Abstract
Glycogen synthase plays a key role in regulating glycogen metabolism. In a search for regulators of glycogen synthase, a yeast two-hybrid study was performed. Two glycogen synthase-interacting proteins were identified in human skeletal muscle, glycogenin-1 and nebulin. The interaction with glycogenin was found to be mediated by the region of glycogenin which contains the 33 COOH-terminal amino acid residues. The regions in glycogen synthase containing both NH2- and COOH-terminal phosphorylation sites are not involved in the interaction. The core segment of glycogen synthase from Glu21 to Gly503 does not bind COOH-terminal fragment of glycogenin. However, this region of glycogen synthase binds full-length glycogenin indicating that glycogenin contains at least one additional interacting site for glycogen synthase besides the COOH-terminus. We demonstrate that the COOH-terminal fragment of glycogenin can be used as an effective high affinity reagent for the purification of glycogen synthase from skeletal muscle and liver.
Introduction
Glycogen is a branched polymer of glucose that is covalently attached to the specialized initiator protein, glycogenin, which attaches the first dozen or so glucose residues. Bulk biosynthesis of glycogen is mediated by glycogen synthase which catalyzes the formation of the α-1,4-glycosidic linkages of the polymer, and branching enzyme which forms the α-1,6-glycosidic branchpoints (reviewed in 1). Glycogen synthase is controlled by multisite phosphorylation and by several allosteric effectors, of which the activator glucose 6-P is most important (2, 3). Two phosphorylation sites (2 and 2a) are present in the first ten amino acids at the NH2-terminus of muscle isoforms of glycogen synthase, while other sites (3a, 3b, 3c, 4, 5, 1a and 1b) are located in a stretch of 100 amino acids at the COOH-terminus (2, 3). These sites are phosphorylated by several protein kinases and dephosphorylated by glycogen-associated type 1 protein phosphatase (reviewed in 1, 4). Therefore, control of glycogen synthase by multisite phosphorylation implies that glycogen synthase interacts with a variety of protein kinase/phosphatase signaling complexes.
Two-hybrid analysis of yeast proteins capable of interacting with Gsy2, the major nutritionally regulated form of glycogen synthase in yeast, revealed two clones derived from the genes PIG1 and PIG2 (5). The products of both genes are putative type-1 protein phosphatase targeting subunits that could tether the yeast Glc7 type-1 phosphatase to Gsy2 (6). These findings demonstrated that two-hybrid screening could be a useful approach for the identification of new players in the regulation of glycogen synthase by phosphorylation. Another protein identified in the two-hybrid screen for proteins that interact with Gsy2 was the self-glucosylating protein Glg2 (5), a yeast homolog of mammalian glycogenin which mediates the initiation step of glycogen synthesis. In rabbit glycogenin-1, self-glucosylation results in the formation of a C-1-O-tyrosyl linkage between glucose and Tyr194 (7, 8). Self-glucosylation continues with the formation of α-1,4-glycosidic linkages, until a chain of 8 to 12 residues has formed. This form of glycogenin serves as a substrate for glycogen synthase.
In an effort to identify other proteins involved in the regulation of glycogen synthesis in mammalian cells, we used the yeast two-hybrid system with rabbit glycogen synthase as bait to screen a human skeletal muscle library. We found that glycogen synthase interacts with glycogenin-1 and identified the regions for interaction in both proteins.
