Abstract
Intermittent, chronically delivered electrical stimulation of the vagus nerve (VNS) is an FDA-approved procedure for the treatment of refractory complex/partial epilepsy in humans. Stimulation of the vagus has also been shown to enhance memory storage processes in laboratory rats and human subjects. Recent evidence suggests that some of these effects of VNS may be due to the activation of neurons in the nucleus locus coeruleus resulting in the release of norepinephrine (NE) throughout the neuraxis. Because antagonism of NE systems has been shown to delay recovery of function following brain damage, it is possible that enhanced release of NE in the CNS may facilitate recovery of function. To evaluate this hypothesis the lateral fluid percussion injury (LFP) model of traumatic brain injury was used and a variety of motor and cognitive behavioral tests were employed to assess recovery in pre-trained stimulated, control, and sham-injured laboratory rats. Two hours following moderate LFP, vagus nerve stimulation (30.0-sec trains of 0.5 mA, 20.0 Hz, biphasic pulses) was initiated. Stimulation continued in each animal’s home cage at 30-min intervals for a period of 14 days, with the exception of brief periods when the animals were disconnected for behavioral assessments. Motor behaviors were evaluated every other day following LFP and tests included beam walk, locomotor placing, and skilled forelimb reaching. In each measure an enhanced rate of recovery and/or level of final performance was observed in the VNS-LFP animals compared to non-stimulated LFP controls. Behavior in the Morris water maze was assessed on days 11–14 following injury. Stimulated LFP animals showed significantly shorter latencies to find the hidden platform than did controls. Despite these behavioral effects, neurohistological examination did not reveal significant differences in lesion extent, density of fluorojade positive neurons, reactive astrocytes or numbers of spared neurons in the CA3 subarea of the hippocampus, at least at the one time point studied 15 days post-injury. These results support the idea that vagus nerve stimulation enhances the neural plasticity that underlies recovery of function following brain damage and provides indirect support for the hypothesis that enhanced NE release may mediate the effect. Importantly, since VNS facilitated both the rate of recovery and the extent of motor and cognitive recovery, these findings suggest that electrical stimulation of the vagus nerve may prove to be an effective non-pharmacological treatment for traumatic brain injury.
Keywords: fluid percussion, memory, norepinephrine, recovery of function, skilled reaching, traumatic brain injury, vagus nerve stimulation
INTRODUCTION
AN ESTIMATED TWO MILLION head injuries occur each year in the United States, and 500,000 cases require hospitalization (Goldstein, 1990). Further, the long-term outcome for many survivors of head injury is poor, with around 70,000 individuals annually facing a life-long debilitating loss of function. Accordingly, a large amount of research focused on reducing neural cell death and/or enhancing recovery of function following traumatic brain injury (TBI) has occurred in the past decade. Aided by the availability of the clinically relevant and valid lateral fluid percussion (LFP) animal model of TBI (Thompson et al., 2005), exciting new information concerning the induction, up-regulation, or activation of neurochemical cascades following TBI has paved the way for testing the potential of pharmacological (Feeney, 1998; Goldstein, 1998a; McIntosh, 1993; McIntosh et al., 1998) and non-pharmacological (Dietrich, 1996) treatment strategies that may have the potential to reduce damage and/or enhance recovery of function.
This paper describes facilitated recovery of function afforded by vagus nerve stimulation in rats which received experimentally induced LFP. Chronic, intermittent unilateral vagus nerve stimulation (VNS) is FDA approved for use in the treatment of refractory complex-partial epilepsy and it has been shown to be effective with only minimal side effects (Schachter and Saper, 1998; Fisher and Handforth, 1999). Previously, we demonstrated that VNS (0.4 or 0.5 mA, 20 Hz, 0.5 msec, biphasic stimulation applied for 30 sec immediately after a learning experience) enhances memory in laboratory rats (Clark et al., 1995, 1998) and human epileptics (Clark et al., 1999). However, using the same stimulation parameters, VNS delivered at intensity of 0.8 mA or above had no effect on retention performance, despite the fact that this stimulation intensity effectively suppresses seizures.
Of central importance to the present research is the literature on drugs that influence noradrenergic systems and their effects on recovery. Considerable evidence indicates that drugs which increase brain norepinephrine (NE) concentrations promote recovery of function from TBI whether the drugs are applied shortly after brain injury or at some later time (Feeney, 1998; Feeney and Sutton, 1987, 1988; Gladstone and Black, 2000; Goldstein, 1998b; McIntosh, 1993). Initially, these reports concerned the return of simple reflexes, such as visual and tactile placing (Meyer et al., 1963; Feeney and Hovda, 1983) and depth perception (Feeney and Hovda, 1985), following ablation of visual cortex when the animals were given injections of D-amphetamine. Additionally, Hovda and Fenney (1984) and Sutton et al. (1989) reported that D-amphetamine hastened an enduring recovery of beam walking performance following unilateral sensorimotor or bilateral frontal lesions in cats. Likewise, in rats given unilateral sensorimotor lesions, Boyeson and Feeney (1990) demonstrated that intraventricular infusions of NE, but not dopamine, accelerated beam walking performance. Further, Feeney and Westerberg (1990) demonstrated that α1 receptor antagonists retard balance beam motor recovery following unilateral fluid percussion injury centered over the sensorimotor cortex and depletion of catecholamines retards plasticity in the visual cortex of kittens (Kasamatsu and Pettigrew, 1976). Recently, Kikuchi et al. (2000) documented a role for the precursor of norepinephrine (L-theo-3,4 dihydroxyphenyl-serine [L-DOPS]) in accelerating recovery of beam walking performance following unilateral sensorimotor cortex ablation. Taken together, these studies strongly suggest that the noradrenergic systems of the brain play an important role in recovery of function following TBI in laboratory animals.
Importantly, D-amphetamine administration in hemiplegic human patients enhances recovery following stroke (Crisostomo et al., 1988; Walker-Batson et al., 1995), at least when paired with physical therapy (Feeney, 1998). The findings from these and other studies led to the development of the NE hypothesis of recovery (Feeney and Sutton, 1987, 1988; Feeney et al., 1985; Sutton et al., 1987).
The NE hypothesis predicts that increased efferent output from the locus coeruleus (LC) will enhance recovery, while reduced output from the LC will retard recovery from the hemiplegia produced by sensorimotor cortex contusions or ablation (Feeney et al., 1985, Goldstein and Bullman, 1997). There is evidence from our group and others that VNS activates neurons in the LC. The evidence is (1) electrical stimulation of the vagus nerve in the rat activates a subpopulation of neurons in the locus coeruleus measured eletrophysiologically (Takigawa and Mogenson, 1977; Hermann and Rogers, 1985; Krahl et al., 1994, Groves et al., 2005); (2) vagus nerve stimulation induces fos protein labeling in the LC and A5 noradrenergic nucleus (Naritoku et al., 1995); (3) 6-OHDA lesions or inactivation of the LC decreases the anticonvulsant effectiveness of VNS (Krahl et al., 1998); (4) VNS is associated with NE release in the amygdala (Hassert et al., 2004); and (5) recent microdialysis experiments in our laboratory demonstrated increased release of NE in the hippocampus and cerebral cortex of rats receiving VNS (Roosevelt et al., 2004). Thus, the NE hypothesis clearly predicts that VNS should enhance recovery of motor and cognitive behaviors following experimental TBI. The research described in this report demonstrates that under the stimulation parameters employed, VNS enhances both the rate and extent of motor and cognitive recovery following experimental TBI in the rat.
MATERIALS AND METHODS
Subjects
Fifty-seven male Long Evans hooded rats weighing 425–475 g at the time of injury were housed individually in standard clear acrylic cages and maintained at 22°C on a 12:12-h light/dark cycle. Five animals died following vagus nerve (VN) stimulating electrode implantation surgery. Three animals died from respiratory failure post-fluid percussion injury. The data from these animals were not included in subsequent analyses. All animals were well handled prior to behavioral training which began at 90 days of age. One week prior to surgery, the animals were moved into modular test chambers (ENV-008-VP, Med Associates, Inc, St. Albans, VT). All rats were fed standard rat diet ad libitum until they reached 450 g, at which time they were maintained at approximately 85% of the weight of an age- and sex-matched group fed ad libitum. Following surgery, animals were fed a liquid diet for 5 days after which standard rat dry food was reinstated. Animal care, surgical protocols and anesthesia were conducted in strict accordance with Federal regulations as outlined in the NIH Guide for the Care and Use of Laboratory Animals. All experimental procedures were reviewed and approved by the Southern Illinois University Institutional Animal Care and Use Committee.
Vagus Nerve Stimulating Electrodes
Helical electrodes (Fig. 1) were constructed in the laboratory using two 7 mm sections of pure silver wire (0.25 mm diameter) covered with polyethylene tubing (PE-10). Each section was soldered to a 30-gallon. Teflon-insulated, silver-plated stranded wire 90 mm in length and then inserted into a 3.5-mm section of polyethylene tubing (PE-160) to keep the electrodes stable and separated (1.5 mm). All seams and open ends were sealed with Epoxy. Prior to surgery, a 2-mm-long strip of tubing was stripped from the inner side of each PE-10 tube to expose a segment of the silver wire for contact with the vagus nerve. Gold-plated connector pins were soldered to the free ends of the stranded wires. Each VN stimulating electrode was sterilized prior to being implanted in the rat.
FIG. 1.
