Summary
Balanced control of neuronal activity is central in maintaining function and viability of neuronal circuits. The endocannabinoid system tightly controls neuronal excitability. Here, we show that endocannabinoids directly target hippocampal glutamatergic neurons to provide protection against acute epileptiform seizures in mice. Functional CB1 cannabinoid receptors are present on glutamatergic terminals of the hippocampal formation, colocalizing with vesicular glutamate transporter 1 (VGluT1). Conditional deletion of the CB1 gene either in cortical glutamatergic neurons or in forebrain GABAergic neurons, as well as virally induced deletion of the CB1 gene in the hippocampus, demonstrate that the presence of CB1 receptors in glutamatergic hippocampal neurons is both necessary and sufficient to provide substantial endogenous protection against kainic acid (KA)-induced seizures. The direct endocannabinoid-mediated control of hippocampal glutamatergic neurotransmission may constitute a promising therapeutic target for the treatment of disorders associated with excessive excitatory neuronal activity.
Introduction
The endocannabinoid system is an important neuromodulatory system involved in a plethora of physiological functions (De Petrocellis et al., 2004; Freund et al., 2003; Piomelli, 2003). It consists of cannabinoid receptors, their endogenous lipid ligands (endocannabinoids), and the enzymatic machinery for their synthesis, release, and degradation. To date, the only molecularly characterized cannabinoid receptor expressed in fore-brain neurons is the type 1 cannabinoid receptor, CB1 (Freund et al., 2003; Howlett et al., 2002), although pharmacological approaches suggest the presence of additional neuronal cannabinoid receptors that have yet to be fully characterized (Freund et al., 2003). Through activation of CB1 receptors, the endocannabinoid system exerts important functions in the central nervous system, such as retrograde inhibition of neurotransmitter release (Alger, 2002; Chevaleyre et al., 2006; Marsicano and Lutz, 2006), control of neuronal excitability (Marsicano et al., 2003), and regulation of various forms of long-term synaptic plasticity (Chevaleyre et al., 2006; Gerdeman and Lovinger, 2003; Marsicano and Lutz, 2006). In cortical areas, CB1 receptors are mainly expressed by specific subpopulations of GABAergic interneurons, where they are proposed to mediate both endocannabinoid-dependent physiological regulation of GABA transmission (Alger, 2002; Chevaleyre et al., 2006; Freund et al., 2003) and pharmacological effects of exogenous cannabinoids on GABA release (Freund et al., 2003). Indeed, immunohistochemical studies in cortical regions find CB1 receptor protein largely associated with GABAergic interneurons (Egertová and Elphick, 2000; Freund et al., 2003; Katona et al., 1999; Tsou et al., 1998; Tsou et al., 1999), although discrepant results have been reported (Domenici et al., 2006; Hoffman et al., 2005; Katona et al., 2006; Kawamura et al., 2006; Ong and Mackie, 1999; Pettit et al., 1998; Takahashi and Castillo, 2006). In fact, evidence was previously reported for the presence of CB1 receptors in other neuronal subtypes too, including glutamatergic principal neurons. For instance, hippocampal principal neurons express significant levels of CB1 mRNA, though at lower levels than GABAergic interneurons (Marsicano and Lutz, 1999; Matsuda et al., 1993). Given the general ability of CB1 receptors to reduce neuronal excitability (Freund et al., 2003; Howlett et al., 2002; Lutz, 2004; Piomelli, 2003) and the increased kainic acid (KA)-induced excitation in hippocampal pyramidal neurons in CB1 mutant mice, a direct control of glutamatergic transmission in the hippocampus has been proposed to mediate the endocannabinoid-dependent on-demand protection against excitotoxicity (Marsicano et al., 2003). However, conclusive evidence for a physiological or pathophysiological function of CB1 receptors on hippocampal glutamatergic neurons has been elusive.
Excessive activation of glutamatergic transmission is considered as a key pathogenic event leading to epileptiform seizures, and glutamatergic circuits in the hippocampal formation are particularly prone to excessive pathological activity (Ben-Ari and Cossart, 2000). Understanding the mechanisms regulating excitatory neurotransmission is mandatory to elucidate the pathophysiology of important neurological disorders, such as epilepsy and neurodegenerative diseases, and to generate novel therapeutic concepts for their treatment. Given the central neuroprotective function of the endocannabinoid system in several models of neuronal insults (Panikashvili et al., 2001; van der Stelt et al., 2002; Lutz, 2004) and the proposed therapeutic exploitation of this system for the treatment of several diseases (Di Marzo et al., 2004; Mackie, 2006), it is of central importance to determine in detail which neuronal subtypes are controlled by the endogenous activation of CB1 receptors. Conditional mutagenesis using the Cre/loxP system represents an optimal tool to dissect the specific involvement of different neuronal populations in living animals (Branda and Dymecki, 2004; Morozov et al., 2003). In this study, we introduce novel conditional mutants lacking the CB1 receptor in specific neuronal populations and use the KA model of seizures (Ben-Ari and Cossart, 2000) to provide functional and anatomical evidence that CB1 receptors on hippocampal glutamatergic neurons are necessary for the CB1-dependent protection against acute excitotoxic seizures.
Results
KA-Induced Seizures in Conditional CB1 Mutants
As compared to wild-type mice (Figures 1A–1D), conditional mutant mice lacking CB1 expression from fore-brain principal neurons express the receptor only in cortical GABAergic interneurons and in nonforebrain regions (CB1f/f;CaMKIIα-Cre mice, here called CaMK-CB1−/− [CB1−/− in cells expressing the calcium/cal-modulin-dependent kinase IIα, CaMKIIα]; Marsicano et al., 2003; Figures 1E–1H) and show stronger seizures following KA treatment as compared to wild-type (CB1f/f) littermates (F1,24 = 13.5, p = 0.0012; Figure 2D). These data confirm our previous results (Marsicano et al., 2003) and suggest that glutamatergic cortical neurons are the main target of CB1-dependent protection against acute excitotoxic seizures. However, CaMK-CB1−/− mice present a deletion of the CB1 gene not only in cortical glutamatergic neurons, but also in many neurons of subcortical and diencephalic regions, including striatum, thalamus, and hypothalamus (Figures 1E and 1F), which might be involved in KA-induced epileptiform seizures (Aparicio et al., 2004; Lee et al., 2002; Nomura et al., 2000). Therefore, to obtain a more selective deletion of CB1 receptors in glutamatergic cortical neurons, we crossed CB1f/f with NEX-Cre mice (Kleppisch et al., 2003; Wu et al., 2005; see Supplemental Data available with this article online; Figure S1), to obtain the double mutants CB1f/f;NEX-Cre. In these mice (hereafter called Glu-CB1−/−), CB1 expression is absent in the majority of cortical glutamatergic neurons, including hippocampus, neocortex, and amygdala, whereas GABAergic interneurons and other subcortical neurons express normal levels of the receptor (Figures 1I–1L). Double in situ hybridization experiments, combining visualization and quantification of CB1 and glutamic acid decarboxylase 65 kDa (GAD65, a marker of GABAergic neurons) mRNA expression in Glu-CB1−/− mice, revealed that more than 99% of CB1-positive cells coexpress GAD65 mRNA in the whole hippocampal formation, indicating a loss of expression in adjacent glutamatergic neurons (Figures 2A and 2B). Furthermore, 39.2% ± 3.8% of GAD65-positive cells express CB1 mRNA, similarly to wild-type (CB1f/f, 38.7% ± 6.9%; see also Marsicano and Lutz [1999]), suggesting that CB1 expression in GABAergic neurons is unaffected in Glu-CB1−/− mice. Treatment of Glu-CB1−/− mice with 30 mg/kg of KA induced stronger seizures as compared to CB1f/f littermates, thus revealing the essential role of cortical glutamatergic principal neurons in CB1-dependent protection against KA-induced seizures (F1,29 = 24.1, p < 0.001; Figure 2E). To definitely exclude the involvement of GABAergic neurons in this function of the endocannabinoid system, we generated an additional conditional CB1 mutant mouse line, in which CB1 expression is abolished in GABAergic neurons. CB1f/f mice were crossed with Dlx5/6-Cre mice (Figures 1M–1P and S2 and S3; Supplemental Data). CB1f/f;Dlx5/6-Cre mice (hereafter called GABA-CB1−/−) lack expression of CB1 in virtually all the GABAergic neurons of the fore-brain (Figures 1M–1P). In particular, in the hippocampal formation, more than 99% of GAD65-positive cells lack coexpression with CB1 mRNA (Figure 2C). Interestingly, treatment of these mice with 30 mg/kg KA induced similar levels of seizures as in CB1f/f littermate controls (F1,18 = 0.1, p = 0.76; Figure 2F).