Materials and methods
Plasmids and Proteins
The cDNA for rabbit muscle glycogen synthase (9) was ligated into pGBDU-C2 (10) to generate the pGBDU-GS vector. The cDNA encoding full-length human muscle glycogen synthase in the pGAD10 vector, pGAD-GS, was obtained from a yeast two-hybrid screen with glycogenin-1 as bait (11). The rabbit muscle glycogen synthase (GS) deletion constructs pGBDU-GS(30-735) (amino acids 30-735), pGBDU-GS(49-735), pGBDU-GS(1-637) and pGBDU-GS(1-369) were made by subcloning the indicated GS fragments from pGBDU-GS vector into pGBDU-C(1, 2 or 3) vectors. The human muscle GS deletion constructs pGBDU-GS (21-662), pGBDU-GS (21-614), pGBDU-GS (21-503), pGBDU-GS (21-578) and pGBDU-GS (21-402) were made by subcloning the indicated GS fragments from pGAD-GS into the pGBDU-C2 vector. For expression of truncated GS with NH2-terminal c-myc epitope tag in COS cells, the fragments of GS cDNA were subcloned into the pCMV-Tag 3B vector (Stratagene) to generate pCMV-GS(21-662), pCMV-GS(21-614), pCMV-GS(21-503) and pCMV-GS(21-402). Construction of a plasmid for the expression of rabbit skeletal muscle glycogenin, pCMV-GN, was described previously (12). The human glycogenin (GN) deletion constructs pGEX-GN(263-333), pGEX-GN(297-333) and pGEX-GN(301-333) were made by subcloning fragments of cDNA for GN obtained from two-hybrid screen into pGEX-4T1 vector (Amersham Pharmacia Biotech). All constructs were sequenced before using for protein expression and in two-hybrid assay.
Yeast Two-hybrid Screen
The yeast strain PJ69-4A (10) was sequentially transformed with pGBDU-GS and the human skeletal muscle matchmaker cDNA library in a pGAD10 plasmid (Clontech) using the lithium-acetate method. The transformants were plated on synthetic medium lacking uracil, leucine and adenine. Processing the two-hybrid clones, rescuing the library plasmids and confirming two-hybrid interaction were performed as described previously (11).
Expression and Purification of Recombinant Proteins
Vectors encoding GST (PGEX-4T1) or COOH-terminal fragments of glycogenin as glutathione-S-transferase (GST)-fusion proteins, pGEX-GN(263-333), pGEX-GN(297-333) and pGEX-GN(301-333), were used to transform E. coli cell BL21/DE3. Transformants were isolated, induced with IPTG and the recombinant proteins were purified over glutathione agarose. Bound proteins were eluted from the resin with 20 mM glutathione and dialyzed.
Cell Culture, Transfection and Immunoprecipitation
COS-M9 cells were transfected by using LipofectAMINE (Invitrogen). Cells were grown for 3 days, lysed and soluble and pellet fractions were prepared as described previously (13). Glycogenin from the soluble fractions of COS cells was immunoprecipitated using anti-glycogenin antibody essentially by the procedure described previously (13).
GST-based Affinity Pull-Down
Mouse skeletal muscle was homogenized in 10 volumes of buffer containing 50 mM Tris-HCl, pH 7.8, 10 mM EDTA, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 2 mM benzamidine and 1 mM dithiothreitol. Homogenates were centrifuged at 14,000 × g for 15 min. Approximately 7 μg of GST or GST-fusion proteins was added to 0.5 ml of muscle supernatant and the mixture was incubated for 15 min at 30°C. Then 40 μl of glutathione-Agarose (50% suspension) was added and incubation was continued for 1 h at 25°C. Beads were extensively washed with phosphate-buffered saline and adsorbed proteins eluted with 5 mM glutathione. The GST-protein complexes were resolved by SDS-PAGE, and analyzed by silver staining and immunoblotting. Similarly, pull-down was performed with proteins from soluble fractions of COS cells expressing truncated glycogen synthase.
Results and Discussion
Two-hybrid analysis of proteins which interact with glycogen synthase
To search for cDNA clones encoding proteins that interact with glycogen synthase, we fused the coding sequence for rabbit skeletal muscle glycogen synthase with the Gal4p DNA binding domain and screened a human skeletal muscle library expressed from the pGAD-10 vector. From 1.01 × 106 transformants harboring bait and library cDNA, eleven clones were isolated representing two different cDNAs as judged by cDNA sequencing. One clone contained a fragment of cDNA for nebulin, a muscle structural protein (14). Analysis of the interaction between glycogen synthase and nebulin will be described elsewhere. Ten other clones contained fragments of cDNA for glycogenin-1 encoding carboxy-terminal parts of the protein corresponding to residues 301–333, 297–333 and 263–333. To confirm the interactions, the plasmids isolated from two-hybrid clones were introduced into yeast that had been pre-transformed with pGBDU-GS. The resulting transformants were able to express both the ADE2 and HIS3 genes, which are under control of the GAL4 upstream activating sequence in the PJ69-4A yeast strain (data not shown). The activity of β-galactosidase in transformants, calculated in Miller units, was increased at least 30-fold indicating that the interaction between glycogen synthase and COOH-terminal fragments of glycogenin is relatively strong (data not shown).