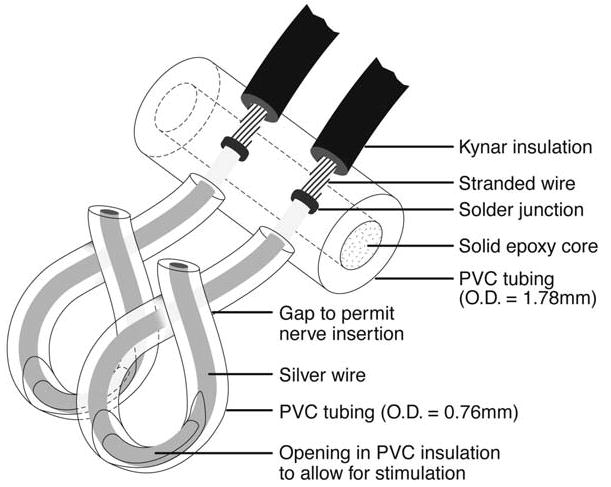
Diagram of vagus nerve stimulating electrode.
Vagus Nerve Electrode Implant Surgery
All rats underwent two surgical procedures conducted under sterile conditions. In the first surgery, the animals were implanted with a VN stimulating electrode. All electrodes were placed on the left VN because fewer efferents go to the heart from the left VN than from the right and stimulation of the left VN produces little bradycardia with the 0.5-mA current intensity employed in this study (Ardell and Randall, 1986; Woodbury and Woodbury, 1990; Groves and Brown, 2005). Animals were anesthetized with a ketamine (90 mg/kg)–xylazine (10 mg/kg) mixture administered i.p. The head and neck were shaved and prepped with Betadine. To access the left VN at the cervical level, a small incision was made on the left ventral side of the neck just lateral to the midline. The layer of subcutaneous fat caudal to the salivary gland and the underlying muscle were blunt dissected. The sternohyoid and sternomastoid muscles were separated longitudinally and retracted laterally to reveal the left vagus nerve which lies lateral to the carotid artery within the carotid sheath. The VN was isolated from the surrounding connective tissue and the vagosympathetic trunk. A 5 mm length of the VN was exposed and each pole of the VN stimulating electrode was coiled around the nerve in a helical fashion. Contact with the exposed VN by the electrode poles was verified with a continuity test using an ohmmeter. In order to prevent displacement of the nerve or electrode, the electrode was loosely sutured to the mediodorsal surface of the sternomastoid muscle. The electrode leads were threaded through the fascia to the dorsal aspect of the neck and tucked subcutaneously for retrieval during the LFP surgery. Both incisions were then sutured and the animals were placed on a warming bed to recover prior to returning to their home cages. Following surgery, each animal received 200,000 units of penicillin G suspension i.m. Animals were allowed to recuperate for 5 days prior to continuing behavioral testing.
Fluid Percussion Surgery
One week after stimulating electrode implantation surgery, rats were anesthetized using an Isoflurane (2–4%), nitrous oxide (0.2 L/min), and oxygen (0.8 L/min) mixture. During the surgery, the animals were kept at a stage III-4 level of anesthesia (Guedel, 1920; Friedberg et al., 1999). In this stage, respiratory rate was approximately 60 breaths/min, and the rats were unresponsive (no vibrissae movement or hind limb withdrawal response). Body temperature was maintained at 37°C throughout the surgery with a heating pad and a rectal probe feedback device (YSI model 73ATA). While under anesthesia, animals were placed in a stereotaxic frame and a midline incision was made in the scalp and underlying fascia. A unilateral 4.0-mm circular craniotomy (epicenter: 4.4 mm posterior to bregma, 2.4 mm lateral to the midline) was made over the left hemisphere keeping the dura intact. An injury cap consisting of a plastic female Luer coupling (06359-40; Cole-Parmer Instrument, Co) was attached to the skull over the craniotomy using Temp Bond (Kerr Manufacturing Co, Romulus, MI) and dental acrylic. The injury cap was filled with sterile saline and the animals were then attached to a LFP device (VCU Biomedical Engineering, Richmond, VA). The LFP device is a 4.5-cm-diameter fluid-filled Plexiglas cylinder (60 cm long) with a 90° curved 10-mm stainless steel tube (2.6 mm internal diameter) attachment on one end and a piston on the other. The steel tube is connected to the injury cap attached to the rat’s skull. A brain injury is induced when the piston is struck by a pendulum weight, creating a pressure pulse approximately 23 msec in duration. The magnitude of the pressure pulse is measured by a pressure transducer, registered on a storage oscilloscope, and converted to units of atmospheres of pressure (atm). The pressure pulse was calibrated prior to each injury to ensure that each animal received an equivalent moderate fluid percussion injury (mean = 1.82 atm; SD = 0.09).
To ensure that all animals were in the same stage of anesthesia during the LFP, the pressure pulse was delivered only when the animals had regained a stage III-1 level of anesthesia (increased respiratory rate, vibrissae movement, and reappearance of the hind limb withdrawal response). At the moment of impact, each animal’s respiration halted, a brief seizure ensued, and all reflexes were absent. Duration of apnea (defined as the latency in seconds to resume spontaneous breathing) and unconsciousness (defined as number of seconds to display a hind-limb withdrawal response) were recorded for each animal (Li et al., 2004). Following the return of the hind-limb withdrawal response, the animals were re-anesthetized and placed back into the stereotaxic frame. The injury cap and dental acrylic were removed and the craniotomy covered with sterile gelfoam. The electrical leads of the VN stimulating electrode were then extracted from under the skin and inserted into a 6-pin pedestal (MS363; Plastics One, Roanoke, VA) that was affixed to the skull using stainless steel screws and dental acrylic. Following recovery the animals were returned to their living chambers and attached to a commutator using a 40-cm-long 6-conductor spring-covered cable. Vagus nerve stimulation (VNS) was delivered via a programmable constant current pulse generator (model 102; Cyberonics, Houston, TX) mounted on a counter balanced swivel arm and connected to the commutator. The VNS parameters used in this study were a 30-sec train of 0.5-msec biphasic pulses delivered at 20 Hz at an intensity of 0.5 mA. The 30-sec stimulation trains were delivered once every 30 min beginning 2 h post-injury. Stimulation continued for 14 days except for brief periods when the animals were being tested on behavioral and cognitive measures. Immediately prior to sacrifice, electrode impedance was measured to ensure that it was still within acceptable constant current operating range (<16 kΩ) of the Cyberonics model 102 stimulator.
An initial index of injury severity was calculated by summing the durations of apnea and unconsciousness. For a moderate level of injury the duration of apnea plus unconsciousness ranges from 120 to 300 sec, while durations greater than 300 sec were taken to indicate a severe level of injury. Assignment of each animal to either the VNS (treatment) or the unstimulated injured (control) group was done using the combined apnea and duration of unconsciousness score. This matching was conducted to ensure that each group was equated as to the initial severity of injury.
To ensure that all testers were blind to the treatment status of the animals, inoperative pulse generators that appeared identical to the functional stimulators (only the serial numbers were different) were used for injured unstimulated control and sham-operated animals. Sham-operated animals underwent the same two surgical procedures including implantation of a VN electrode, but did not receive a brain injury following the craniotomy.
Behavioral Assessments
All animals were pre-trained on the skilled forelimb reaching and beam walk tasks prior to the VN surgery. Following the recuperation period after VN surgery, pre-injury criterion scores were recorded for the reaching and beam walk tests. In addition, pre-injury scores were obtained for the inclined plane, forelimb flexion, and locomotor placing tasks. Post-injury behavioral performance was assessed every 2 days beginning at day 2 until day 14. On test days, a single test trial was conducted for each of the beam walk, inclined plane, forelimb flexion and locomotor placing tasks. Each reaching test session consisted of the presentation of 20 pellets. Animals were tested in the Morris water maze (MWM) on post-injury test days 11–14. Experienced personnel who were blind to the injury and treatment status of each animal conducted all behavioral and cognitive assessments.
Skilled forelimb reaching test
The skilled forelimb reaching task has been shown to be sensitive to changes in pyramidal tract function (Whishaw and Metz, 2002) and to cortical contusion injury (Whishaw et al., 2004). All animals were trained to retrieve 20 sucrose pellets (45 mg; Noyes Precision Pellets, Research Diets, Inc., New Brunswick, NJ) consecutively using only the right forelimb. The reaching apparatus and shaping methods were similar to those employed by Bury and Jones (2002) with modifications to the scoring procedures. For the purpose of this study, only the well closest to the front wall and contralateral to the reaching limb was used. A movement was defined as a reach each time the rat inserted his right paw through the aperture of the cage (Whishaw et al., 2004). Each subsequent pellet was offered only after the rat had turned away from the food aperture, returned and repositioned itself ready for the next retrieval. A successful retrieval in which a rat reached, grasped the pellet and then drew it back into the chamber to be consumed was given a score of 6 points. If the animal reached and touched the pellet but did not successfully grasp or retrieve it, the reach was given a score of 3 points. When the animal reached but missed the pellet completely, that attempt was given a score of 1. In the event when the animal was unable to reach, a score of 0 was assigned. Pre-injury criterion was 17 successful retrievals out of 20 pellets for 3 consecutive days. Training to criterion usually took 10–14 days. Animals that did not reach criterion were omitted from this behavioral task.