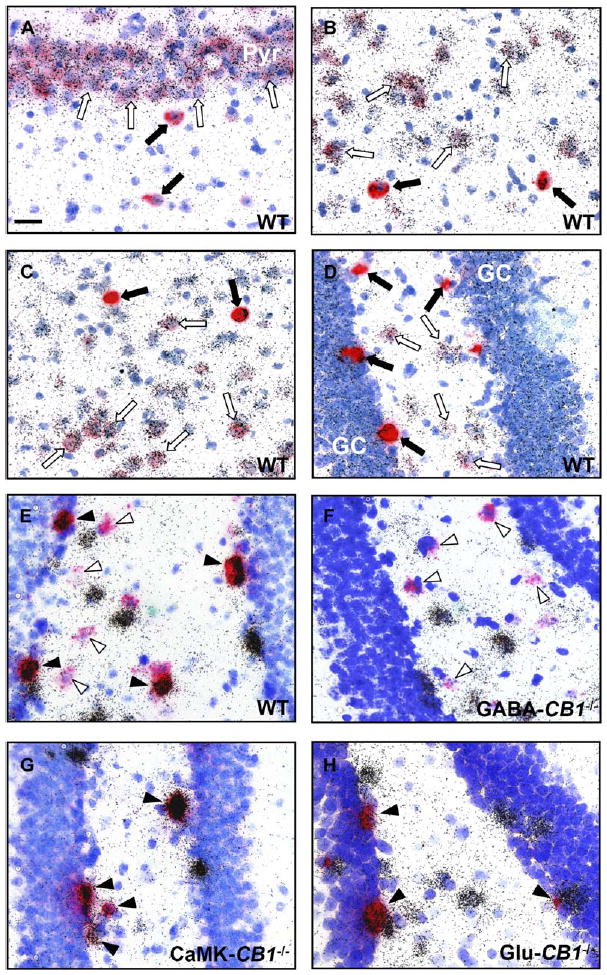
Figure 1.
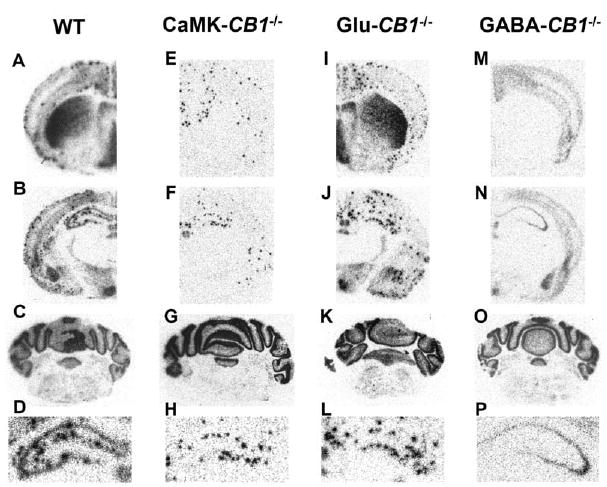
Specific Deletion of CB1 in Different Neuronal Subpopulations in Conditional CB1 Mutant Mice as Revealed by In Situ Hybridization with a Specific Riboprobe for CB1 mRNA
Micrographs showing CB1 mRNA expression in the brain of CB1f/f (wt, [A]–[D]), CaMK-CB1−/− (E–H), Glu-CB1−/− (I–L), and GABA-CB1−/− mice (M–P). Sections were stained throughout the brain and micrographs were taken at the level of caudate putamen (A, E, I, and M), dorsal hippocampus (B, F, J, and N) and cerebellum (C, G, K, and O).
(D, H, L, and P) Detailed enlargements of the dorsal hippocampus of (B), (F), (J), and (N), respectively. In all three mutant lines, deletion of CB1 is mainly restricted to the forebrain, with little or no alterations in the hindbrain (C, G, K, and O). In CaMK-CB1−/− mice, CB1 mRNA is absent from all principal neurons and expressed only in GABAergic interneurons (intense scattered dots; E, F, and H). In Glu-CB1−/− mice, CB1 mRNA is absent in the majority of cortical glutamatergic neurons (I, J, and L). In GABA-CB1−/− mice, CB1 mRNA is absent in all GABAergic neurons and it is expressed only in non-GABAergic cells (low uniform gray staining; M, N, and P).
Figure 2.
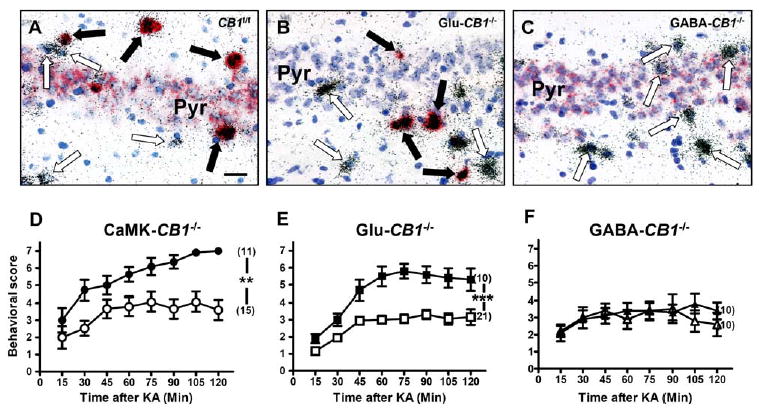
Deletion of CB1 in Glutamatergic Cortical Neurons, but Not in GABAergic Neurons, Increases Susceptibility to KA-Induced Seizures
(A–C) Micrographs showing double in situ hybridization of CB1 mRNA (red staining) together with GAD65 mRNA (silver grains) in the CA3 region of CB1f/f (A), Glu-CB1−/− (B), and GABA-CB1−/− mice (C). In (A), CB1 is present in both GABAergic interneurons and pyramidal neurons. In (B), CB1 is present only in GABAergic interneurons. In (C), CB1 is present only in pyramidal neurons. Blue staining, toluidine blue nuclear counter-staining. Filled arrows, GABAergic interneurons (GAD65-positive) expressing CB1 mRNA. Open arrows, GABAergic interneurons lacking CB1 expression. Bar, 25 μm. For similar analysis of CB1 expression in CaMK-CB1−/− mice, see Figure 2 of Marsicano et al. (2003).
(D–F) Seizure scoring (30 mg/kg KA) of conditional CB1 mutant mice (filled symbols), as compared to control CB1f/f littermates (open symbols). Seizures are worsened in CaMK-CB1−/− (D) and in Glu-CB1−/− (E), whereas they do not differ from wild-type controls in GABA-CB1−/− mice (F). In brackets, number of animals for each experimental group. Data are presented as mean ± SEM. **p < 0.005; ***p < 0.001 (by two-way ANOVA, factor genotype).
These results clearly show that expression of CB1 receptors in GABAergic cells does not play a significant role in the physiological protection against acute KA-induced seizures, whereas cortical glutamatergic neurons mediate the protective functions of the endocannabinoid system.
CB1 Receptors Directly Control GABAergic Transmission but Are Dispensable for Pharmacological GABA-Mediated Protection against KA-Induced Seizures
As CB1 receptors are well known to control GABAergic transmission (Chevaleyre et al., 2006; Alger, 2002; Freund et al., 2003), the absence of a phenotype in GABA-CB1−/− mice is a surprising result. Therefore, we performed experiments aimed at confirming (1) the absence of CB1-mediated effects on GABAergic transmission in GABA-CB1−/−mice and (2) the independence from CB1 receptors of GABA-mediated protection against KA-induced seizures, as induced by the allosteric GABA-A receptor enhancer diazepam (Möhler et al., 2002).
To this end, we first tested the CB1-mediated retrograde regulation of GABAergic transmission (Wilson et al., 2001) in hippocampal slices derived from GABA-CB1−/− and CaMK-CB1−/− mice and the respective CB1f/f controls. Pharmacologically isolated GABAergic currents were recorded in CA1 hippocampal pyramidal neurons. After a 5 s postsynaptic depolarization step, a reliable short-term decrease of inhibitory currents (depolarization-induced suppression of inhibition, DSI; Wilson et al., 2001) was observed in the two CB1f/f control groups (Figures 3A–3D). As expected, in CaMK-CB1−/− slices, DSI was undistinguishable from CB1f/f controls (Figures 3A and 3B). However, DSI was abolished in GABA-CB1−/− mice (Figures 3C and 3D). Therefore, retrograde CB1-dependent short-term control of GABAergic transmission in the hippocampus entirely relies on the expression of CB1 on GABAergic interneurons and, importantly, these data demonstrate that this function is abolished in GABA-CB1−/− mice.