Glycogenin COOH-terminus is sufficient for interaction with glycogen synthase
The fact that all the identified glycogenin clones corresponded to the COOH-terminus suggested its importance for the interaction. To confirm that the interaction occurs not only in the two-hybrid system, we performed in vitro studies. The fragments of glycogenin were fused with glutathione S-transferase (GST) to obtain proteins GST-GN263-333, GST-GN297-333 and GST-GN301-333, and tested for their ability to pull down glycogen synthase from mouse skeletal muscle homogenates. All fusion proteins were able to precipitate glycogen synthase in the presence of glutathione-Agarose (Fig. 1B). Incubation of muscle proteins with GST-GN297-333 led to almost complete removal of glycogen synthase from the tissue extract (Fig. 1A) indicating strong binding of glycogen synthase with GN297-333. SDS-PAGE analysis of proteins eluted from glutathione-Agarose beads with glutathione revealed a predominant band with Mr~85 kDa, corresponding to the molecular weight of glycogen synthase (Fig. 1C). Similar results were obtained with the liver isoform of glycogen synthase, pulled down from mouse liver (not shown). These data indicate that the amino acid residues 301–333 in glycogenin are engaged in interaction with glycogen synthase and furthermore could be used as an effective reagent for affinity purification of glycogen synthase from tissue extracts.
Figure 1. Interaction of COOH-terminal fragments of glycogenin with mouse muscle glycogen synthase.
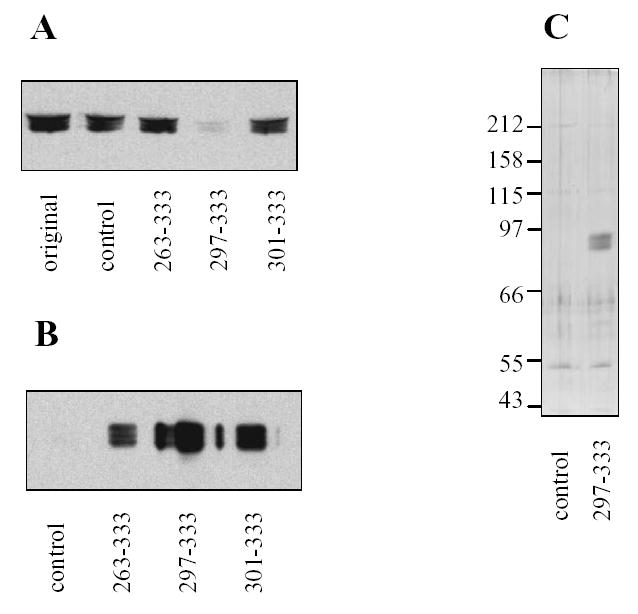
Mouse skeletal muscle was homogenized, centrifuged and supernatant (original) prepared. The recombinant GST-fusion proteins with C-terminal parts of glycogenin (amino acids 263–333, 297–333 and 301–333) or native GST (control) were added. GST-fusion proteins were precipitated by adding glutathione-agarose, and the resulting supernatants (A) and beads (B) were analyzed for the presence of glycogen synthase using immunoblotting. Glycogen synthase purified with GST-GN297-333 was eluted from beads by glutathione and analyzed by silver staining (C). The numbers to the left show the molecular masses and migration of protein standards.