Beam walk
Vestibulomotor function and motor coordination were evaluated using the beam walk (Feeney et al., 1982; Schmanke et al., 1996). The animals were trained to escape a bright light and enter a darkened box by traversing a 120-cm-long elevated beam (2.5 cm wide) for 3 trials a day for 5 days. Pre-surgery criterion was assessed as the ability to traverse the beam with no more than two foot slips on a single trial for 3 consecutive days. Post-injury performance was rated on a seven point scale (7 = animal traversed the beam normally with no more than two foot slips; 6 = traversed the beam and used the affected limb to aid more than 50% of its steps; 5 = traversed the beam, but used the affected limb less than half of its steps; 4 = traversed the beam and at least once placed the affected hind paw on the horizontal surface of the beam; 3 = traversed the bean while dragging the affected limbs; 2 = unable to traverse the beam, but placed affected hind limb on horizontal surface and maintained balance; 1 = unable to transverse beam and could not place affected limb on horizontal surface).
Forelimb flexion test
The extent of flexion and adduction of the forelimbs after injury was assessed (McIntosh et al., 1989; Hoover et al., 2004). Rats were placed on a horizontal surface and lifted by the base of the tail. The amount of flexion of each forelimb was rated on a four-point scale (4 = normal, no flexion; 3 = less than 50% of flexion of the contralateral forelimb with adduction clearly present; 2 = more than 50% of flexion; and 1 = complete adduction of the forelimb).
Inclined plane
This task assessed the animal’s ability to maintain its position on an inclined plane (McIntosh et al., 1989; Hoover et al., 2004). Rats were placed horizontally on a board (60 × 40 cm) with a smooth surface with the right side, contralateral to the injury, being lowest. The left side of the board was slowly raised until the rat could no longer maintain its position. The angle of the slope at which the animal was unable to maintain its position was recorded.
Locomotor placing test
Coordination of limb placing during locomotion was assessed (Hoane et al. 1997, 2004). Fifteen test tube racks (Nalge Nunc International, product no. 5970-0330) were bound together to create an 86 × 55 cm grid surface with 3 × 3 cm square openings. Rats were allowed to freely traverse the grid for 3 min. A foot-fault occurred when one of the rat’s limbs fell through an opening during locomotion. The total number of foot-faults and the total number of sectors entered was recorded.
Cognitive ability test
MWM acquisition procedures were conducted on post-injury days 11–14 (Floyd et al., 2002). A circular 120 cm diameter pool was painted white and filled with water to a depth of 41 cm. The water was colored white using nontoxic white tempera paint and water temperature was maintained at 24°C. A submerged 11-cm-diameter and 39-cm-high escape platform (2 cm below the water surface) was placed in the north–east quadrant of the pool for all trials. The pool was located in a room with multiple extra-maze visual cues of different shapes and sizes. Path length, latency, and swim patterns were recorded using a computerized video tracking system (Chromotrack version 4.02b, Prototype Systems Ltd.). Animals were trained for 4 trials per day, starting from one of the four different directions on each trial (north, south, east, west). The order of the starting position was randomized across training days. On each trial, the animal was placed into the water facing the wall of the pool. The animal was allowed to swim until the escape platform was found and mounted, or when 60 sec had elapsed. The animal remained or was manually placed on the platform for 30 sec. Each animal was placed in a heated cage during the 2-min inter-trial interval.
Histology
Histological studies were aimed at determining whether enhanced recovery of function associated with the delivery of VNS was paralleled by reduced neuropathology. All measures and observations were performed using a single blind approach. Sections were collected at systematic and consistent intervals between animals and thus represent a type of systematic random sampling according to standard methods of morphometry.
On day 15 post-injury, following completion of all behavioral testing, all animals were anesthetized with urethane (3.0 g/kg, 0.5 g/mL, i.p.) and transcardially perfused for 90 seconds with chilled 0.01 M phosphate buffered saline (0.9%, PBS) followed immediately by 4% paraformaldehyde in PBS. Following removal, brains were post fixed in 4% paraformaldehyde for 24 h and cryoprotected by being immersed in 30% sucrose for 2 days prior to frozen sectioning. In every brain, four serial sets of six coronal sections were collected every 1.2 mm starting 0.04 mm posterior to bregma and extending to 5.0 mm posterior to bregma (Paxinos and Watson, 1998). Two sets of 30-μm sections were thaw mounted on slides, dried and alternately stained with formal thionine or Fluoro-Jade B. One 30-μm set was collected into PBS for free-floating immunohistochemistry of glial fibrillary acidic protein (GFAP); one set was collected at 10 μm thickness and stained with formal thionin for hippocampal CA-2/3 cell counts.
Lesion cavity size
With computer-aided image-capture microscopy (Scion Image, Frederick, MD) coupled to NIH Image analysis, actual surface areas of the cerebral cortices ipsilateral and contralateral to the injury were traced in six serial coronal sections and for each animal (n = 15 rats/group). It is noted that these measurements were of cerebral cortex tissue and differences between hemispheres represent loss of cerebral cortex tissue and often cavity formation. This is a gross measure of cortical tissue loss but not necessarily lesion size. From these data, a percent difference score was computed between the injured and non-injured sides of the cerebral cortices, and this score was used for a more standardized comparison. This technique allowed for statistical comparison between groups of animals, including the sham surgery group that did not exhibit cortical lesions (i.e., the difference between left and right sides of the cortex was less than 0.6%).
Neurodegeneration
Fluorojade B (FJ, Chemicon, AG-310, Temecla, CA) histo-fluorescence staining was used to determine the extent of ongoing neurodegeneration 15 days post-injury according to the methods of Schmued et al. (1997). Slides were examined with a fluorescence microscope using a FITC fluorescence filter cube at ×20 magnification. FJ-stained sections were examined for degenerating neuron clusters in the cerebral cortex and thalamus. A relative intensity of staining rating scale was developed (0 = no FJ positive cells, 1 = scattered FJ cells and/or 1 FJ positive cluster, 2 = more numerous scattered cells and/or 2 FJ-positive clusters, and 3 = densely scattered and more than 2 clusters of FJ positive neurons in the cerebral cortex).
Hippocampal pyramidal neuron death
Manual cell counts were made in 4 contiguous predefined areas along a linear grid over CA-2/3 of the dorsal hippocampus underlying the cerebral cortex caudal to the epicenter of the injury site but still within the window of the injury site (AP = bregma −4.4). This measure quantified the neuronal density along a consistent 4-window corridor of the CA-3 layer that is particularly sensitive to excitotoxicity and cell loss in TBI.
Reactive astrocytosis
Staining intensity of astrocytes was assessed using GFAP immunoreactivity at 15 days post-injury. Briefly, floating sections were blocked with 1.5% normal goat serum for 30 min and incubated with a GFAP anti-rabbit primary antibody (Sigma G-9269, diluted 1:1000) for 48 h, rinsed in PBS (10 times) and incubated with biotinylated anti-rabbit IgG (Vector Labs, BA-1000) for 1 h. Sections were then rinsed with Tris-buffered saline and incubated in the ABC substrate (Vector, PK-6100) for 1 h, rinsed in Tris-buffered saline, and reacted in a nickel and cobalt-intensified solution containing 0.1% CoCl2, 0.1% NiNH3(SO4), 0.04 % 3,3′-di-aminobenzidine tetrahydrochloride and 0.04% H2O2. Of the 10 collected 30-μm sections per site, six randomly selected sections were mounted, dehydrated, and cover slipped. Slides were visualized with brightfield microscopy at ×10 and ×20 magnifications. GFAP staining intensities were measured in the cerebral cortex and corpus callosum by two independent investigators blind to the treatment group using a rating scale (1 = low staining, 2 = medium staining, and 3 = heavy staining).
Inclusion/Exclusion Criteria
Only those animals which had attained criterion levels of performance prior to brain damage were included in subsequent analyses for the skilled forelimb reaching (n = 30) and beam walk (n = 51) tasks. Different subsets of animals were assessed in the locomotor placing (n = 45), inclined plane (n = 42) and forelimb flexion (n = 33) tests, and all animals tested on each of these tests were included in the analyses. Thirty-nine animals were included in the MWM analyses. Animals which were unable to acquire this task (i.e., never found the platform) by the fourth day of training were excluded (n = 5 for both VN stimulated and non-stimulated LFP animals, n = 2 for sham controls).
Statistical Analysis
All measures were analyzed using repeated measures ANOVA. The within-group factor was day of testing (post-injury days 2, 4, 6, 8, 10, 12, 14), and the between-factor was group (VN stimulated, non-stimulated, sham). Greenhouse-Geisser corrections and Fischer’s Least Significant Difference Test (LSD) were used to control for Type I error in the repeated measures and post hoc means comparisons, respectively. A significance level of p < 0.05 was used for all the statistical tests conducted. Inter-rater reliabilities were analyzed using Pearson’s correlations and Cronbach’s alpha (Cronbach, 1951). All data are shown as the mean ± SEM.
RESULTS
Severity of Injury Index
Mean apnea and duration of unconsciousness scores were analyzed with a one-way ANOVA. The LFP VN stimulated group was not significantly different from LFP controls on apnea [F (1, 39) = 3.054, p > 0.05] and duration of unconsciousness [F (1, 39) = 1.675, p > 0.05]. The combined apnea and duration of unconsciousness score was also not significantly different between the stimulated and unstimulated control groups [F (1, 39) = 2.410, p > 0.05]. These observations indicate that the groups were equally matched in the severity of injury sustained. Using the definitions of injury severity, based on duration of unconsciousness reported by Li et al. (2004) and Lee et al. (personal communication), the animals in this study were moderately injured (mean duration of unconsciousness = 180.03 sec, SD = 58.10; mean apnea = 19.19 sec, SD = 13.78). Thompson et al. (2005) suggest that the duration of unconsciousness and the absence of reflexes are a more accurate assessment of the severity of injury the animal received as opposed to the pressure readings obtained from the fluid percussion device.