Figure 3.
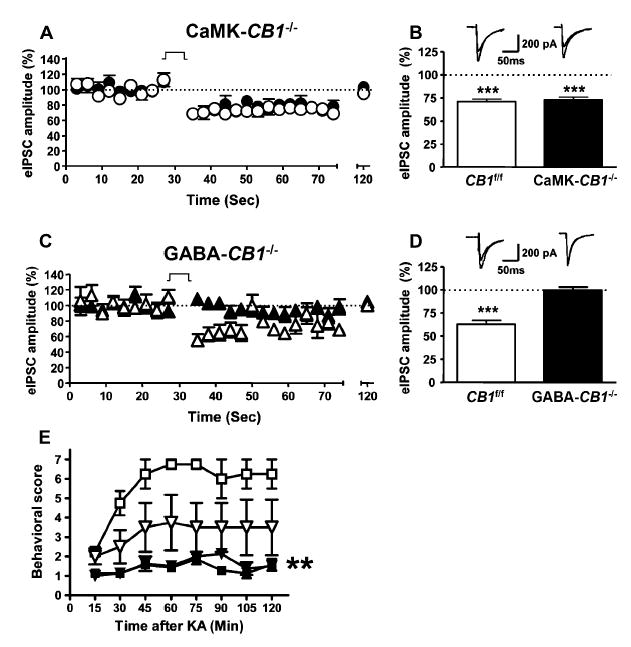
Functions of CB1 Receptors in the Control of GABAergic Transmission in Conditional CB1 Mutant Mice and in the Development of KA-Induced Seizures
(A) Depolarization-induced suppression of inhibitory currents (DSI) in CA1 hippocampal pyramidal neurons of CaMK-CB1−/− mice (filled circles, n = 11 cells from six mice) and of respective CB1f/f littermate controls (open circles, n = 13 cells from six mice). The 5 s depolarization step is indicated (see Experimental Procedures). Data are mean ± SEM.
(B) Summary of DSI data obtained by calculating the mean of the five eIPSCs before and after the depolarization step. Data are mean ± SEM. Top, representative traces before and after depolarization step for each genotype, respectively. Data are mean ± SEM. ***p < 0.001 versus respective baseline before depolarization (100%, dotted line).
(C) DSI in CA1 hippocampal pyramidal neurons of GABA-CB1−/− (filled triangles, n = 13 cells from six mice) and of respective CB1f/f littermate controls (open triangles, n = 10 cells from six mice). Data are mean ± SEM. The 5 s depolarization step is indicated.
(D) Summary of DSI data obtained by calculating the mean of the five eIPSCs before and after the depolarization step. Top, representative traces before and after depolarization step for each genotype, respectively. Data are mean ± SEM. ***p < 0.001 versus respective baseline before depolarization (100%, dotted line).
(E) Effects of vehicle (open symbols) and of the allosteric modulator of GABA-A receptor diazepam (6 mg/kg, filled symbols) on KA-induced seizures in CB1+/+ (triangles) and on CB1−/− littermates (squares). Data are mean ± SEM. **p < 0.01 as compared to respective vehicle-treated groups (by two-way ANOVA, factor treatment).
We next asked whether the KA-induced increase in inhibitory GABAergic transmission in the hippocampus (Ben-Ari and Cossart, 2000) is influenced by the endocannabinoid system. Benzodiazepines are allosteric modulators of GABA-A receptors that act as potent anticonvulsants by enhancing the inhibitory effects of GABAergic transmission (Möhler et al., 2002). If the endocannabinoid system modulates release of GABA during KA-induced seizures, the anticonvulsant effects of the prototypical benzodiazepine diazepam should be altered in mutant mice lacking CB1 expression in all cells (Marsicano et al., 2002). In accordance with our previous findings (Marsicano et al., 2003), CB1−/− mice were highly susceptible to KA-induced seizures as compared to control wild-type littermates (CB1+/+; Figure 3E). However, the injection of 6 mg/kg diazepam protected both CB1+/+ and CB1−/− littermates to the same extent against KA-induced seizures (Figure 3E). Pretreatment with diazepam might cause a general strong increase of basal GABAergic transmission, which might mask the CB1-dependent influence during KA-induced seizures. To rule out this possibility, we treated CB1+/+ and CB1−/− mice with 6 mg/kg diazepam after the occurrence of first seizures (behavioral score 4). In these conditions too, diazepam protected both genotypes to similar extent and reduced mortality of CB1−/− mice to levels indistinguishable from CB1+/+ controls (Figure S4).
Altogether, these results confirm that physiological endocannabinoid-dependent control of GABAergic transmission depends on intact CB1 signaling in GABAergic interneurons and suggest that the endocannabinoid system does not influence GABAergic transmission during the development of KA-induced seizures. Therefore, direct modulation of glutamatergic transmission by CB1 receptors expressed on cortical glutamatergic neurons appears to be the major mechanism of endocannabinoid-mediated protection against KA-induced seizures.
The Inner Molecular Layer of Dentate Gyrus Contains CB1 Receptor Protein
In order to investigate the location of CB1 receptors involved in the protection against KA-induced seizures, we performed a detailed analysis of CB1 expression in the hippocampal formation of GABA-CB1−/−, CaMK-CB1−/−, and CB1f/f controls (where CB1 expression is not different from wild-type C57BL/6N mice, see Experimental Procedures). In CB1f/f mice, immunohistochemistry for CB1 protein revealed the well-described wild-type expression pattern, with intense staining of GABAergic terminals mainly belonging to basket cells and lack of CB1 expression in pyramidal and granule cell bodies (Egertová and Elphick, 2000; Freund et al., 2003; Katona et al., 1999; Tsou et al., 1998; Tsou et al., 1999; Figures 4A–4C). In conditional CaMK-CB1−/− hippocampi, CB1 protein expression is still present in GABAergic terminals surrounding pyramidal and granule cell bodies (Figures 4D–4F). However, clear differences emerge in the pattern of CB1 expression in CaMK-CB1−/− mice as compared to wild-type. In the conditional mutant mice, the staining appears much less uniform and much more localized to punctae and fibers (Figures 4D–4F). The intensity of the signal is also lower as compared to wild-type, particularly in extrapyramidal CA1 and CA3 regions and extragranular dentate layers, such as the strata molecularis (Figures 4D–4F), radiatum, and oriens (Figures 4D and 4F). In the hippocampal formation of GABA-CB1−/− mice, the overall CB1 staining is strongly decreased as compared to wild-type. In particular, the intense network of CB1 staining, corresponding to interneuron basket cell terminals (Egertová and Elphick, 2000; Freund et al., 2003; Katona et al., 1999; Tsou et al., 1998; Tsou et al., 1999), surrounding pyramidal and granule cells is absent (Figures 4G–4I). However, residual CB1 labeling is present in the hippocampal formation of GABA-CB1−/− mice (for comparison, see staining of complete CB1 knockout mice, Figures 4J–4L). The staining appears much more diffuse and less localized to easily identifiable fibers than in wild-type and CaMK-CB1−/− hippocampi. Weak CB1 labeling is present in the strata molecularis (Figures 4G and 4H), radiatum, and oriens of the CA1 and CA3 region of GABA-CB1−/− mice (Figures 4G and 4I). Interestingly, these areas are the same in which a decreased CB1 expression is observed in CaMK-CB1−/− mice, suggesting that the complementary CB1 expression in these two mutant lines observed at mRNA level (Figure 1), is also present at protein level. The strongest CB1 expression in the hippocampal formation of GABA-CB1−/−mice is present in the inner third of the molecular layer of dentate gyrus, represented by diffuse immuno-labeling throughout the whole subregion (Figures 4G and 4H). Interestingly, the expression of CB1 in this subregion of CaMK-CB1−/− mice (Figure 4E) is not only strongly reduced as compared to wild-type (Figure 4B), but is also different in terms of staining pattern. By careful observation, one can notice that the “sum” of GABA-CB1−/− (low and diffuse; Figure 4H) and CaMK-CB1−/− patterns (high and punctate; Figure 4E) would approximate wild-type staining, where both these features are present (Figure 4B). Semiquantitative analysis of immunostaining in the inner molecular layer revealed that the intensity of staining in the inner molecular layer reached 150.4 ± 5.3 relative intensity units in the wild-type hippocampus and was reduced to 85.0 ± 1.5 and to 52.2 ± 1.0 in CaMK-CB1−/− and in GABA-CB1−/−, respectively. Therefore, in the inner third of the molecular layer, mice lacking CB1 on GABAergic neurons (GABA-CB1−/−mice) still retain CB1 protein expression, strongly suggesting that CB1 receptors are expressed on glutamatergic terminals in this area.
