOI] [PubMed] [Google Scholar]
- 14.Miller JM, Ren TY, Nuttal AL. Studies of inner ear blood flow in animals and human beings. Otolaryngol Head Neck Surg. 1995;112:101–13. doi: 10.1016/S0194-59989570308-X. [DOI] [PubMed] [Google Scholar]
- 15.Mom T, Avan P, Bonfils P, et al. A model of cochlear function assessment during reversible ischemia in the Mongolian gerbil. Brain Res Protocol. 1999;4:249–57. doi: 10.1016/s1385-299x(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 16.Mom T, Telischi FF, Martin GK, et al. Measuring the cochlear blood flow and distortion-product otoacoustic emissions during reversible cochlear ischemia: a rabbit model. Hear Res. 1999;133:40–52. doi: 10.1016/s0378-5955(99)00056-8. [DOI] [PubMed] [Google Scholar]
- 17.Widick MP, Telischi FF, Lonsbury-Martin BL, et al. Early effects of cerebellopontine angle compression on rabbit distortion-product otoacoustic emissions: A model for monitoring cochlear function during acoustic neuroma surgery. Otolaryngol Head Neck Surg. 1994;111:407–16. doi: 10.1177/019459989411100404. [DOI] [PubMed] [Google Scholar]
- 18.Segawa H, Saito I, Okada T, et al. Efficacy of intracisternal papaverine on symptomatic vasospasm. No Shinkei Geka. 1986;14:847–54. [PubMed] [Google Scholar]
- 19.Vollmer DG, Jane JS, Torner JC, et al. Intracisternal papaverine reverses experimental cerebral vasospasm in a rabbit model. In: Wilkins RH, editor. Cerebral Vasospasm. New York: Raven Press; 1986. pp. 425–31. [Google Scholar]
- 20.Shiokawa K, Kasuya H, Miyajima M, et al. Prophylactic effect of papaverine prolonged-release pellets on cerebral vasospasm in dog. Neurosurgery. 1998;42:109–16. doi: 10.1097/00006123-199801000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Kuwayama A, Zervas NT, Shintani A, et al. Papaverine hydrochloride and experimental hemorrhagic cerebral arterial spasm. Stroke. 1972;3:27–33. doi: 10.1161/01.str.3.1.27. [DOI] [PubMed] [Google Scholar]
- 22.Kaku Y, Yonekawa Y, Tsukahara T, et al. Superselective intra-arterial infusion of papaverine for the treatment of cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg. 1992;77:909–15. doi: 10.3171/jns.1992.77.6.0842. [DOI] [PubMed] [Google Scholar]
- 23.Kassell NF, Helm GH, Simmons N, et al. Treatment of cerebral vasospasm with intra-arterial papaverine. J Neurosurg. 1992;77:848–52. doi: 10.3171/jns.1992.77.6.0848. [DOI] [PubMed] [Google Scholar]
- 24.Telischi FF, Mom T, Agrama M, et al. Comparison of the auditory-evoked brainstem response wave I to distortion-product otoacoustic emissions resulting from changes to inner ear blood flow. Laryngoscope. 1999;109:186–191. doi: 10.1097/00005537-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Brechtelsbauer PB, Prazma J, Garrett CG, et al. Catecholaminergic innervation of the inner ear. Otolaryngol Head Neck Surg. 1990;103:566–74. doi: 10.1177/019459989010300407. [DOI] [PubMed] [Google Scholar]
- 26.Carlisle L, Aberdeen J, Forge A, et al. Neural basis for regulation of cochlear blood flow: peptidergic and adrenergic innervation of the spiral modiolar artery of the guinea pig. Hear Res. 1990;43:107–13. doi: 10.1016/0378-5955(90)90219-f. [DOI] [PubMed] [Google Scholar]
- 27.Pillsbury HC, Pulver S, Carrasco VN, et al. Glyoxylic acid in the study of autonomic innervation in the gerbil cochlea. Arch Otolaryngol Head Neck Surg. 1992;118:413–16. doi: 10.1001/archotol.1992.01880040079013. [DOI] [PubMed] [Google Scholar]
- 28.Laurikainen EA, Kim D, Didier A, et al. Stellate ganglion drives sympathetic regulation of cochlear blood flow. Hear Res. 1993;64:199–204. doi: 10.1016/0378-5955(93)90006-m. [DOI] [PubMed] [Google Scholar]
- 29.Gruber DD, Dang H, Shimozono M, et al. 1A-Adrenergic receptors mediate vasoconstriction of the isolated spiral modiolar artery in vitro. Hear Res. 1998;119:113–24. doi: 10.1016/s0378-5955(98)00036-7. [DOI] [PubMed] [Google Scholar]
