Abstract
The GABA-B agonist baclofen reduces drug self-administration in rats and has shown promise clinically in the treatment of substance abuse. Baclofen generally does not reduce food intake in non-binge feeding protocols. In this study, baclofen was tested in a fat-binge protocol. Thirty male rats were divided into three groups (B: binge; FM: fat-matched; C: chow). B received a bowl of vegetable shortening for 2 h on Monday, Wednesday, and Friday (MWF) and continuous access to powdered chow (regular chow) in all phases. FM had continuous access to a regular chow+shortening mixture (FM chow) that provided the same proportion of shortening and regular chow that the B rats consumed in all phases. In addition, FM had the following: phase 1: no separate bowl of shortening; phase 2: 2-h MWF access to a separate bowl of shortening; phase 3, daily 2-h access to a separate bowl of shortening; C rats had continuous access to the regular chow in all phases. In addition, C had the following: phase 1: no separate bowl of shortening; phase 2: 2-h MWF access to a separate bowl of shortening; in phase 3, daily 2-h access to a separate bowl of shortening. Baclofen (1.0, 1.8 mg/ kg, i.p.) reduced shortening intake regardless of access condition. Baclofen had no effect on, or stimulated, FM and regular chow intake. These results demonstrate that baclofen can reduce fat intake in rats under binge-type conditions. Furthermore, these results indicate that bingeing, as modeled in our protocol, is different from other forms of food intake and may share similarities with substance abuse.
Keywords: Baclofen, Binge eating, Eating disorders, GABA-B
1. Introduction
Binge eating is an intermittent excessive behavior with negative effects on both physical and mental health [1–3]. Prevalence estimates range from 1%–2% of the population to ~30% of those seeking treatment for weight loss [4]. There are few effective treatments for binge eating, and relapse rates are high [5,6].
Binge eating shares co-morbidity with other intermittent excessive behaviors, particularly those involving substance abuse [7–18]. This suggests that neural mechanisms involved in binge eating and substance abuse may overlap. Recent research indicates that GABA-B receptors may be involved in substance abuse disorders. Specifically, the GABA-B agonist baclofen reduces cocaine, heroin, d- and methamphetamine, ethanol, and nicotine self-administration in animals [19–28] and has shown promise clinically in the treatment of cocaine, opioid, alcohol, and nicotine dependence [29–35]. When tested in animal feeding studies using non-binge protocols, baclofen generally had no effect on or increased food intake [21,22,36–50]. The effects of baclofen under binge-type eating conditions, however, have never been tested.
Therefore, in the present study, the effects of baclofen were tested in rats maintained on a limited-access protocol of binge-type eating [51–55]. We report baclofen-induced reductions in consumption of the binge food at dosages that had no effect on or stimulated consumption of chow.
2. Methods
2.1. Rats and diets
Thirty 2-month old male Sprague Dawley rats (Harlan Sprague Dawley, Inc., Indianapolis, IN; body weight 267.8 ± 1.8 g) were individually housed in hanging wire cages in a temperature- and humidity-controlled vivarium under a 12:12 light:dark cycle. All rats had ad libitum access to water and powdered (regular) chow [Lab Diet 5001 Rodent Diet, PMI Nutrition, International, LLC, Brentwood, MO; 3.3 kcal/g; macronutrient (% energy): protein (28%), fat (12.1%), carbohydrate (59.8%)] throughout all studies unless otherwise noted.
Three days after being introduced into the vivarium, rats were given overnight access to a bowl of vegetable shortening clipped to the front of the cage (Crisco All-Vegetable Shortening, The J.M. Smucker Co., Orrville, OH; 9.17 kcal/g). This was done to prevent neophobia during the study. Chow and water were also available during this overnight access period. The rats were then divided into three groups of ten matched for two-day average chow intake [ F(2, 27) < 1.0], overnight shortening intake [ F(2,27) <1.0], and body weight [ F(2,27) <1.0]. The same rats were used in all phases of the study. All procedures were approved by the Pennsylvania State University Institutional Animal Care and Use Committee.