Figure 4.
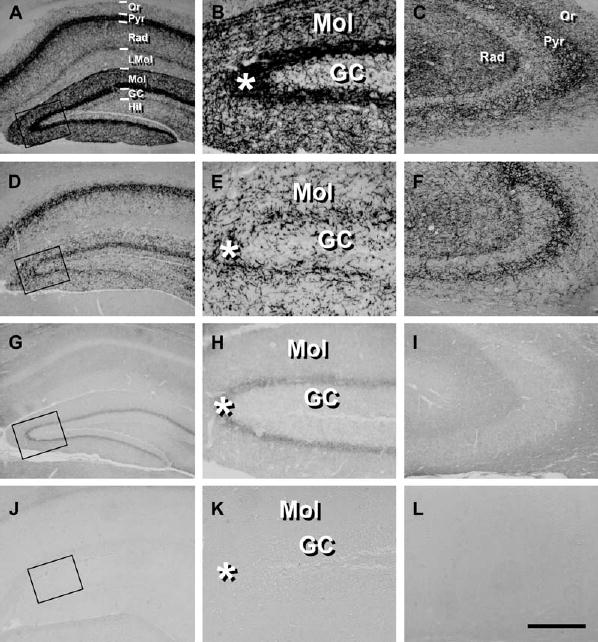
CB1 Protein Is Present in Glutamatergic Hippocampal Neurons
Micrographs showing the immunohistochemical analysis of CB1 expression in wild-type (A–C), CaMK-CB1−/− (D–F), GABA-CB1−/− (G–I) and complete CB1 knock-out mice (J–L).
(B, E, H, and K) Higher magnification micrographs of the areas enclosed in the square in (A), (D), (G), and (J), respectively.
(C, F, I, and L) Detail of the CA3 hippocampal region.
GC, granule cell layer of dentate gyrus; Hil, hilar region of dentate gyrus; LMol, stratum lacunosum-molecularis; Mol, stratum molecularis; Or, stratum oriens; Pyr, CA1/CA3 pyramidal cell layer of hippocampus; Rad, stratum radiatum. Asterisks indicate the inner third of the molecular layer. Bar, 100 μm (A, C, D, F, G, I, J, and L); 25 μm (B, E, H, and K).
Dentate Gyrus Hilar Mossy Cells Express Functional CB1 Receptors
The inner third of the dentate gyrus molecular layer consists of sparse interneurons and of the initial part of the dendritic arbor of the granule cells of dentate gyrus (Johnston and Amaral, 2004). Granule cells receive glutamatergic inputs primarily from perforant path fibers originating in the entorhinal cortex, from a portion of glutamatergic neurons of the CA3 region (Li et al., 1994) and from mossy cells, which represent the great majority of non-GABAergic neurons in the hilus of dentate gyrus (Johnston and Amaral, 2004). These projections to granule cell dendrites are spatially segregated, with perforant path terminals mainly occupying the outer two-thirds of the molecular layer and mossy cells mainly contributing to the glutamatergic afferent fibers in the inner third of this region (Johnston and Amaral, 2004). Therefore, we hypothesized that the intense CB1 band in this subregion (Figures 4B, 4C, and 4H) might in part belong to mossy cell terminals or axons, which, therefore, should express CB1 mRNA in their cell bodies. To test this notion, double in situ hybridization experiments were performed in wild-type mice using a combination of riboprobes specific for CB1 and vesicular glutamate transporter 1 (VGluT1) mRNA. VGluT1 is a protein that participates in the recycling of glutamate into synaptic vesicles of glutamatergic neurons, and it currently represents the best available marker for cortical glutamatergic neurons (Fremeau et al., 2004). Double in situ hybridization staining of wild-type mouse brains revealed a very high degree of coexpression of CB1 and VGluT1 in all cortical regions, as exemplified in CA3 hippocampal pyramidal neurons, basolateral amygdala, and entorhinal cortex (Figures 5A–5C). VGluT1 mRNA is present in virtually all neurons expressing low levels of CB1 mRNA, which are known as non-GAD65-expressing putative glutamatergic cells (Marsicano and Lutz, 1999). Detailed analysis of the hilus of dentate gyrus, where cell bodies of mossy cells are located, also revealed high degree of coexpression in glutamatergic mossy cells (Figure 5D). The great majority (92.0% ± 7.3%) of VGluT1-positive mossy cells expresses low levels of CB1 mRNA and 96.6% ± 3.2% of low CB1-expressing cells contain VGluT1 mRNA, suggesting that much of the CB1 protein observed in the inner third of the molecular layer is on mossy cell terminals. Indeed, glutamatergic mossy cells express CB1 mRNA in wild-type and GABA-CB1−/− mice (Figures 5E and 5F), whereas all CB1-expressing neurons in the hilus of CaMK-CB1−/− and Glu-CB1−/− mice coexpress GAD65 (Figures 5G and 5H), showing loss of CB1 expression in glutamatergic mossy cells in these two mouse lines.
Figure 5.
CB1 mRNA Is Expressed in Glutamatergic Cortical Neurons and in Glutamatergic Mossy Cells of the Dentate Gyrus, as Revealed by Double In Situ Hybridization Experiments in Wild-Type C57BL/6N Mice and Conditional Mutants
(A–D) Micrographs showing coexpression of CB1 mRNA (red staining) with VGluT1 mRNA (silver grains) in the CA3 region of hippocampus (A), basolateral amygdala (B), entorhinal cortex (C), and hilus of dentate gyrus (D) of wild-type C57BL/6N adult mice. Filled arrows, GABAergic interneurons, expressing CB1, but not VGluT1. Open arrows, glutamatergic neurons, coexpressing CB1 and VGluT1.
(E–H) Micrographs showing coexpression of CB1 mRNA (red staining) with GAD65 mRNA (silver grains), in the hilus of dentate gyrus in CB1f/f (E), GABA-CB1−/− (F), CaMK-CB1−/− (G), and Glu-CB1−/− mice (H). Filled arrowheads, GABAergic interneurons, coexpressing CB1 and GAD65 mRNAs. Open arrowheads, presumable glutamatergic neurons, not coexpressing CB1 and GAD65 mRNAs.
Note that high CB1-expressing cells do not coexpress VGluT1 mRNA (A–D), but do coexpress GAD65 mRNA (E–H), showing that they are GABAergic interneurons. Conversely, low CB1-expressing cells coexpress VGluT1 mRNA (A–D), but do not contain GAD65 mRNA (E–H), showing that they are glutamatergic neurons. In particular, glutamatergic hilar mossy cells contain CB1 mRNA in wild-type (D), CB1f/f mice (E), and GABA-CB1−/− (F), but not in CaMK-CB1−/− (G) and Glu-CB1−/− mice (H). Blue staining, toluidine blue nuclear counterstaining. GC, granule cells of dentate gyrus. Pyr, CA3 pyramidal neurons. Bar, 25 μm.
To analyze the subcellular localization of CB1 protein in mossy cells, we performed double-label immunohistochemistry using specific antibodies against CB1 and VGluT1. The expression of VGluT1 in the hippocampal formation was similar as previously described (Kaneko et al., 2002), with strong labeling of all hippocampal layers, excluding pyramidal and granule cell bodies (Figure 6A). Confocal analysis of double CB1/VGluT1 staining in the inner third of the molecular layer revealed a very high degree of coexpression of the two proteins (Figures 6B–6D). 62%±2% ofterminals inthisregioncontain CB1 protein, whereas 94% ±1% of visible terminals are VGluT1 positive. Consistently, coexpression of CB1 and VGluT1 was observed in 91% ± 1% of CB1-positive terminals and in 59% ± 2% of VGluT1-positive ones.
Figure 6.