Delineation of interacting regions in glycogen synthase
To identify which region of glycogen synthase interacts with glycogenin, NH2- and COOH-terminal deletions of glycogen synthase were made and analyzed in the GAL4 yeast two-hybrid system with GN297-333. The interaction was scored by growth in the absence of adenine or of histidine in the presence of 3-aminotriazole (3AT) and quantitative β-galactosidase assays. Deletion of the COOH-terminal 98 residues (construct GS 1-637, Fig. 2, Table 1) left a protein capable of interacting, indicating that the region in glycogen synthase containing all COOH-terminal phosphorylation sites is not involved in binding to glycogenin. Deletion of the NH2-terminal 20 residues together with removal of COOH-terminal 73 residues (GS 21-662, Fig. 2, Table 1) also did not abolish the interaction suggesting that the region carrying the NH2-terminal phosphorylation sites is also not important for binding to glycogenin. However, deletion of an additional 10 residues from the NH2 terminus (GS 30-735) led to loss of interaction. Therefore, it is possible that the region in vicinity of Phe30 in glycogen synthase is involved in binding of glycogenin. To determine the shortest region in glycogen synthase capable of interacting with glycogenin, larger fragments of the COOH-terminus of glycogen synthase were deleted. Removal of 120 amino acids from COOH-terminus (GS 21-615) caused a more than 20 fold reduction in β-galactosidase activity in two-hybrid assay (Fig. 2, Table 1) indicating significantly weaker interaction. Removal of an additional 37 amino acids (GS 21-578) completely abolished the interaction. These results indicate that the region adjacent to the COOH-terminal phosphorylation sites might be involved in binding of glycogenin. Another possibility is that truncations of NH2- and/or COOH-terminal regions disrupt a specific overall conformation of the glycogen synthase molecule.
Figure 2. Schematic representation of wild type and truncated glycogen synthase.
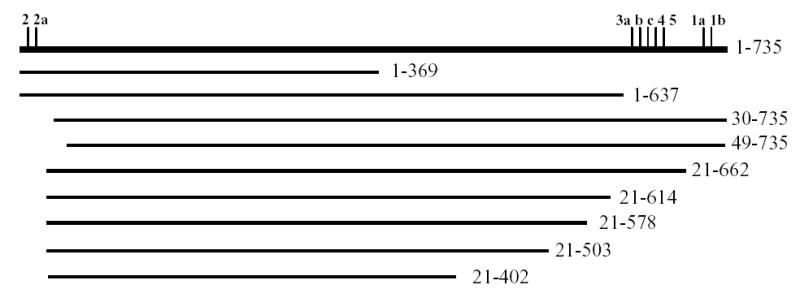
Glycogen synthase protein is shown as horizontal line; the vertical tick marks indicate phosphorylation sites. The numbers to the left indicate the first and the last amino acids in the protein sequence of wild type (1–735) and truncated glycogen synthase.
Table 1.
Two-hybrid analysis of glycogenin-ineracting regions in glycogen synthase
| GS mutanta | Growth on selection mediumb | β-Galactosidase activity (Miller units) |
|---|---|---|
| GS 1-369 | no | 1.6 |
| GS 1-637 | yes | 268 |
| GS 30-735 | no | 0.5 |
| GS 49-735 | no | 0.8 |
| GS 21-662 | yes | 180 |
| GS 21-614 | yes | 7.1 |
| GS 21-578 | no | 0.7 |
| GS 21-503 | no | 0.6 |
| GS 21-402 | no | 0.5 |
Glycogen synthase mutants are described in Figure 1. The mutants and GN297-333 were fused with DNA-binding and DNA-activation domains, respectively.
Selection was on plates SC-Leu-Ura-Ade and SC-Leu-Ura- His+3AT (see Experimental procedures).
To further confirm the results of two-hybrid assay, truncated mutants of glycogen synthase were expressed in COS cells followed by pull down with GST-GN297-333. In previous studies we demonstrated that co-expression of both glycogen synthase and glycogenin was required to produce glycogen synthase in the soluble fraction of COS cells (12, 13). The glycogen synthase mutant GS 21-662 was also detected in the soluble fraction only if co-expressed with glycogenin (Fig. 3A). Interestingly, the solubilizing effect of glycogenin was observed with the mutant GS 21-503, which did not interact with GN297-333 in the two-hybrid assay (Fig. 2, Table 1). Consistent with the two-hybrid results, we were able to pull down glycogen synthase mutant GS 21-662 from the soluble fraction of COS cells using GST-GN297-333 (Fig. 3B). Another glycogen synthase mutant GS 21-614 was poorly precipitated with GST-GN297-333 confirming that interaction between two proteins is relatively weak. Consistent with two-hybrid data, two other mutants, GS 21-503 and GS 21-402, expressed in COS cells did not bind the COOH-terminus of glycogenin (Fig. 3B).