Lesion Analysis
Each of the four histological analyses revealed significant differences between the sham-surgery animals and the LFP-treated animals. An example photomicrograph and a depiction of the cerebral cortex measurement are shown in Figure 2A. Areal measurements of the cerebral cortices confirmed that the LFP-treated animals incurred significant loss of tissue in the cerebral cortex ipsilateral to the injury site compared to the sham-surgery group [F (2, 42) = 10.6, p < 0.001]. The measurements revealed that LFP caused a loss of approximately 12–15% of the ipsilateral cerebral cortex (Fig. 2B). Lesion cavity size in the injured animals averaged approximately 1 mm2 of coronal surface area of cerebral cortex at the epicenter. Based on section thickness and number of sections through the lesion, this represents approximately 3 cubic mm of cerebral cortex. In the present study, despite the beneficial effects of VNS on behavioral recovery, no sparing of gross cortical tissue loss in the VNS animals was observed.
FIG. 2.
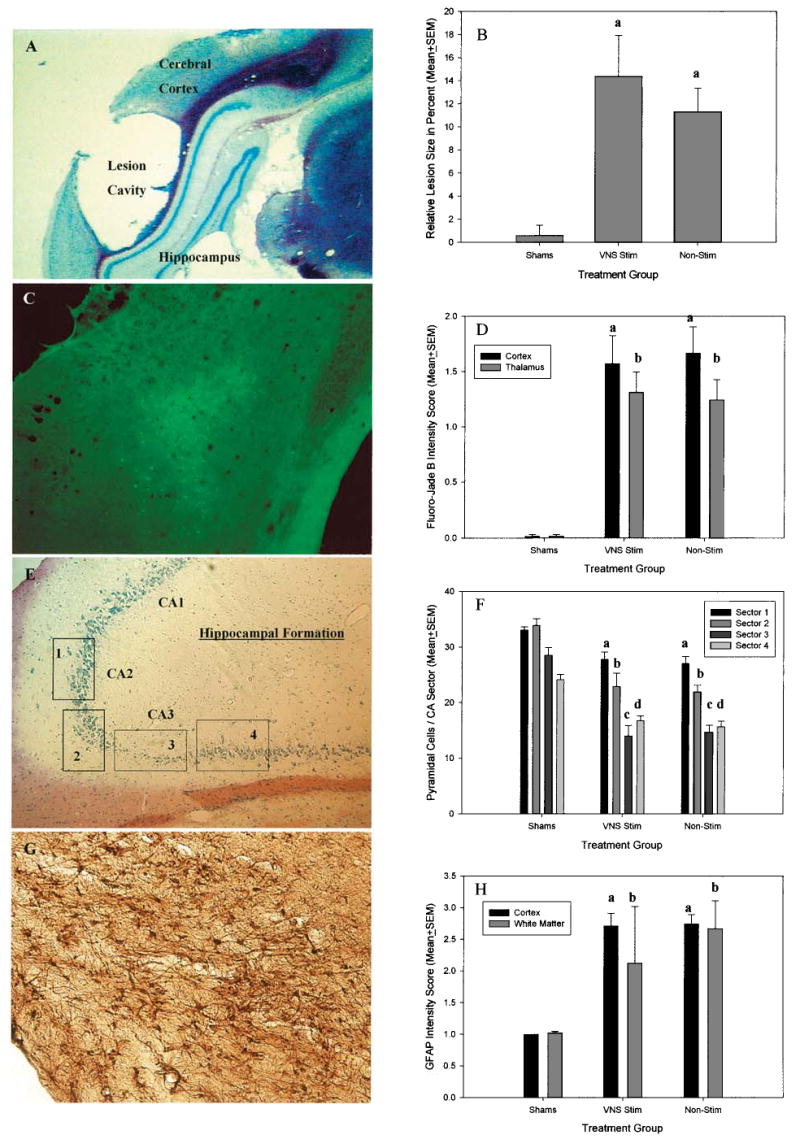
(A) Representative photomicrograph of a formal thionine stained 30 μm coronal section through the injury site 15 days after LFP. The lesion core is contained primarily in the cerebral cortex and underlying white matter. (B) Graph of relative lesion size, expressed as percent size difference between injured and uninjured (contralateral) cortex. a = Non-stimulated injured and VNS stimulated injured animals had significantly larger differences between injured and uninjured hemispheres compared to shams. (C) Representative photomicrograph of a Fluorojade (FJ) stained 30 μm coronal section following LFP. FJ-staining in the cortex ipsilateral to LFP revealed numerous FJ-positive neurons and neuron clusters. Diffuse FJ staining was also observed in deeper structures including the basal ganglia and the thalamus. (D) Graph of fluorojade intensity score in the cortex and thalamus underling the epicenter of the injury. Non-stimulated injured and VNS animals had significantly higher FJ intensity score in the cortex and thalamus than shams (as indicated by a and b, respectively). (E) Representative photomicrograph of 10 μm coronal formal thionine stained section through the hippocampal formation near the injury site following LFP. Pyramidal cell counts were concentrated in subsectors of CA2 and CA3 of the hippocampal formation as indicated by the boxes labeled 1, 2, 3, and 4. (F) Graph of pyramidal cell counts per sector in area CA2 and 3 of the hippocampus underlying the epicenter of injury. Non-stimulated injured and VNS animals had significantly fewer pyramidal cells in all counted subsectors (a–d) but were most severely reduced in subsector 3 of layer CA-3. (G) Representative photomicrograph of 30 μm coronal GFAP section following LFP. GFAP reactive astrocytes can be seen in the cerebral cortex and underlying white matter fiber tracts of the callosum. (H) Graph of GFAP intensity scores in the cortex and white matter underlying the epicenter of injury. Non-stimulated injured and VNS animals had significantly higher ratings of GFAP intensity scores in the cortex and white matter than shams (as indicated by a and b, respectively).
An example photomicrograph of clustered FJ positive staining in the cerebral cortex is shown in Figure 2C. Qualitatively, rats that had undergone LFP showed clusters of degenerating neurons in the cerebral cortices as well as scattered degenerating neurons in the thalamus and basal ganglia as long as 15 days post-injury. Relative intensity scores between the VNS and non-stimulated groups of rats that experienced LFP showed no significant differences in the profile of degenerating neurons. (Fig. 2D). The inter-rater reliability scores on measures of relative intensity showed a 98% concordance between observers.
An example composite photomicrograph of the measured CA-3 region of the dorsal hippocampus is shown in Figure 2E. Hippocampal pyramidal neuron death associated with LFP was confirmed throughout the measured CA-3 region although the heaviest loss was seen in one circumscribed region (linear quadrant 3). As shown in Figure 2F, no significant differences were observed in CA-3 cell loss between the VNS and non-stimulated groups of LFP rats though both were significantly different from the uninjured animals.
An example photomicrograph of GFAP staining in the cerebral cortex is shown in Figure 2G. Qualitatively, all LFP rats showed intensely stained clusters of GFAP positive cells in the cerebral cortices as well as the underlying white matter 15 days post-injury. However, based on relative intensity scores between treatment groups, both the VNS and non-stimulated groups of rats that experienced LFP showed no significant differences in the profile of GFAP-stained cells 15 days post-LFP although both were significantly different from the uninjured animals (Fig. 2H). The inter-rater reliability scores on measures of relative intensity showed a 99% concordance between observers. Thus, as with cortical lesion size, FJ-staining, and hippocampal CA-3 cell number, facilitation of behavioral recovery from LFP by VNS was not paralleled by an obvious change in GFAP staining intensity 15 days post-LFP.
Behavioral Results
Skilled forelimb reaching test
Difference scores for each post-injury test day were calculated by subtracting the post-injury reaching test score from the pre-injury reaching criterion and analyzed with a repeated measures ANOVA. VNS animals performed significantly better than injured controls across test days [F (12, 162) = 2.82, p < 0.02]. Post hoc comparisons showed that by day 8 post-injury, the LFP animals receiving VNS were performing significantly better than the non-stimulated animals (p < 0.02). This effect persisted through the last day of testing at day 14 (Fig. 3). Further, while the non-stimulated animals were significantly impaired on the reaching task across all test days compared to sham operated animals, the VNS animals were not significantly different from shams on days 8 and 14 (p > 0.05). Inter-rater scores were correlated at 0.987 (p < 0.0001), Cronbach’s alpha = 0.992.
FIG. 3.
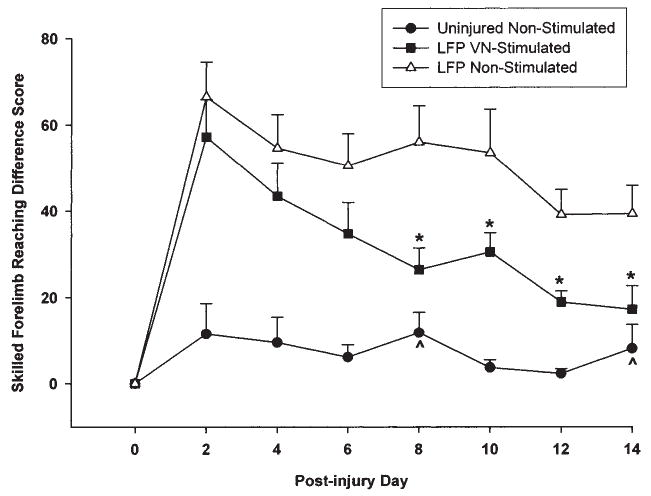
Graph of skilled forelimb reaching (pre-injury criteria—post-injury) difference scores. Non-stimulated injured animals were significantly impaired on all test days compared to uninjured sham animals. VNS stimulated injured animals performed significantly more poorly than shams except on days 8 and 14 (^). In contrast with non-stimulated injured animals, VNS stimulation significantly improved reaching performance beginning at post-injury day 8–14 (*).