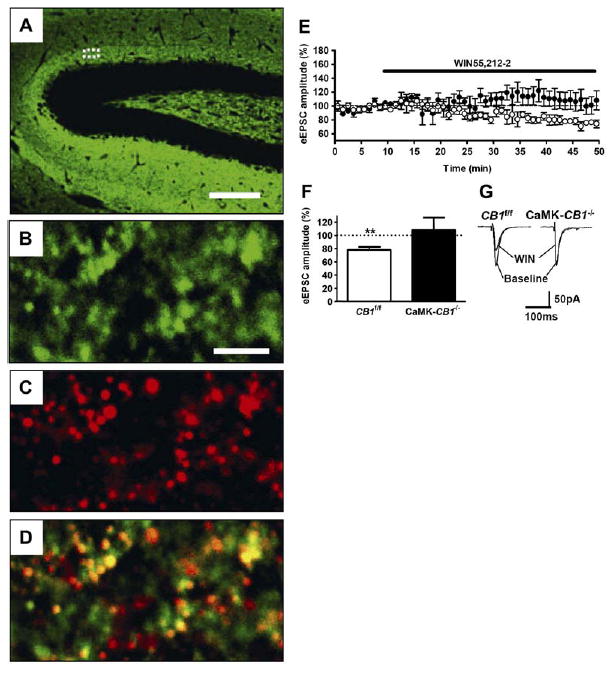
Glutamatergic Terminals of Mossy Cells Contain Functional CB1 Receptors
(A) Low-magnification micrograph showing expression of VGluT1 protein in the hippocampal formation.
(B–D) High-magnification confocal micrographs showing detailed expression of CB1 and VGluT1 protein in the inner third of the molecular layer (approximately corresponding to the dotted area in [A]). Expression of VGluT1 (green, [B]), CB1 (red, [C]), and merged image (D) are shown. Yellow areas indicate colocalization of the two proteins.
(E–G) Activation of CB1 receptors reduces glutamatergic inputs onto dentate gyrus granule cells.
(E) Time-course analysis of the effects of 5 μM of the CB1 agonist WIN55,212-2 on eEPSCs in wild-type (CB1f/f, open circles, n = 6 slices from three mice) and CaMK-CB1−/− littermates (filled circles, n = 6 cells from three mice).
(F) Summary of eEPSCs data obtained by calculating the average of the measurements obtained during the last 5 min of experiment (35–40 min after drug application).
(G) Representative electrophysiological traces before (baseline) and 40 min after application of WIN 55,212-2 (WIN).
Bars, 75 μm (A), 3.5 μm (B–D); **p < 0.01 as compared to baseline (average of the measurements during the last 5 min before drug application, dotted line).
To test for the functionality of these CB1 receptors, evoked glutamatergic currents in granule cells of the dentate gyrus in hippocampal slices were recorded by whole-cell patch-clamp. To generate evoked excitatory postsynaptic currents (eEPSCs), the stimulation electrode was placed next to the recorded granule cell in the inner molecular layer. Bath application of the CB1 agonist WIN55,212-2 to slices prepared from wild-type mice (CB1f/f) induced a progressive decrease in eEPSC amplitude, which reached 77.9% ± 4.5% of baseline 40 min after drug application (p < 0.01 as compared to baseline; Figures 6E–6G). Conversely, bath application of WIN55,212-2 to slices prepared from CaMK-CB1−/− mice did not significantly affect granule cell eEPSC amplitude (108.4% ± 18.4% of baseline, p = 0.66; Figures 6E–6G).
Altogether, these data show that functional CB1 protein is abundantly present on glutamatergic terminals in the inner third of the molecular layer of dentate gyrus, which represent a very well-suited locus to exert tight regulation of hippocampal excitability.
Virally Induced Deletion of CB1 Gene in the Hippocampus and KA-Induced Seizures
The residual expression of CB1 in glutamatergic hippocampal terminals in GABA-CB1−/− mice might account for the normal protection against KA-induced seizures in this mutant mouse line. However, in GABA-CB1−/− mice, CB1 is still expressed in other cortical and subcortical neurons (Figures 1M and 1N), leaving open the possibility that glutamatergic hippocampal neurons play only a secondary role in this function of the endocannabinoid system. To rule out this possibility, we injected an adeno-associated virus expressing Cre-recombinase (AAV-Cre) or green fluorescent protein (AAV-GFP), as a negative control, bilaterally into the hilus of dentate gyrus of CB1f/f mice. Injection of AAV-GFP did not induce any change in the expression of CB1 mRNA (Figure 7A), whereas AAV-Cre induced a complete deletion of CB1 gene in the hilus of dentate gyrus and in part of the CA3 and CA1 regions of the hippocampus (Figures 7B and 7C). Importantly, expression of the receptor in adjacent regions, such as the neocortex, or the lateral habenula was unaffected (Figures 7A and 7B).
Figure 7.
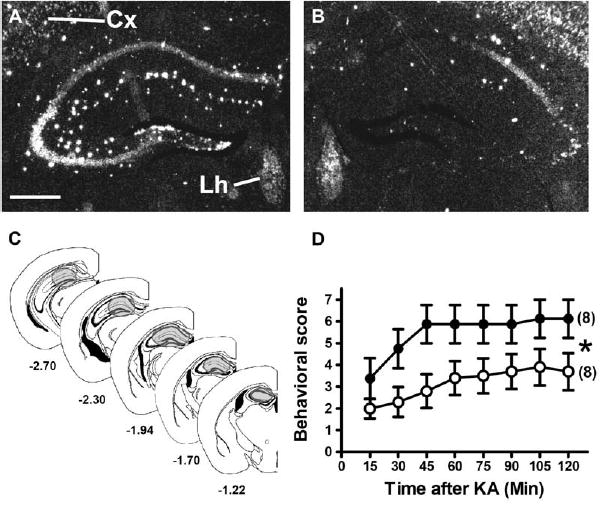
Virally Induced Deletion of CB1 Gene in the Hippocampal Formation Increases the Sensitivity to KA-Induced Seizures
(A and B) Dark-field micrographs showing CB1 mRNA expression in CB1f/f mice 9 weeks after intrahilar injections of AAV-GFP (A) and of AAV-Cre (B), showing the Cre-mediated deletion of CB1 in the hilus of the dentate gyrus and in part of the CA1 and CA3 hippocampal regions. Cx, neocortex; Lh, lateral habenula; bar, 500 μm.
(C) Schematic diagrams showing the approximate extension of Cre-mediated recombination of CB1 (gray shading) in AAV-Cre-injected CB1f/f mice (see Experimental Procedures). Numbers, distance from bregma (Paxinos and Franklin, 2001).
(D) Seizure scoring (30 mg/kg of KA) of AAV-GFP-injected (open circles) and AAV-Cre-injected CB1f/f mice (filled circles). In brackets, number of mice in each experimental group. Data are presented as mean ± SEM. *p < 0.05, by two-way ANOVA.
CB1f/f mice injected with either virus were treated with 30 mg/kg KA, and seizures were monitored. AAV-GFP-injected mice showed levels of seizures very similar to wild-type mice (Figure 7D, see also Figures 2D–2F), whereas hippocampal injection of AAV-Cre induced a strong worsening of seizures (F1,16 = 4.6; p < 0.05; Figure 7D). Importantly, we previously showed that expression of Cre-recombinase in principal forebrain neurons per se does not alter susceptibility to KA-induced seizures (Marsicano et al., 2003). Altogether, these results indicate that local deletion of CB1 in the hippocampal formation strongly reduces on-demand protection against KA-induced acute seizures.
Discussion
Normal neuronal computation in the brain requires intense ongoing excitatory transmission. However, excessive excitatory activity results in neuronal damage and death, through a mechanism known as excitotoxicity (Ben-Ari and Cossart, 2000). The spreading of excessive uncontrolled glutamatergic neurotransmission eventually leads to sustained, paroxysmal network activity culminating in behaviorally observable symptoms, such as seizures. Similar mechanisms might underlie important human neurological diseases, including various forms of epilepsy (Ben-Ari and Cossart, 2000). Therefore, neuronal circuits need very efficient control systems to limit the danger of excessive excitatory activity. The endocannabinoid system represents one of these systems. Indeed, KA-induced seizures are accompanied by a rapid increase of production of the endocannabinoid anandamide in the hippocampus and activation of CB1 cannabinoid receptors, necessary for the protection against acute excitotoxicity and for the induction of protective intracellular signaling cascades (Marsicano et al., 2003). However, the nature of the neuronal subpopulations under the functional control of the endocannabinoid system has been subject of considerable debate (Freund et al., 2003; Hájos et al., 2001; Katona et al., 1999; but see Domenici et al., 2006; Katona et al., 2006; Kawamura et al., 2006; Marsicano and Lutz, 1999; Matsuda et al., 1993; Takahashi and Castillo, 2006). In the present study, we used a systematic genetic approach aimed at dissecting the roles of different neuronal subpopulations in the protective functions of the endocannabinoid system against KA-induced seizures.