Figure 3. Interaction of glycogenin and glycogen synthase expressed in COS cells.
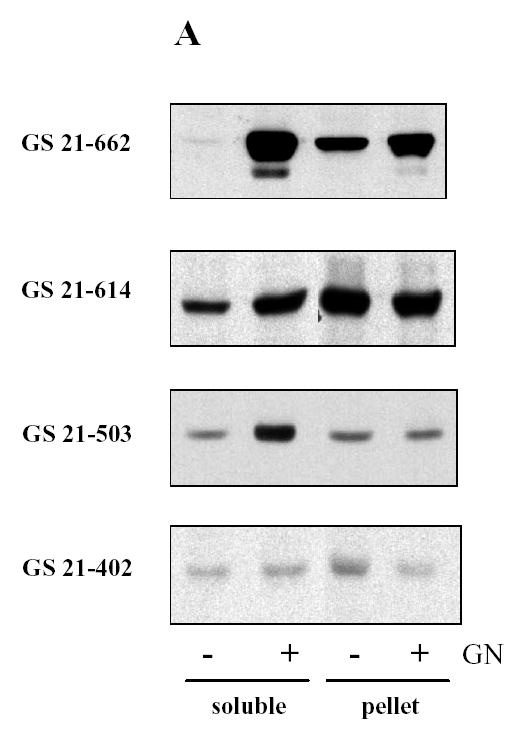
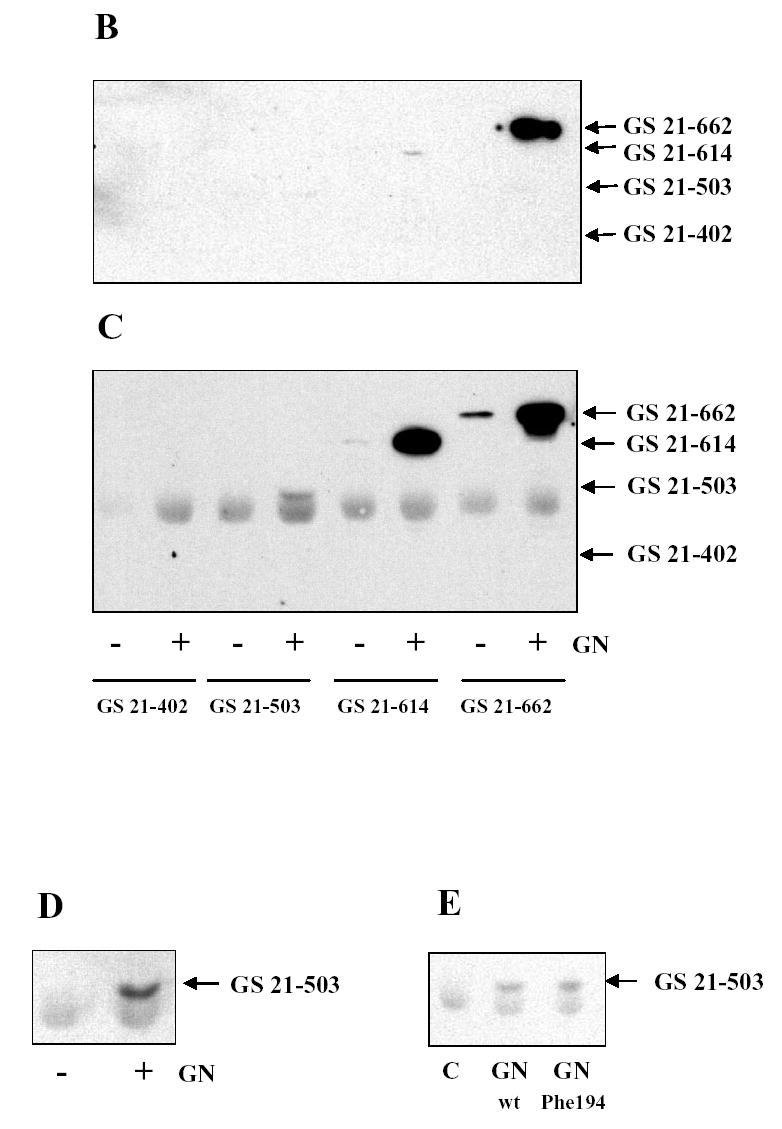
Glycogen synthase mutants with an NH2-terminal myc epitope tag were expressed in COS cells with (+) or without (−) glycogenin (GN). Proteins in the soluble and pellet fractions of COS cells were analyzed by immunoblotting (A). Glycogen synthase mutants were pulled down from the soluble fraction of COS cells using GST-GN297-333 (B) or immunoprecipitated with anti-glycogenin antibody (C) and detected by immunoblotting. To better separate glycogen synthase GS 21-503 from the immunoglobulin band, SDS-PAGE was performed in a gel with lower percentage of acrylamide (D). The glycogen synthase GS 21-503 was co-expressed with wild type or the Phe194 mutant of glycogenin followed by immunoprecipitation with anti-glycogenin antibodies and immunoblotting (E). All immunoblots were performed with anti-myc antibodies.
As an alternative approach to detect the interaction between glycogen synthase and glycogenin, we used antibody against glycogenin to perform immunoprecipitations. Glycogen synthase mutant GS 21-662, which interacts with the COOH-terminus of glycogenin, coimmunoprecipitated with full length glycogenin from COS cells co-transfected with both proteins (Fig. 3C). Unexpectedly, a significant amount of glycogen synthase mutant GS 21-614, which interacted poorly with GN297-333, was found in glycogenin immunoprecipitate (Fig. 3C). Another mutant GS 21-503, which did not interact with GN297-333 in two-hybrid and pull down assays, was also present in immunoprecipitate (Fig. 3D). The shortest mutant of glycogen synthase, GS 21-402, did not interact with full-length glycogenin (Fig. 3C). Our