Beam walk test
Analysis of the difference in the rats’ ability to traverse the beam after LFP as compared to performance prior to injury revealed a significant effect of VNS across post-injury test days [F (12, 288) = 13.067, p < 0.0001] (Fig. 4). The non-stimulated group performed significantly more poorly than uninjured rats on all test days (p < 0.05). Conversely, the VNS group was not significantly different from the uninjured group for all post-injury test days starting at day 6 through day 14. In addition, the VNS animals were significantly better able to traverse the beam on all post-injury test days compared to the non-stimulated injured animals (p < 0.01). Inter-rater scores were correlated at 0.994 (p < 0.0001), Cronbach’s alpha = 0.997.
FIG. 4.
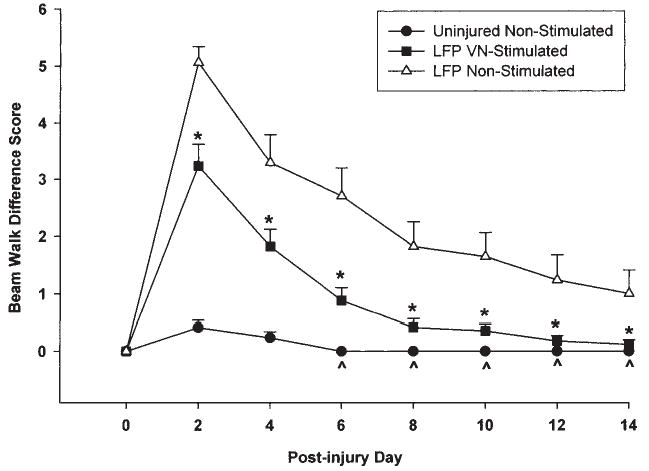
Graph of beam walking (pre-injury criteria—post-injury) difference scores. The injured non-stimulated group was significantly impaired on the beam walk compared to both shams and VNS animals on all post-injury test days. The VNS animals performed significantly better than the injured non-stimulated animals on all test days 2–14 (*). The ability of the VNS stimulated animals to traverse the beam was not significantly different from shams beginning at day 6 post-injury through day 14 (^).
Forelimb flexion test
Flexion differences were calculated by subtracting the right limb (contralateral to injury site) score from the left limb score on each test day. VN stimulated animals showed significantly less adduction of the contralateral forelimb over time in comparison with the injured controls [F (14, 210) = 7.82, p < 0.0001]. Subsequent post hoc analyses revealed that the injured non-stimulated animals were significantly more impaired in comparison to the uninjured animals on all test days (p < 0.01; Fig. 5). The VNS animals also showed greater flexion as compared to the shams (p < 0.01) but this difference was not significant by the last two days of testing at days 12 and 14 (p > 0.05). Further, the VNS animals were significantly less impaired than the non-stimulated injured animals at days 6 (p < 0.001) and 8 (p < 0.05) but were not statistically different on all other test days (p > 0.05). Inter-rater scores were correlated at 0.987 (p < 0.0001), Cronbach’s alpha = 0.994.
FIG. 5.
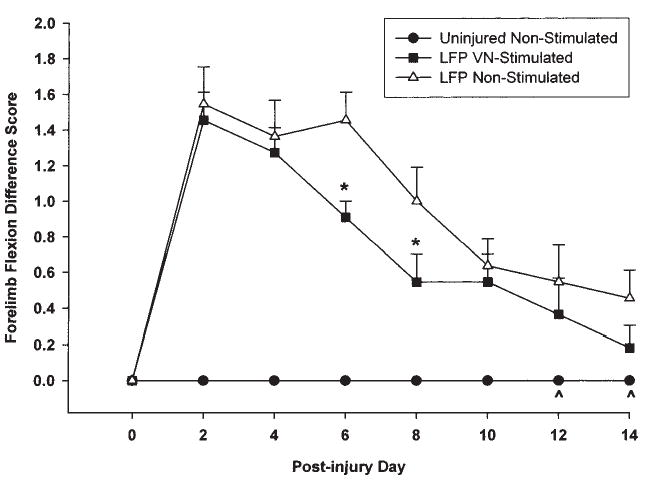
Graph of forelimb flexion (ipsilateral—contralateral) difference scores. The non-stimulated group showed significantly greater flexion of the contralateral limb following injury on all test days compared to shams. The VNS group also showed greater flexion compared to shams up to day 10 post-injury. The amount of flexion shown by the VNS group was not significantly different from shams at days 12 and 14 (^). VNS animals were not significantly different from non-stimulated animals on all days except on days 6 and 8 post-injury (*) when the VNS group showed significantly less flexion of the contralateral limb.
Inclined plane test
Each animal’s ability to maintain its position on the inclined plane after LFP was compared to its baseline performance prior to injury. A significant difference in the recovery rate of the VNS animals as compared to injured controls was revealed in the interaction effect [F (14, 266) = 3.06, p < 0.001] (Fig. 6). Post hoc comparisons showed that the non-stimulated injured group performed significantly worse than the uninjured group on all test days following injury (p < 0.01). In contrast, the VNS animals were not significantly different from the uninjured shams in their ability to maintain position on the inclined plane at every test starting at test day 2 (p > 0.05). Moreover, the VNS group performed significantly better than the non-stimulated group on all test days (p < 0.05) except day 12 (p > 0.05). Inter-rater scores were perfectly correlated, Cronbach’s alpha = 1.0.
FIG. 6.

Graph of inclined plane (baseline—post-injury) difference scores. The non-stimulated injured animals were severely impaired as compared to shams on the incline plane on all test days. The performance of the VNS animals was not significantly different from shams on all test days (^). The VNS stimulated animals were significantly better at maintaining position on the incline plane compared to the non-stimulated group on all test days (*) except day 12.
Locomotor placing test
VNS animals showed a more rapid rate of recovery than the non-stimulated injured controls in limb placing during locomotion as compared with pre-injury baseline performance [F (14, 294) = 3.31, p < 0.002]. Post hoc tests revealed that the non-stimulated animals were severely impaired in locomotor placing as compared to uninjured shams on all test days (p < 0.03) except on test day 10 (p > 0.05; Fig. 7). The VNS animals were not significantly different from uninjured animals on all test days (p > 0.05). Further, the VNS animals performed significantly better than non-stimulated injured animals on days 2, 4, and 6 (p < 0.01), but were not significantly different from the non-stimulated group on days 8–14 (p > 0.05). Inter-rater scores were correlated at 0.995 (p < 0.0001), Cronbach’s alpha = 0.998.
FIG. 7.
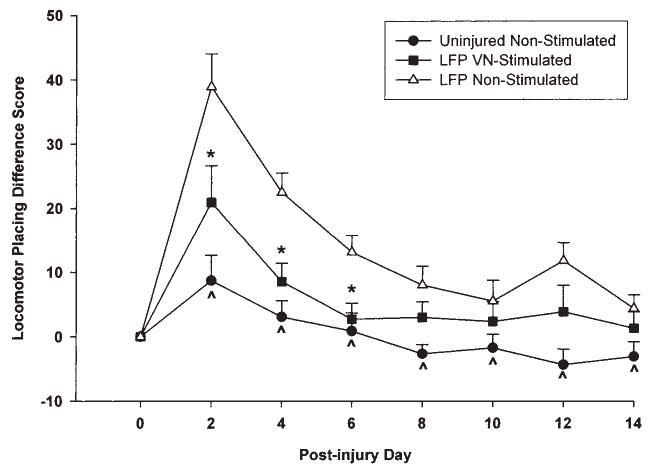
Graph of locomotor placing (baseline—post-injury) difference scores. Non-stimulated injured animals made significantly more foot faults compared to shams on all test days except day 10. VNS animals were not significantly different from shams on all test days (^). The VNS group performed significantly better than the non-stimulated group at days 2, 4, and 6 (*) but was not significantly different on days 8–14.
Cognitive ability test
Differences in latency to reach the platform of each treatment group were reflected in a significant main effect for group [F (2,36) = 24.76, p < 0.0001] (Fig. 8). Post hoc comparisons revealed that the brain-injured animals were slower to find the hidden platform on all test days compared to the uninjured animals (p < 0.0001). Nonetheless, the VNS animals were less impaired than the non-stimulated injured animals overall (p < 0.006). The main effect for test day was significant [F (3, 108) = 77.59, p < 0.0001] indicating that animals in all treatment groups became more efficient at locating the platform over the 4-day period of testing. The acquisition rate of the animals in different treatment conditions was not significantly different, as indicated in the group by test day interaction effect [F (6,108) = 1.62, p > 0.05]. Path length measures were correlated at 0.98 (p < 0.01) with latency measures and hence yielded similar analysis results. There were no significant differences between the swim speeds of the animals (mean 12.64 cm/sec [F (2, 38) = 1.806, p > 0.10]).
FIG. 8.

Graph of MWM latency to locate the hidden platform. By training days 13 and 14 post-injury, the VNS animals were faster than the non-stimulated group at locating the hidden platform (*) and their performance was also not significantly different from shams (^).