Our results clearly show that hippocampal glutamatergic neurons, where CB1 receptors are present at low but detectable levels, are the central mediators of on-demand endocannabinoid-dependent protection against KA-induced acute excitotoxic seizures. Furthermore, although the presence of CB1 receptors on GA-BAergic neurons is necessary for endocannabinoid-dependent forms of short-term plasticity of inhibitory transmission (DSI), the abundant expression of CB1 on GABAergic interneurons does not play a role in the endocannabinoid-dependent protection against KA-induced seizures.
The pharmacological protection given by the GABA-A allosteric enhancer diazepam does not seem to be influenced by CB1 receptors. This further suggests that GABAergic transmission during acute KA-induced seizures is independent from CB1 receptor-mediated control. However, given the only partial inhibition of GABA release by CB1 receptor activation, the possibility that the strong enhancement of postsynaptic GABA signaling by diazepam could compensate for the CB1 receptor-mediated reduction of GABA release cannot be ruled out at this point.
Dentate gyrus mossy cells and hippocampal pyramidal neurons express CB1 mRNA and functional CB1 protein is present in the inner molecular layer of dentate gyrus, associated with glutamatergic terminals. The presence of CB1 protein in this area in GABA-CB1−/− mice suggests that this location might be an important target for on-demand endocannabinoid-mediated protection against KA-induced seizures. Mossy cells are centrally involved in epileptiform seizure development (Ratzliff et al., 2002). These glutamatergic neurons are situated in the hilar portion of the dentate gyrus and receive intense glutamatergic inputs from dentate granule cells (Johnston and Amaral, 2004). In turn, they innervate ipsilateral and contralateral GABAergic interneurons and send back projections to a large number of granule cells along the longitudinal axis of the hippocampal formation (Johnston and Amaral, 2004; Ratzliff et al., 2002). This strategic position suggests that the glutamatergic loop between granule and mossy cells is centrally involved in the modulation of excitatory activity of hippocampal circuits and in the regulation of information influx into the hippocampal formation. Our finding that the inner molecular layer of the dentate gyrus, where mossy cell terminals synapse onto granule cells, contains functional CB1 receptors represents a novel step in understanding the progression of acute excitotoxic seizures and the development of epileptic states. However, it should be mentioned that the inner molecular layer of dentate gyrus receives glutamatergic inputs not only from mossy cells (Johnston and Amaral, 2004), but also from CA3 pyramidal neurons (Li et al., 1994). Both these neuronal types express CB1 mRNA (Figures 2A and 5A), indicating that the presence of CB1 protein in the inner molecular layer is carried by axonal terminals of both these glutamatergic hippocampal neuronal populations. Moreover, low but significant levels of CB1 protein are still present in extrapyramidal layers of the CA1 and CA3 hippocampal region of GABA-CB1−/− mice. Therefore, a parsimonious interpretation of the present data is that the endocannabinoid system strongly controls key glutamatergic circuitry in the hippocampal formation during KA-induced seizures, likely regulating both mossy cell and pyramidal neuron transmission.
The presence of CB1 receptors in cortical glutamatergic neurons has been vigorously debated in recent years, and the cannabinoid effects on glutamatergic transmission have been proposed to depend on novel non-CB1/non-CB2 cannabinoid receptors (Freund et al., 2003; Hájos et al., 2001; but see Domenici et al., 2006; Takahashi and Castillo, 2006). Our data clearly show that CB1 on hippocampal glutamatergic neurons plays a central role during acute KA-induced behavioral seizures, thus demonstrating the functional importance of this location of CB1 receptors.
Deletion of CB1 receptors in GABAergic neurons in GABA-CB1−/− mice, where endocannabinoid-mediated DSI in the hippocampus is abolished, does not alter the behavioral seizure response to KA. Considering that the endocannabinoid system controls a portion of GABA-ergic transmission in the hippocampus (Chevaleyre et al., 2006; Alger, 2002; Freund et al., 2003; Marsicano and Lutz, 2006; present results) and that GABAergic interneurons are intensely activated during KA-induced seizures (Ben-Ari and Cossart, 2000), this is a surprising result. The reasons of this lack of involvement of GABAergic interneurons in the CB1 receptor-dependent control of behavioral KA-induced seizures by the endocannabinoid system are not clear at the moment. However, a recent publication by Földy et al. (2006) showed that the CB1 receptor-dependent control of GABAergic transmission in the hippocampus is strictly regulated by the firing rate of the presynaptic GABAergic interneuron. CB1 agonist- and endocannabinoid-dependent reduction of GABAergic transmission is strongly reduced when the firing rate of the presynaptic interneuron is above a certain threshold. Kainic acid, mainly through the activation of GluR5-containing KA receptors, directly and strongly increases the firing rate of GABAergic inter-neurons (Ben-Ari and Cossart, 2000). Therefore, it is possible that, in the presence of seizure-inducing doses of KA, GABAergic interneurons fire at frequencies that are sufficient to free them from the control of the endocannabinoid system (Földy et al., 2006).
Altogether, these observations support a hypothetical scenario in which acute KA-induced excitotoxic seizures would activate the endocannabinoid system in respect to its ability to inhibit only “harmful” glutamatergic transmission, but not “protective” GABAergic release.
In conclusion, our study reveals a mechanism through which the endocannabinoid system is able to provide on-demand protection against acute behavioral seizures. CB1 expression on hippocampal glutamatergic circuits accounts for this protection and might represent a suitable target for the treatment of neurological disorders associated with excessive neuronal excitation.
Experimental Procedures
Animals
Male mice were used in all experiments, aged 2–5 months, maintained in standard conditions with food and water ad libitum. All experimental procedures were approved by the Committees on Animal Health and Care of local governments. C57BL/6NCrlBR mice were purchased from Charles River. CD1 mice (for colocalization of VGluT1 and CB1) were purchased from Jackson Laboratory. Conditional CB1 mutant mice were obtained by crossing the respective Cre-expressing mouse line with CB1f/f mice (Marsicano et al., 2003), using a three-step breeding protocol. CB1f/f;CaMKIIα-Cre mice (here called CaMK-CB1−/−) were obtained as described (Marsicano et al., 2003). CB1f/f;NEX-Cre mice (here called Glu-CB1−/−) were obtained by crossing CB1f/f with NEX-Cre mice (Kleppisch et al., 2003; Schwab et al., 2000; Wu et al., 2005). Genotyping was performed by PCR as described for CaMK-CB1−/− (Marsicano et al., 2003), for Glu-CB1−/− (Kleppisch et al., 2003), and for CB1f/f (Marsicano et al., 2003). CB1+/+ and CB1−/−mice were generated and genotyped as described (Marsicano et al., 2002). All lines were in a mixed genetic background, with a predominant C57BL/6NCrlBR contribution. All animals used in single experiments were littermates. Experimenters were always blind to genotype and/or treatment.
Generation of GABA-CB1−/− Mice
In order to express the Cre-recombinase with patterns that resemble that of the mouse Dlx5/Dlx6 genes, we placed the Cre gene under the control of the I56i and I56ii intergenic enhancer sequences (Zerucha et al., 2000). A 1.4 kb EcoRI-XhoI fragment of the zebrafish dlx5a/dlx6a locus, which recapitulates with a high degree of precision the endogenous Dlx5 expression patterns in transgenic mice (Zerucha et al., 2000), was placed into a plasmid containing the Cre coding sequence downstream of a 3.5 kb fragment from the immediate 5′-flanking region of zebrafish dlx6a, including part of the 5′UTR (Ghanem et al., 2003). The dlx6a upstream fragment does not, by itself, produce any tissue-specific expression in transgenic animals but was shown to increase the activity of the Dlx5/Dlx6 intergenic enhancers. Transgenic mice (Dlx5/6-Cre) were produced as previously described (Zerucha et al., 2000), and their spatial patterns of Cre activity were determined by mating them with mice carrying the Z/AP double reporter transgene (Lobe et al., 1999), Z/EG double reporter transgene (Novak et al., 2000), and Rosa26 reporter transgene (Soriano, 1999). Dlx5/6-Cre mice were crossed with CB1f/f mice to obtain CB1f/f;Dlx5/6-Cre (here called GABA-CB1−/−) and CB1f/f littermate controls.
Induction of Acute Excitotoxic Seizures
KA was purchased from Ocean Produce International. Seizures were induced by injecting 30 mg/kg of KA in 0.9% saline i.p. in a volume of 10 ml/kg body weight. Diazepam was purchased from Sigma, dissolved in saline, and injected s.c. at 20 ml/kg either 30 min before the injection of KA or immediately after the appearance of first observable seizures (score 4, see below). Seizures were monitored by a trained observer blind to the genotype or treatment of the mice for 2 hr and scored every 15 min according to the following scale (Marsicano et al., 2003; Schauwecker and Steward, 1997): 0—no response; 1—immobility and staring; 2—forelimb and/or tail extension, rigid posture; 3—repetitive movements, head bobbing; 4—rearing and falling; 5—continuous rearing and falling: 6—severe clonic-tonic seizures; 7—death.