observation that GS 21-503 does not interact and GS 21-614 does poorly interact with GN297-333 but both mutants bind to full length glycogenin indicate that glycogenin contains more than one site for binding of glycogen synthase. The first site is located at COOH terminus. We hypothesized that second site might be associated with the oligosaccharide chain attached to Tyr194 of glycogenin. Therefore, we used the mutant of glycogenin with Tyr194 substituted by Phe194 which cannot catalyze the reaction of self-glucosylation (12). However, both wild type and mutated glycogenins coimmunoprecipitated with GS 21-503 (Fig. 3E) indicating that carbohydrate moiety of glycogenin is not important for interaction with glycogen synthase.
Although a crystal structure of glycogenin has been reported, the COOH-terminal portion of the molecule was not detected and was most likely disordered (15). Nor is the C-terminus of glycogenin overall highly conserved between species. There is a short motif W-E-X2-4D-Y-L/M present in most glycogenins and positioned less than 20 residues from the COOH-terminus. Mutation of the Tyr residue in the yeast glycogenins had some functional effects but direct influence on glycogen synthase binding was not tested (16). Whether or not the motif noted above is critical for the interaction remains to be determined. Our results do suggest, however, that although the extreme COOH-terminus can be sufficient for a strong interaction with glycogen synthase, there may be other regions of contact. The COOH-terminal binding domain of glycogenin does provide a useful reagent for the isolation of glycogen synthase from tissue extracts.
Acknowledgments
Supported by NIH grants DK27221 (P.J.R.) and the American Diabetes Association (A.V.S.)