DISCUSSION
Chronic, intermittent electrical stimulation of the vagus nerve (VNS) beginning 2 h after a moderate fluid percussion injury enhanced the rate of recovery on neurological measures of forelimb flexion and capacity to maintain position on an inclined plane. The deficits in these neurological measures observed in the non-stimulated controls were consistent with those typically reported by others following moderate levels of LFP in a combined neurological score (McIntosh, et al., 1989; Saatman et al., 1997). The present results indicate that these neurological signs separately demonstrate a significant improvement in the rate of recovery in the VNS animals and, for the inclined plane test, differences in the final level of performance reached 14 days post-injury. Likewise, in the beam walk (vestibulomotor) and locomotor placing (sensory/motor) tests, VNS produced a significant facilitation of the rate of recovery, while for the beam walk task both the rate of recovery and final level of performance were enhanced. More demanding, probably cortically controlled (Wishaw and Metz, 2002), skilled reaching performance was also enhanced both in the rate of recovery and final level of performance. Importantly, following 14 days of VNS the stimulated group’s performance was not different from that of uninjured animals. Since we did not perform high-speed motion analysis of the reaching movements (Metz and Wishaw, 2000; Wishaw and Metz, 2002; Wishaw et al., 2004) it is not known whether these reaching movements were the same as they were prior to the LFP; however, the VNS animals did demonstrate significant improvements in this skilled reaching test and, regardless of how these skilled movements are performed, the clinical implications of once again being able to manipulate and control objects is of considerable importance. Finally, cognitive memory abilities as assessed in the MWM demonstrated significant stimulation-induced improvement in both measures (latencies and path length) measured 11–14 days post-injury.
Behavioral differences in both the rate of recovery and final level of performance might suggest that VNS has a neuroprotective effect. However, despite the facilitation of recovery afforded by VNS, the neurohistological measures used in this study did not reveal significant effects on lesion volume, the extent of reactive gliosis, hippocampal cell loss, or in neurodegeneration as assessed by the number of Fluoro-Jade positive cells. Our inability to demonstrate any apparent anatomical neuroprotective effects may have been due to the behavioral testing requirements which restricted our neurohistological assessment to only one time point, 15 days following injury. It is well established that the histopathological effects of LFP change over time and even continue for over a year (Hicks et al., 1996; Smith et al., 1997; Pierce et al., 1998; Bramlett and Dietrich, 2002). While the histopathological effects of LFP demonstrated in the present results are in agreement with those reported by these authors following LFP, it remains to be determined if evidence of a neuroprotective effect can be found by a more rigorous examination at several time-points post-injury.
Prior to discussion of the potential mechanisms of the effectiveness of VNS in promoting recovery it is necessary to address potential concerns regarding the data. Any experiment that induces LFP might inadvertently place more severely injured animals in the control group and, thus, bias the results. Three observations argue against the possibility that this occurred. First the histological findings discussed above revealed no differences between the VNS and non-stimulated LFP groups in terms of size of the cortical injury, a measure that correlates well with the severity of behavioral deficits (Thompson et al., 2005). Second, another primary indicator of the severity of damage, the duration of unconsciousness and/or apnea (Li et al., 2004; Thompson et al., 2005) were directly matched between groups and assessed and there were no significant differences observed between the stimulated and unstimulated LFP groups. Third, both VNS and non-stimulated LFP controls demonstrated significant impairments in the neurological measures, beam walking and skilled reaching immediately post-injury, as well as in the initial trials in the MWM that did not differ in magnitude. Thus, we are confident that the more rapid and more extensive recovery of these behaviors in the stimulated animals reflects the effects of VNS.
The present study was not designed to identify possible mechanisms underlying the effectiveness of VNS in promoting behavioral recovery following TBI. However, there are some highly testable hypotheses we are pursuing to address the potential mechanisms. First, as described in the introduction, we predicted VNS would enhance recovery based on the effects of NE agonists and antagonists on recovery following TBI. There is now direct microdialysis evidence from our laboratory and others that VNS increases the release of NE in the amygdala (Hassert, et al., 2004) and the hippocampus and neocortex (Roosevelt et al., 2004) for at least the first 24 h VNS is applied. Nevertheless, it is not currently known whether the chronic 14 days of VNS (over 670 stimulations) applied in the current study results in any decrements or enhancement of NE release.
To evaluate the degree to which NE is the mechanism of action of VNS-induced recovery of function, the effects of systematic manipulation of noradrenergic systems need to be studied. For example, Feeney and Westerberg (1990) demonstrated that α1 receptor antagonist’s retards motor recovery in the beam balance task following unilateral fluid percussion centered over the sensorimotor cortex. On this basis administration of a selective α1 antagonist would be predicted to attenuate the effectiveness of VNS in promoting recovery following LFP. We are currently testing this prediction. Furthermore, there is very good evidence that amphetamine delivered 24 h following injury partially reverses the hypometabolism and cerebral inhibition that accompanies unilateral contusion injury (Queen et al., 1997; Sutton et al., 2000). Importantly, following unilateral contusion injury, or LFP, this diaschisis-like state is associated with widespread depletion of cortical and subcortical NE 6–48 h following injury (Dunn-Meynell et al., 1994; Levin et al., 1995) which persists up to two weeks in the hypothalamus following LFP (McIntosh et al., 1994). It is thus very possible that VNS and its associated release of NE serves to reduce this hypometabolic state and thus promotes more rapid and sustained behavioral recovery and we are actively investigating this possibility.
It is important to note that most studies in the literature which have demonstrated the effectiveness of NE agonists on behavioral recovery, applied these agents 24 h or more after injury. In contrast, we initiated VNS 2 h post-injury and this might be thought of as an acute, as opposed to a delayed, traumatic intervention that might create important temporal difference issues. However, we now have evidence (Smith et al., 2005) that delaying the onset of VNS for 24 h post-injury facilitates the recovery in all behavioral measures reported here. Nevertheless, we continue to investigate the temporal window of effectiveness of VNS in promoting recovery. One of our most important aims is to determine the effects of termination of VNS after behavioral recovery has already been facilitated to see if the behavioral facilitation is enduring or requires continued, chronic VNS.
An additional potential mechanism mediating the effectiveness of VNS in promoting behavioral recovery may be its antiepileptic effects. It is well-established that VNS suppresses seizures in several animal models of epilepsy as well as in humans. We originally reported that Long-Evans hooded rats develop electrographic and sometimes motor convulsive seizures following LFP (Roosevelt et al., 2000). The appearance of such seizures following LFP has now been documented in other laboratories and strains of rats (D’Ambrosio et al., 2004, 2005). It is well documented that the nature of the brain injury affects both the probability of appearance of post-traumatic seizures and also the quality of the functional outcome following TBI in humans, although in experimental animals the predicted relationship between seizures and functional outcome is less clear (Hernandez and Naritoku, 1997). As an effective antiepileptic treatment in both humans and experimental models of epilepsy, VNS could be expected to reduce seizure frequency and possibly the behavioral deficits associated with such seizures. As we did not monitor either EEG or motor seizures in the current experiment such a relationship remains speculative; however, it is certainly possible that the enhancement in neurological, motor and cognitive recovery found in the present study reflects an attenuation of seizures and/or a facilitation of learning and memory (Clark et al., 1995, 1998, 1999), either or both of which would be predicted to enhance functional outcome following TBI. Interestingly, related to the NE hypothesis of recovery discussed above, NE has also been shown to suppress seizures in most experimental models of epilepsy except for the tottering mouse model of absence epilepsy (Browning, 1987; Jobe et al., 1986; Jobe, 2003; Maynert et al., 1975). Only additional research can evaluate the role of separate potential mechanisms in the effects of VNS on functional recovery following TBI and we are currently examining these.
Regardless of the actual mechanisms underlying the effectiveness of VNS in promoting recovery from TBI, the present findings suggest that VNS represents a potential non-pharmacological post-injury treatment that may prove to be useful in the treatment of TBI. Further, human clinical trials of the enhancement of recovery with several drugs shown to be effective in animal models of TBI have been totally disappointing, in large part due to the side effects of these drugs (Hatton, 2001; Marklund et al., 2004; Statler et al., 2001). Certainly a single study such as ours does not establish the pre-clinical efficacy of VNS as a treatment for TBI; nevertheless, our results clearly demonstrate that VNS is effective in facilitating recovery in the LFP model and, as such, requires further examination.
Much remains to be determined. However, the results reported here represent a promising first step in the search for a non-pharmacological treatment without major side effects in the treatment of TBI.
Acknowledgments
We gratefully acknowledge the consultations of Drs. David Hovda, Stefan Lee, and Rich Sutton, who provided valuable training and advice to D.C.S. in the fluid percussion technique. Further, several students played immensely important roles in helping with the behavioral training and testing: Luke Sherrill, Andrea Duke, Sarah Turner, Julie Schneider, and Abranda VonLanken, as well as Derrick Hassert, Jerome Klope, and Faith Hall in early pilot experiments. We also thank Cyberonics, Inc. for providing the Cyberonics model 102 functioning and dummy vagus nerve stimulators. Finally, Steve Mueller of the IMAGE facility at SIUC did the illustration of the VNS electrode in Figure 1. Supported by a National Institute of Neurological Disorders and Stroke grant (NS 41551) to D.C.S.