- 30.Johnson RJ, Potter JM, Reid RG. Arterial spasm in subarachnoid hemorrhage. Mechanical considerations. J Neurol Neurosurg Psych. 1958;21:68–9. [Google Scholar]
- 31.Kapp J, Mahaley MS, Odom GL. Cerebral arterial spasm. Part II: Experimental evaluation of mechanical and humoral factors in pathogenesis. J Neurosurg. 1968;29:339–49. doi: 10.3171/jns.1968.29.4.0339. [DOI] [PubMed] [Google Scholar]
- 32.Arutinunov AI, Baron MA, Majorova NA. The role of mechanical factors in the pathogenesis of short term and prolonged spasm of the cerebral arteries. J Neurosurg. 1974;40:451–58. doi: 10.3171/jns.1974.40.4.0459. [DOI] [PubMed] [Google Scholar]
- 33.Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Ann Rev Pharmacol Toxicol. 1990;30:535–60. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- 34.Winder SJ, Allen BG, Clement-Chomienne O, et al. Regulation of smooth muscle actin-myosin interaction and force by calponin. Acta Physiol Scand. 1998;164:415–26. doi: 10.1111/j.1365-201x.1998.tb10697.x. [DOI] [PubMed] [Google Scholar]
- 35.Dietrich HH, Dacey RG. Molecular keys to the problems of cerebral vasospasm. Neurosugery. 2000;46:517–30. doi: 10.1097/00006123-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg S, Curro FA, Tanaka TP. Regulation of vascular smooth muscle of the microcirculation. In: Mortillaro NA, editor. The Physiology and Pharmacology of the Microcirculation. Vol 1. New York: Harcourt Brace Jovanovich; 1983. [Google Scholar]
- 37.Goodman L, Gilman A. The Pharmacological Basis of Therapeuthics. 7th Ed. New York: MacMillan Publishing; 1985. [Google Scholar]
- 38.Ohlsen KA, Didier A, Baldwin D, et al. Cochlear blood flow in response to dilating agents. Hear Res. 1992;58:19–25. doi: 10.1016/0378-5955(92)90004-7. [DOI] [PubMed] [Google Scholar]
- 39.Perlman HB, Kimura RS. Experimental obstruction of venous drainage and arterial supply of the inner ear. Ann Otol Rhinol Laryngol. 1957;66:537–46. [PubMed] [Google Scholar]
- 40.Billett TE, Thorne PR, Gavin JB. The nature and progression of injury in the organ of Corti during ischemia. Hear Res. 1989;41:189–98. doi: 10.1016/0378-5955(89)90010-5. [DOI] [PubMed] [Google Scholar]
- 41.Puel JL, Pujol R, Tribillac F, et al. Excitatory amino acid antagonists protect cochlear auditory neurons from excitotoxicity. J Comp Neurol. 1994;341:241–56. doi: 10.1002/cne.903410209. [DOI] [PubMed] [Google Scholar]
- 42.Fechter LD, Thorne PR, Nuttall AL. Effects of carbon monoxide on cochlear electrophysiology and blood flow. Hear Res. 1987;27:37–45. doi: 10.1016/0378-5955(87)90024-4. [DOI] [PubMed] [Google Scholar]
- 43.Tabuchi K, Ito Z, Wada T, et al. The effect of mannitol upon cochlear dysfunction induced by transient local anoxia. Hear Res. 1998;126:28–36. doi: 10.1016/s0378-5955(98)00142-7. [DOI] [PubMed] [Google Scholar]
- 44.Tabuchi K, Tsuji S, Ito Z, et al. Does xanthine oxidase contribute to the hydroxyl radical generation in ischemia and reperfusion of the cochlea? Hear Res. 2001;153:1–6. doi: 10.1016/s0378-5955(00)00247-1. [DOI] [PubMed] [Google Scholar]
- 45.Colletti V, Fiorino FG, Mocella S, et al. EcochG, CNAP and ABR monitoring during vestibular Schwannoma surgery. Audiology. 1998;37:27–37. doi: 10.3109/00206099809072959. [DOI] [PubMed] [Google Scholar]
- 46.Colletti V, Fiorino FG. Vulnerability of hearing function during acoustic neuroma surgery. Acta Otolaryngol (Stockh) 1994;114:264–70. doi: 10.3109/00016489409126054. [DOI] [PubMed] [Google Scholar]
- 47.Colletti V, Fiorino FG. Advances in monitoring of seventh and eighth cranial nerve function during posterior fossa surgery. Am J Otol. 1998;19:503–12. [PubMed] [Google Scholar]