Electrophysiology
For DSI experiments in the CA1 region (Wilson et al., 2001), sagittal hippocampal slices (300 μm thick) were obtained from P21-30 mice and maintained in artificial cerebrospinal fluid (ACSF) for at least 40 min prior to recording. The ACSF contained 125 mM NaCl, 1.25 mM NaH2PO4, 25 mM glucose, 2.5 mM KCl, 2.5 mM CaCl2, 2 mM MgCl2, 25 mM NaHCO3, 0.005 mM NBQX, 0.05 mM APV, gassed with carbogen (95% O2/5% CO2) at 25°C. Neurons were recorded in the whole-cell voltage-clamp mode (holding potential, −60 mV), using electrodes (open tip resistance, 2–5 MΩ) filled with a solution containing 100 mM CsCH3SO3, 60 mM CsCl, 10 mM HEPES, 0.2 mM EGTA, 1 mM MgCl2, 1 mM MgATP, 0.3 mM Na3GTP, 5 mM QX-314, (pH 7.3; 275 mOsm). Recordings were accepted only if the holding current was <100 pA. The current was low-pass filtered at 1 kHz and digitized at 3 kHz. Inhibitory postsynaptic currents (IPSCs) in CA1 pyramidal cells were evoked using a bipolar tungsten electrode placed within the CA1 stratum radiatum. DSI tests consisted of 30 stimuli at 0.33 Hz and a depolarization step to 0 mV for 5 s after the thirteenth stimulus. DSI was calculated using the mean of the five eIPSCs just before and after depolarization. For studying the effects of CB1 activation on mossy cell-granule cell synapses, sagittal hippocampal slices were obtained as described above. Granule cells of the dentate gyrus were recorded using the same extracellular and intracellular solutions as in DSI experiments, with the following differences: the intracellular solution did not contain QX-314 and the ACSF did not contain any glutamate receptor inhibitor but, in order to isolate evoked excitatory postsynaptic glutamatergic currents (eEPSCs), contained the GABAA and GABAB receptor antagonists bicuculline (10 μM) and CGP35348 (200 μM), respectively. Granule cells were recorded from the middle part of the dorsal “arm” of the dentate gyrus, and the electrode for stimulation (square pulse stimuli at 0.066 Hz, 5–20V, 100 μs) was positioned in their close vicinity (toward the septum) in the inner third of the molecular layer, where glutamatergic afferents to granule cell dendrites belong in majority to mossy cell terminals (Johnston and Amaral, 2004) and partially to CA3 terminals (Li et al., 1994). WIN55,212-2 (5 μM, purchased from Tocris) was dissolved in DMSO and directly added to the circulating ACSF at the indicated time points. The final concentration of DMSO was 0.01%, and preliminary experiments showed that this vehicle has no effect on eEPSCs. The reduction of eEPSCs was calculated by comparing the mean of the last five measurements before application of WIN55,212-2 (baseline) and the last five measurements 40 min after application of the drug. Evoked EPSCs were completely abolished by the application of the glutamate receptor antagonists NBQX (5 μM) and APV (50 μM; data not shown).
In Situ Hybridization
Single and double in situ hybridization were performed as described (Marsicano et al., 2003; Marsicano and Lutz, 1999), using riboprobes against mouse CB1 (Marsicano and Lutz, 1999), GAD65 (Marsicano and Lutz, 1999), and VGluT1 (kind gift of Prof. Edwards, San Francisco, California; Fremeau et al., 2004). CB1 mRNA expression in CB1f/f mice was not different from genetically wild-type C57BL/6NCrlBR mice (data not shown). Cell counting was performed as described in Marsicano and Lutz (1999). For quantification of GAD65-CB1 mRNA coexpression, a total of 3027 cells were counted in 22 hippocampal sections of CB1f/f and 2565 cells in 15 hippocampal sections of Glu-CB1−/− mice. For quantification of VGluT1-CB1 mRNA coexpression in the hilus of dentate gyrus, a total of 624 cells expressing low levels of CB1 mRNA (known as non-GABAergic neurons, see Marsicano and Lutz [1999]) and of 642 cells expressing VGluT1 mRNA were counted in 26 sections corresponding to the hilus of dentate gyrus.
Immunohistochemistry
Single immunohistochemistry for CB1 protein in different conditional mutants was performed as described (Egertová and Elphick, 2000). Semiquantitative evaluation of CB1 staining in the inner molecular layer of dentate gyrus was performed using the NIH Image software as described (Marsicano et al., 2003). Double immunohistochemistry for CB1 and VGluT1 was performed on 40 μm sections from adult mouse brains prepared as described (Wager-Miller et al., 2002), using an antibody raised in goat against the final 72 amino acids of rat CB1 (Harkány et al., 2003) and a rabbit antibody against VGluT1 (Synaptic Systems). Sections were imaged with a Leica SL confocal microscope and processed using Adobe Photoshop. For analyzing VGluT1 and CB1 colocalization, well-defined circular structures were considered as terminals. A total of 2226 terminals were counted in 24 fields corresponding to the inner third of the molecular layer of dentate gyrus.
Generation of Recombinant Adeno-Associated Virus and Intrahilar Injections
The nuclear localization signal (nls) of the simian virus 40 large T antigen and the Cre-recombinase coding region (Gu et al., 1993) was fused downstream of the hemagglutinin (HA)-tag, in a rAAV plasmid backbone containing the 1.1kb CMV immediate early enhancer/chicken β-actin hybrid promoter (CBA), the woodchuck posttranscriptional regulatory element (WPRE; Paterna et al., 2000), and the bovine growth hormone poly(A) (bGH) to obtain rAAV-CBA-WPRE-bGH carrying Cre-recombinase (AAV-Cre). The rAAV-CBA-WPRE-bGH backbone carrying the renilla GFP cDNA (Stratagene) was used as negative control. rAAV chimeric vectors (virions containing a 1:1 ratio of AAV1 and AAV2 capsid proteins with AAV2 ITRs) were generated as described (Klugmann et al., 2005). Briefly, HEK293 cells were transfected with the AAV cis plasmid, the AAV1 and AAV2 helper plasmids, and the adenovirus helper plasmid by standard calcium phosphate transfection methods. Sixty hours after transfection, cells were harvested and the vector purified using heparin affinity columns (Sigma). Genomic titers were determined using the ABI 7700 real time PCR cycler (Applied Biosystems) with primers designed to WPRE (Clark et al., 1999). CB1f/f mice (6–9 weeks old at the time of surgery) were anesthetized with isoflurane. One microliter of either AAV-Cre or AAV-GFP (titres—2 × 1016 viral genomes/ml) were bilaterally injected at 0.05 μl/min by glass capillaries with tip resistance of 2–4 MΩ in a stereotactic apparatus, aiming at the dorsal hilus of the dentate gyrus (Paxinos and Franklin, 2001; 1.6 mm posterior to bregma, 1 mm lateral from midline, 1.8 mm below the surface of the skull). Mice quickly recovered from surgery and were tested 9 weeks after virus injection. Evaluation of the extension of the deletion of CB1 mRNA was done on radioactive in situ hybridization autoradiographic films containing sequential coronal sections of AAV-GFP- and AAV-Cre-injected mice. Considering the normal expression of CB1 in the forebrain (Marsicano and Lutz, 1999), the extent of CB1 mRNA deletion in AAV-Cre-injected mice was approximately calculated using coordinates from Paxinos and Franklin (2001). Only data from mice bearing CB1 mRNA deletion limited to the hippocampal formation were considered for analysis of KA-induced seizures.
Statistical Analysis
Data from seizure scoring were analyzed using two-way ANOVA for repeated measurements. Only statistical parameters referring to the factor genotype or treatment are presented. Electrophysiological data were analyzed by Student’s t test. Software Graph Pad Prism 4.0 was used for statistics and generation of graphs.