References
- ARDELL JL, RANDALL WC. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am J Physiol. 1986;251:H764–H773. doi: 10.1152/ajpheart.1986.251.4.H764. [DOI] [PubMed] [Google Scholar]
- BOYESON MG, FEENEY DM. Intraventricular norepinephrine facilitates motor recovery following sensorimotor cortex injury. Pharmacol Biochem Behav. 1990;35:497–501. doi: 10.1016/0091-3057(90)90279-q. [DOI] [PubMed] [Google Scholar]
- BRAMLETT HM, DIETRICH WD. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol (Berl) 2002;103:607–614. doi: 10.1007/s00401-001-0510-8. [DOI] [PubMed] [Google Scholar]
- BROWNING RA. The role of neurotransmitters in electroshock seizure models. In: Jobe PC, Laird HE II, editors. Neurotransmitters and Epilepsy. Clifton, NJ: Humana Press; 1987. pp. 277–320. [Google Scholar]
- BURY SD, JONES TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22:8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK KB, KRAHL SE, SMITH DC, JENSEN RA. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol Learn Mem. 1995;63:213–216. doi: 10.1006/nlme.1995.1024. [DOI] [PubMed] [Google Scholar]
- CLARK KB, NARITOKU DK, SMITH DC, BROWNING RA, JENSEN RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- CLARK KB, SMITH DC, HASSERT DL, BROWNING RA, NARITOKU DK, JENSEN RA. Post-training electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol Learn Mem. 1998;70:364–373. doi: 10.1006/nlme.1998.3863. [DOI] [PubMed] [Google Scholar]
- CRISOSTOMO EA, DUNCAN PW, PROPST M, DAWSON DV, DAVIS JN. Evidence that amphetamine with physical therapy promotes recovery of motor function in stroke patients. Ann Neurol. 1988;23:94–97. doi: 10.1002/ana.410230117. [DOI] [PubMed] [Google Scholar]
- CRONBACH LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- D’AMBROSIO R, FAIRBANKS JP, FENDER JS, BORN DE, DOYLE DL, MILLER JW. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’AMBROSIO RD, FENDER JS, FAIRBANKS JP, et al. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 2005;128:174–188. doi: 10.1093/brain/awh337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIETRICH WD. Non-pharmacological strategies—hypothermia. In: Narayan RK, Wilberger JE, Povlishock JT, editors. Neurotrauma: A Comprehensive Textbook on Head and Spinal Injury. McGraw-Hill; New York: 1996. pp. 1491–1506. [Google Scholar]
- DUNN-MEYNELL A, PAN S, LEVIN BE. Focal traumatic brain injury causes widespread reductions in rat brain norepinephrine turnover from 6 to 24 h. Brain Res. 1994;660:88–95. doi: 10.1016/0006-8993(94)90842-7. [DOI] [PubMed] [Google Scholar]
- FEENEY DM. Mechanisms of noradrenergic modulation of physical therapy: effects on functional recovery after cortical injury. In: Goldstein LB, editor. Restorative Neurology: Advances in Pharmacotherapy for Recovery after Stroke. Futura Publishing; New York: 1998. pp. 35–78. [Google Scholar]
- FEENEY DM, HOVDA DA. Amphetamine and apomorphine restore tactile placing after motor cortex injury in the cat. Psychopharmacology. 1983;79:67–71. doi: 10.1007/BF00433018. [DOI] [PubMed] [Google Scholar]
- FEENEY DM, HOVDA DA. Reinstatement of binocular depth perception by amphetamine and visual experience after visual cortex ablation. Brain Res. 1985;342:352–356. doi: 10.1016/0006-8993(85)91135-7. [DOI] [PubMed] [Google Scholar]
- FEENEY DM, SUTTON RL. Pharmacotherapy for recovery of function after brain injury. Crit Rev Neurobiol. 1987;3:135–197. [PubMed] [Google Scholar]
- FEENEY DM, SUTTON RL. Catecholamines and recovery of function after brain damage. In: Sabel B, Stein D, editors. Pharmacological Approaches to the Treatment of Brain and Spinal Cord Injury. Plenum; New York: 1988. pp. 121–142. [Google Scholar]
- FEENEY DM, WESTERBERG VS. Norepinephrine and brain damage: alpha noradrenergic pharmacology alters functional recovery after cortical trauma. Can J Psychol. 1990;44:233–252. doi: 10.1037/h0084243. [DOI] [PubMed] [Google Scholar]
- FEENEY DM, GONZALEZ A, LAW WA. Amphetamine, haloperidol and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- FEENEY DM, SUTTON RL, BOYESON MG, HOVDA DA, DAIL W. The locus coeruleus and cerebral metabolism: recovery of function after cortical injury. Physiol Psych. 1985;13:197–203. [Google Scholar]
- FISHER RS, HANDFORTH A. Reassessment: vagus nerve stimulation for epilepsy: a report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1999;53:666–669. doi: 10.1212/wnl.53.4.666. [DOI] [PubMed] [Google Scholar]
- FLOYD CL, GOLDEN KM, BLACK RT, HAMM RJ, LYETH BG. Craniectomy position affects morris water maze performance and hippocampal cell loss after parasagittal fluid percussion. J Neurotrauma. 2002;19:303–316. doi: 10.1089/089771502753594873. [DOI] [PubMed] [Google Scholar]
- FRIEDBERG MH, LEE SM, EBNER FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol. 1999;81:2243–2252. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- GLADSTONE DJ, BLACK SE. Enhancing recovery after stroke with noradrenergic pharmacotherapy: a new frontier? Can J Neurol Sci. 2000;27:97–105. [PubMed] [Google Scholar]
- GOLDSTEIN LB. In: Restorative Neurology: Advances in Pharmacotherapy for Recovery after Stroke. Goldstein LB, editor. Futura Publishing; New York: 1998a. [Google Scholar]
- GOLDSTEIN LB. Potential impact of drugs on recovery. In: Goldstein LB, editor. Restorative Neurology: Advances in Pharmacotherapy for Recovery after Stroke. Futura Publishing; New York: 1998b. pp. 241–256. [Google Scholar]
- GOLDSTEIN LB, BULLMAN S. Effects of dorsal noradrenergic bundle lesions on recovery after sensorimotor cortex injury. Pharmacol Biochem Behav. 1997;58:1151–1157. doi: 10.1016/s0091-3057(97)00324-9. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN M. Traumatic brain injury: a silent epidemic. Ann Neurol. 1990;27:327. doi: 10.1002/ana.410270315. [DOI] [PubMed] [Google Scholar]
- GROVES DA, BOWMAN R, BROWN VJ. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anesthetized rat. Neurosci Lett. 2005;379:174–179. doi: 10.1016/j.neulet.2004.12.055. [DOI] [PubMed] [Google Scholar]
- GROVES DA, BROWN VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev. 2005;29:493–500. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- GUEDEL AE. Signs of inhalational anesthesia. A fundamental guide. In: Guedel AE, editor. Inhalation Anesthesia. MacMillian; New York: 1920. pp. 10–52. [Google Scholar]
- HASSERT DL, MIYASHITA T, WILLIAMS CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci. 2004;118:79–88. doi: 10.1037/0735-7044.118.1.79. [DOI] [PubMed] [Google Scholar]
- HATTON J. Pharmacological treatment of traumatic brain injury: a review of agents in development. CNS Drugs. 2001;15:553–581. doi: 10.2165/00023210-200115070-00005. [DOI] [PubMed] [Google Scholar]
- HERMANN GE, ROGERS RC. Convergence of vagal and gustatory afferent input within the parabrachial nucleus of the rat. J Auton Nerv Syst. 1985;13:1–17. doi: 10.1016/0165-1838(85)90002-5. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ TD, NARITOKU DK. Seizures, epilepsy, and functional recovery after traumatic brain injury: a reappraisal. Neurology. 1997;48:803–806. doi: 10.1212/wnl.48.4.803. [DOI] [PubMed] [Google Scholar]
- HICKS R, SOARES H, SMITH D, McINTOSH T. Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol. 1996;91:236–246. doi: 10.1007/s004010050421. [DOI] [PubMed] [Google Scholar]
- HOANE MR, BECERRA GD, SHANK JE, et al. Transplantation of neuronal and glial precursors dramatically improves sensorimotor function but not cognitive function in the traumatically injured brain. J Neurotrauma. 2004;21:163–174. doi: 10.1089/089771504322778622. [DOI] [PubMed] [Google Scholar]
- HOANE MR, RAAD C, BARTH TM. Non-competitive NMDA antagonists and anti-oxidant drugs reduce striatal atrophy and facilitate recovery of function following lesions of the rat cortex. Restor Neurol Neurosci. 1997;11:71–82. doi: 10.3233/RNN-1997-111208. [DOI] [PubMed] [Google Scholar]
- HOOVER RC, MOTTA M, DAVIS J, et al. Differential effects of the anticonvulsant topiramate on neurobehavioral and histological outcomes following traumatic brain injury in rats. J Neurotrauma. 2004;21:501–512. doi: 10.1089/089771504774129847. [DOI] [PubMed] [Google Scholar]
- HOVDA DA, FEENEY DM. Amphetamine with experience promotes recovery of locomotor function after unilateral frontal cortex injury in the cat. Brain Res. 1984;298:358–361. doi: 10.1016/0006-8993(84)91437-9. [DOI] [PubMed] [Google Scholar]