References
- 1.Roach PJ, Skurat AV, Harris RA. Regulation of glycogen metabolism. In: Jefferson LS, Cherrington AD, editors. Handbook of Physiology, Vol. 2: The endocrine pancreas and regulation of metabolism. Oxford University Press; New York: 2001. pp. 609–647. [Google Scholar]
- 2.Cohen P. Muscle Glycogen Synthase. The Enzymes. 1986;17:461–497. [Google Scholar]
- 3.Roach PJ. Control of glycogen synthase by hierarchal protein phosphorylation. FASEB J. 1991;4:2961–2968. [PubMed] [Google Scholar]
- 4.Skurat AV, Roach PJ. Regulation of glycogen synthesis. In: LeRoith D, Olefsky JM, Taylor SI, editors. Diabetes Mellitus: A Fundamental and Clinical Text. third edition. Lippincott-Raven Publishers; Philadelphia: 2004. pp. 317–334. [Google Scholar]
- 5.Cheng C, Mu J, Farkas I, Huang D, Goebl MG, Roach PJ. Requirement of the self-glucosylating initiator proteins Glg1p and Glg2p for glycogen accumulation in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:6632–6640. doi: 10.1128/mcb.15.12.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng C, Huang D, Roach PJ. Yeast PIG genes: PIG1 encodes a putative type 1 phosphatase subunit that interacts with the yeast glycogen synthase Gsy2p. Yeast. 1997;13:1–8. doi: 10.1002/(SICI)1097-0061(199701)13:1<1::AID-YEA49>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez IR, Whelan WJ. A novel glycosyl-amino acid linkage: rabbit-muscle glycogen is covalently linked to a protein via tyrosine. Biochem Biophys Res Commun. 1985;132:829–836. doi: 10.1016/0006-291x(85)91206-9. [DOI] [PubMed] [Google Scholar]
- 8.Smythe C, Caudwell FB, Ferguson M, Cohen P. Isolation and structural analysis of a peptide containing the novel tyrosyl-glucose linkage in glycogenin. EMBO J. 1988;7:2681–2686. doi: 10.1002/j.1460-2075.1988.tb03121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Browner MF, Fletterick RG, DePaoli-Roach AA, Roach PJ. Primary structure of rabbit skeletal muscle glycogen synthase deduced from cDNA clones. FASEB J. 1989;3:2532–2536. doi: 10.1096/fasebj.3.13.2509275. [DOI] [PubMed] [Google Scholar]
- 10.James P, Halladay J, Craig EA. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skurat AV, Dietrich AD, Zhai L, Roach PJ. GNIP, a novel protein that binds and activates glycogenin, the self-glucosylating initiator of glycogen biosynthesis. J Biol Chem. 2002;277:19331–19338. doi: 10.1074/jbc.M201190200. [DOI] [PubMed] [Google Scholar]
- 12.Skurat AV, Cao Y, Roach PJ. Glucose control of rabbit muscle glycogenin expressed in COS cells. J Biol Chem. 1993;268:14701–14707. [PubMed] [Google Scholar]
- 13.Skurat AV, Wang Y, Roach PJ. Rabbit skeletal muscle glycogen synthase expressed in COS cells: identification of regulatory phosphorylation sites. J Biol Chem. 1994;269:25534–25542. [PubMed] [Google Scholar]
- 14.McElhinny AS, Kazmierski ST, Labeit S, Gregorio CC. Nebulin: the nebulous, multifunctional giant of striated muscle. Trends Cardiovasc Med. 2003;13:195–201. doi: 10.1016/s1050-1738(03)00076-8. [DOI] [PubMed] [Google Scholar]
- 15.Hurley TD, Stout S, Miner E, Zhou J, Roach PJ. Requirements for catalysis in mammalian glycogenin. J Biol Chem. 2005;280:23892–23899. doi: 10.1074/jbc.M502344200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mu J, Cheng C, Roach PJ. Initiation of glycogen synthesis in yeast. Requirement of multiple tyrosine residues for function of the self-glucosylating Glg proteins in vivo. J Biol Chem. 1996;271:26554–26560. doi: 10.1074/jbc.271.43.26554. [DOI] [PubMed] [Google Scholar]