Acknowledgments
We thank M. Reents, T. Orschmann, and B. Fackelmeier for valuable technical help; G. Hatch for assistance with the generation of transgenic Dlx5/6-Cre mice; E. Casanova and G. Schütz for providing the CaMKIIα-Cre mouse line; P. Osten and A. Mederer for help in virus injection; R. Edwards for providing the mouse cDNA of VGluT1; C. Yee for providing the anti-beta galactosidase antibody; M. Melis, T. Deller and S.W. Schwarzacher for critically reading the manuscript and for valuable suggestions. Grant support was as follows: Marie Curie Fellowship QLG1-CT-2002-51742 (to K. Monory); CIHR MOP14460 (to M. Ekker); Nina Ireland, MH49428 and MH065670 (to J.R.); DA00286, DA11322, and DA15916 (to K. Mackie); BBSRC S19916 (to M.R.E.); Deutsche Forschungsgemeinschaft (DFG) CMPB to K.-A.N. and LU755/1-3 and a scholarship from the Hertie Foundation (to B.L.).
References
- Alger E. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Aparicio LC, Candeletti S, Binaschi A, Mazzuferi M, Mantovani S, Di BM, Landuzzi D, Lopetuso G, Romualdi P, Simonato M. Kainate seizures increase nociceptin/orphanin FQ release in the rat hippocampus and thalamus: a microdialysis study. J Neurochem. 2004;91:30–37. doi: 10.1111/j.1471-4159.2004.02633.x. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004;6:7–28. doi: 10.1016/s1534-5807(03)00399-x. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Clark KR, Liu X, McGrath JP, Johnson PR. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther. 1999;10:1031–1039. doi: 10.1089/10430349950018427. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141:765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- Domenici MR, Azad SC, Marsicano G, Schierloh A, Wotjak CT, Dodt HU, Zieglgänsberger W, Lutz B, Rammes G. Cannabinoid receptor type 1 located on presynaptic terminals of principal neurons in the forebrain controls glutamatergic synaptic transmission. J Neurosci. 2006;26:5794–5799. doi: 10.1523/JNEUROSCI.0372-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertová M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB1. J Comp Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Földy C, Neu A, Jones MV, Soltész I. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci. 2006;26:1465–1469. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Lovinger DM. Emerging roles for endocannabinoids in long-term synaptic plasticity. Br J Pharmacol. 2003;140:781–789. doi: 10.1038/sj.bjp.0705466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem N, Jarinova O, Amores A, Long Q, Hatch G, Park BK, Rubenstein JL, Ekker M. Regulatory roles of conserved intergenic domains in vertebrate Dlx bigene clusters. Genome Res. 2003;13:533–543. doi: 10.1101/gr.716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Zou YR, Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993;73:1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- Hájos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Harkány T, Hartig W, Berghuis P, Dobszay MB, Zilberter Y, Edwards RH, Mackie K, Ernfors P. Complementary distribution of type 1 cannabinoid receptors and vesicular glutamate transporter 3 in basal forebrain suggests input-specific retrograde signalling by cholinergic neurons. Eur J Neurosci. 2003;18:1979–1992. doi: 10.1046/j.1460-9568.2003.02898.x. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Macgill AM, Smith D, Oz M, Lupica CR. Species and strain differences in the expression of a novel glutamate-modulating cannabinoid receptor in the rodent hippocampus. Eur J Neurosci. 2005;22:2387–2391. doi: 10.1111/j.1460-9568.2005.04401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, et al. International union of pharmacology. XXVII Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Johnston D, Amaral DG. Hippocampus. In: Shepherd GM, editor. The synaptic organization of the brain. ed. Oxford: Oxford University Press; 2004. pp. 455–498. [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlágh B, Sik A, Köfalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urbán GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppisch T, Wolfsgruber W, Feil S, Allmann R, Wotjak CT, Goebbels S, Nave KA, Hofmann F, Feil R. Hippocampal cGMP-dependent protein kinase I supports an age- and protein synthesis-dependent component of long-term potentiation but is not essential for spatial reference and contextual memory. J Neurosci. 2003;23:6005–6012. doi: 10.1523/JNEUROSCI.23-14-06005.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugmann M, Wymond SC, Leichtlein CB, Klaussner BK, Dunning J, Fong D, Young D, During MJ. AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol Cell Neurosci. 2005;28:347–360. doi: 10.1016/j.mcn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Lee J, Park K, Lee S, Whang K, Kang M, Park C, Huh Y. Differential changes of calcium binding proteins in the rat striatum after kainic acid-induced seizure. Neurosci Lett. 2002;333:87–90. doi: 10.1016/s0304-3940(02)00987-4. [DOI] [PubMed] [Google Scholar]
- Li XG, Somogyi P, Ylien A, Buszáki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev Biol. 1999;208:281–292. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- Lutz B. On-demand activation of the endocannabinoid system in the control of neuronal excitability and epileptiform seizures. Biochem Pharmacol. 2004;68:1691–1698. doi: 10.1016/j.bcp.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Neuromodulatory functions of the endocannabinoid system. J Endocrinol Invest. 2006;29:27–46. [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutiérrez SO, van der Stelt M, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Morozov A, Kellendonk C, Simpson E, Tronche F. Using conditional mutagenesis to study the brain. Biol Psychiatry. 2003;54:1125–1133. doi: 10.1016/s0006-3223(03)00467-0. [DOI] [PubMed] [Google Scholar]
- Nomura M, Ueta Y, Hannibal J, Serino R, Yamamoto Y, Shibuya I, Matsumoto T, Yamashita H. Induction of pituitary adenylate cyclase-activating polypeptide mRNA in the medial parvocellular part of the paraventricular nucleus of rats following kainic-acid-induced seizure. Neuroendocrinology. 2000;71:318–326. doi: 10.1159/000054552. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Ong WY, Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in primate brain. Neuroscience. 1999;92:1177–1191. doi: 10.1016/s0306-4522(99)00025-1. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Simeonidou C, Ben Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- Paterna JC, Moccetti T, Mura A, Feldon J, Bueler H. Influence of promoter and WHV post-transcriptional regulatory element on AAV-mediated transgene expression in the rat brain. Gene Ther. 2000;7:1304–1311. doi: 10.1038/sj.gt.3301221. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Pettit DA, Harrison MP, Olson JM, Spencer RF, Cabral GA. Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res. 1998;51:391–402. doi: 10.1002/(SICI)1097-4547(19980201)51:3<391::AID-JNR12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Ratzliff AH, Santhakumar V, Howard A, Soltész I. Mossy cells in epilepsy: rigor mortis or vigor mortis? Trends Neurosci. 2002;25:140–144. doi: 10.1016/s0166-2236(00)02122-6. [DOI] [PubMed] [Google Scholar]
- Schauwecker PE, Steward O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc Natl Acad Sci USA. 1997;94:4103–4108. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab MH, Bartholomae A, Heimrich B, Feldmeyer D, Druffel-Augustin S, Goebbels S, Naya FJ, Zhao S, Frotscher M, Tsai MJ, Nave KA. Neuronal basic helix-loop-helix proteins (NEX and BETA2/Neuro D) regulate terminal granule cell differentiation in the hippocampus. J Neurosci. 2000;20:3714–3724. doi: 10.1523/JNEUROSCI.20-10-03714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Takahashi KA, Castillo PE. The CB1 cannabinoid receptor mediates glutamatergic synaptic suppression in the hippocampus. Neuroscience. 2006;139:795–802. doi: 10.1016/j.neuroscience.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tsou K, Mackie K, Sanudo-Pena MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing gabaergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Veldhuis WB, Maccarrone M, Bar PR, Nicolay K, Veldink GA, Di Marzo V, Vliegenthart JF. Acute neuronal injury, excitotoxicity, and the endocannabinoid system. Mol Neurobiol. 2002;26:317–346. doi: 10.1385/MN:26:2-3:317. [DOI] [PubMed] [Google Scholar]
- Wager-Miller J, Westenbroek R, Mackie K. Dimerization of G protein-coupled receptors: CB1 cannabinoid receptors as an example. Chem Phys Lipids. 2002;121:83–89. doi: 10.1016/s0009-3084(02)00151-2. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Wu SX, Goebbels S, Nakamura K, Nakamura K, Kometani K, Minato N, Kaneko T, Nave KA, Tamamaki N. Pyramidal neurons of upper cortical layers generated by NEX-positive progenitor cells in the subventricular zone. Proc Natl Acad Sci USA. 2005;102:17172–17177. doi: 10.1073/pnas.0508560102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerucha T, Stuhmer T, Hatch G, Park BK, Long Q, Yu G, Gambarotta A, Schultz JR, Rubenstein JL, Ekker M. A highly conserved enhancer in the Dlx5/Dlx6 intergenic region is the site of cross-regulatory interactions between Dlx genes in the embryonic forebrain. J Neurosci. 2000;20:709–721. doi: 10.1523/JNEUROSCI.20-02-00709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