- JOBE PC. Common pathogenic mechanisms between depression and epilepsy: an experimental perspective. Epilepsy Behav. 2003;4(Suppl 3):S14–S24. doi: 10.1016/j.yebeh.2003.08.020. [DOI] [PubMed] [Google Scholar]
- JOBE PC, DAILEY JW, RIEGEL CE. Noradrenergic and serotonergic determinants of seizure susceptibility and severity in genetically epilepsy-prone rats. Life Sci. 1986;39:775–82. doi: 10.1016/0024-3205(86)90455-8. [DOI] [PubMed] [Google Scholar]
- KASAMATSU T, PETTIGREW JD. Depletion of brain catecholamines: failure of ocular dominance shift after monocular occlusion in kittens. Science. 1976;194:206–209. doi: 10.1126/science.959850. [DOI] [PubMed] [Google Scholar]
- KIKUCHI K, NISHINO K, OHYU H. Increasing CNS norepinephrine levels by the precursor L-DOPS facilitates beam-walking recovery after sensorimotor cortex ablation in rats. Brain Res. 2000;860:130–135. doi: 10.1016/s0006-8993(00)02034-5. [DOI] [PubMed] [Google Scholar]
- KRAHL SE, BROWNING RA, CLARK KB, SMITH DC. Possible mechanism of seizure attenuating effects of vagal stimulation. Soc Neurosci Abstr. 1994;20:1453. [Google Scholar]
- KRAHL SE, CLARK KB, SMITH DC, BROWNING RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39:709–714. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- LEVIN BE, Brown KL, PAWAR G, DUNN-MEYNELL A. Widespread and lateralized effects of acute traumatic brain injury on norepinephrine turnover in the rat brain. Brain Res. 1995;674:307–313. doi: 10.1016/0006-8993(95)00032-l. [DOI] [PubMed] [Google Scholar]
- LI HH, LEE SM, CAI Y, SUTTON RL, HOVDA DA. Differential gene expression in hippocampus following experimental brain trauma reveals distinct features of moderate and severe injuries. J Neurotrauma. 2004;21:1141–1153. doi: 10.1089/neu.2004.21.1141. [DOI] [PubMed] [Google Scholar]
- MARKLUND N, STOVER JF, McINTOSH TK. Excitotoxicity and traumatic brain injury: pathology, treatment approaches and controversies. In: Ferrarese C, Beal M, editors. Excitotoxicity in Neurological Diseases. Kluwer Academic Publishers; Boston: 2004. pp. 189–216. [Google Scholar]
- MAYNERT EW, MARCZYNSKI TJ, BROWNING RA. The role of neurotransmitters in the epilepsies. Adv Neurol. 1975;13:79–147. [PubMed] [Google Scholar]
- McINTOSH TK. Novel pharmacologic therapies in the treatment of experimental traumatic brain injury: a review. J Neurotrauma. 1993;10:215–261. doi: 10.1089/neu.1993.10.215. [DOI] [PubMed] [Google Scholar]
- McINTOSH TK, YU T, GENNARELLI TA. Alterations in regional brain catecholamine concentrations after experimental brain injury in the rat. J Neurochem. 1994;63:1426–1433. doi: 10.1046/j.1471-4159.1994.63041426.x. [DOI] [PubMed] [Google Scholar]
- McINTOSH TK, JUHLER M, WIELOCH T. Novel pharmacologic strategies in the treatment of experimental traumatic brain injury. J Neurotrauma. 1998;15:731–769. doi: 10.1089/neu.1998.15.731. [DOI] [PubMed] [Google Scholar]
- McINTOSH TK, VINK R, NOBLE L, YAMAKAMI I, FERNYAK S, FADEN AI. Traumatic brain injury in the rat: characterization of a lateral fluid percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- METZ GA, WHISHAW IQ. Skilled reaching an action pattern: stability in rat (Rattus norvegicus) grasping movements as a function of changing food pellet size. Behav Brain Res. 2000;116:111–122. doi: 10.1016/s0166-4328(00)00245-x. [DOI] [PubMed] [Google Scholar]
- MEYER PM, HOREL JA, MEYER DR. Effects of D,L-amphetamine upon placing responses in neo-decorticate cats. J Comp Physiol Psych. 1963;56:402–404. doi: 10.1037/h0049297. [DOI] [PubMed] [Google Scholar]
- NARITOKU DK, TERRY WJ, HELFERT RH. Regional induction of fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epilepsy Res. 1995;22:53–62. doi: 10.1016/0920-1211(95)00035-9. [DOI] [PubMed] [Google Scholar]
- PAXINOS G, WATSON C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- PIERCE JE, SMITH DH, TROJANOWSKI JQ, McINTOSH TK. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998;87:359–369. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- QUEEN SA, CHEN MJ, FEENEY DM. D-Amphetamine attenuates decreased cerebral glucose utilization after unilateral sensorimotor cortex contusion in rats. Brain Res. 1997;777:42–50. doi: 10.1016/s0006-8993(97)00717-8. [DOI] [PubMed] [Google Scholar]
- ROOSEVELT RW, BROWNING RA, JENSEN RA, CLOUGH RW, SMITH DC. Vagus nerve stimulation (VNS) increases extracellular concentrations of norepinephrine in rat hippocampus and cortex. Soc Neurosci. 2004 doi: 10.1016/j.brainres.2006.08.04. Abstract Viewer/Itinerary Planner 45.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROOSEVELT RW, SMITH DC, BROWNING RA. Effects of α-tocopherol, ascorbic acid and ibuprofen on functional recovery following fluid percussion head injury in rats. Soc Neurosci. 2000 Abstract Viewer/Itinerary Planner 862.19. [Google Scholar]
- SAATMAN KE, CONTRERAS PC, SMITH DH, et al. Insulin-like growth factor–1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp Neurol. 1997;147:418–427. doi: 10.1006/exnr.1997.6629. [DOI] [PubMed] [Google Scholar]
- SCHACHTER SC, SAPER CB. Vagus nerve stimulation. Epilepsia. 1998;39:677–686. doi: 10.1111/j.1528-1157.1998.tb01151.x. [DOI] [PubMed] [Google Scholar]
- SCHMANKE TD, AVERY RA, BARTH TM. The effects of amphetamine on recovery of function after cortical damage in the rat depend on the behavioral requirements of the task. J Neurotrauma. 1996;13:293–307. doi: 10.1089/neu.1996.13.293. [DOI] [PubMed] [Google Scholar]
- SCHMUED LC, ALBERTSON C, SLIKKER WJ. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- SMITH DC, NEESE SL, MODGLIN AA, et al. Continuous, intermittent electrical stimulation of the vagus nerve enhances cognitive and motor recovery following fluid percussion injury in the rat when initiated both 2 and 24 hrs post-injury; Washington, D.C.. 2005. Presented at the National Neurotrauma Society Symposium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH DH, CHEN XH, PIERCE JE, et al. Progressive atrophy and neuron death for one year following brain trauma in the rat. J Neurotrauma. 1997;14:715–727. doi: 10.1089/neu.1997.14.715. [DOI] [PubMed] [Google Scholar]
- STATLER KD, JENKINS LW, DIXON CE, CLARK RS, MARION DW, KOCHANEK PM. The simple model versus the super model: translating experimental traumatic brain injury research to the bedside. J Neurotrauma. 2001;18:1195–1206. doi: 10.1089/089771501317095232. [DOI] [PubMed] [Google Scholar]
- SUTTON RL, HOVDA DA, FENNEY DM. Amphetamine accelerates recovery of locomotor function following bilateral frontal cortex ablation in cats. Behav Neurosci. 1989;103:837–841. doi: 10.1037//0735-7044.103.4.837. [DOI] [PubMed] [Google Scholar]
- SUTTON RL, HOVDA DA, SHEN MJ, FEENEY DM. Alleviation of brain injury-induced cerebral metabolic depression by amphetamine: a cytochrome oxidase histochemistry study. Neural Plast. 2000;7:109–125. doi: 10.1155/NP.2000.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTTON RL, WEAVER MS, FEENEY DM. Drug-induced modification of behavioral recovery following cortical trauma. J Head Trauma Rehabil. 1987;2:50–58. [Google Scholar]
- TAKIGAWA M, MOGENSON GJ. A study of inputs to antidromically identified neurons of the locus coeruleus. Brain Res. 1977;135:217–230. doi: 10.1016/0006-8993(77)91027-7. [DOI] [PubMed] [Google Scholar]
- THOMPSON HJ, LIFSHITZ J, MARKLUND N, et al. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- WALKER-BATSON D, SMITH P, CURTIS S, UNWIN H, GREENLEE R. Amphetamine paired with physical therapy accelerates motor recovery after stroke. Further evidence Stroke. 1995;26:2254–2259. doi: 10.1161/01.str.26.12.2254. [DOI] [PubMed] [Google Scholar]
- WHISHAW IQ, METZ GA. Absence of impairments or recovery mediated by the uncrossed pyramidal tract in the rat versus enduring deficits produced by the crossed pyramidal tract. Behav Brain Res. 2002;134:323–336. doi: 10.1016/s0166-4328(02)00051-7. [DOI] [PubMed] [Google Scholar]
- WHISHAW IQ, PIECHARKA DM, ZEEB F, STEIN DG. Unilateral frontal lobe contusion and forelimb function: chronic quantitative and qualitative impairments in reflexive and skilled forelimb movements in rats. J Neurotrauma. 2004;21:1584–1600. doi: 10.1089/neu.2004.21.1584. [DOI] [PubMed] [Google Scholar]
- WOODBURY DM, Woodbury JW. Effects of vagal stimulation on experimentally induces seizures in rats. Epilepsia 31, Suppl. 1990;2:S7–S19. doi: 10.1111/j.1528-1157.1990.tb05852.x. [DOI] [PubMed] [Google Scholar]