2.2. Groups
The groups and different test phases were designed to test the effects of baclofen under different shortening access conditions. In phase 1, rats maintained on a feeding protocol that promoted infrequent, large binges (B group) were compared to rats maintained on feeding protocols that promoted no binges (FM and C groups). In phase 2, rats maintained for an extended period of time on the infrequent, large binge protocol (B group) were compared to rats that had just started the same binge protocol (FM and C groups). In phase 3, rats maintained on the feeding protocol that promoted infrequent, large binges (B group) were compared to rats on a feeding protocol that promoted more frequent, smaller binges (FM and C groups).
The B, FM and C groups were maintained as follows:
Binge (B): These rats had continuous access to the powdered chow and water. In addition, they were given 2-h access to a separate bowl of vegetable shortening clipped to the front of the home cage every Monday, Wednesday, and Friday (MWF), during the 2 h just prior to lights out. During the 2-h shortening access period, the chow and water remained available. Previous work from our laboratory has shown that this protocol results in infrequent, large episodes of binge-type eating in male rats of the same age and strain [51,52]. The MWF protocol is not intended to serve as a comprehensive model of eating disorders such as Bulimia Nervosa or Binge Eating Disorder. Rather, the goal is to model the intermittent excessive eating behavior that characterizes these disorders [55]. The B rats were maintained on this protocol throughout all phases of the study.
Fat-matched (FM): These rats were given the same proportions of chow and shortening that the Binge (B) group consumed except that the shortening was mixed into the powdered chow, which was provided continuously. The proportions of chow and shortening consumed by the Binge group each week were calculated, and the FM group was provided with a fat-matched chow mixed to that proportion the following week. Caloric density of the FM chow ranged from 3.8 to 4.4 kcal/g, and the fat accounted for ~30%–35% of the total energy provided by the FM chow. The FM group had free access to the FM chow and water. This assured that the proportion of fat (and other nutrients) consumed was the same in the Binge and FM groups during phase 1. The FM group was included to control for possible neural or behavioral effects of dietary fat [56–63]. The FM group was maintained on the FM chow throughout all phases of the study. During phase 1, the FM group only had access to the FM chow. During phase 2, the FM group had access to a separate bowl of vegetable shortening for 2-h on Monday, Wednesday, and Friday each week, in addition to the continuously available FM chow. During phase 3, the FM group had 2-h access to the vegetable shortening every day, in addition to the continuously available FM chow. Previous work from our laboratory has shown that the daily access protocol used in phase 3 produces lower intakes during the 2-h shortening access period than does the MWF protocol [51,52]. That is, the daily protocol results in more frequent, smaller episodes of binge-type eating.
Chow/change (C): The rats in this group had continuous access to the regular chow and water throughout all phases of the study. During the first phase, the C group only had access to the regular chow diet. During the second phase, the C group had access to a separate bowl of vegetable shortening for 2-h on Monday, Wednesday, and Friday each week in addition to the continuously available regular chow. During the third phase, the C group had 2-h access to the vegetable shortening every day in addition to the continuously available regular chow.
2.3. Drug testing
Baclofen dose-effect functions were determined during each of the three phases of the study. In phase 1, the effects of baclofen were determined on binge-type consumption of vegetable shortening and on consumption of the regular and FM chow diets (B: MWF access to shortening, FM: FM chow only; C: regular chow only). Rats had been on their respective diets for approximately 6 weeks prior to the initiation of drug testing. In phase 2, the effects of baclofen were assessed in rats that had been bingeing for a relatively long (B group: three months) or short (FM and C groups: 1 day) period of time (all groups had MWF 2-h access to shortening in addition to their assigned regular or FM chow). In phase 3, the effects of baclofen were assessed under conditions of infrequent (B: 2-h MWF) and more frequent (FM and C groups: 2-h daily) shortening access. The FM and C rats were on the daily shortening access schedule for ten days before the first baclofen injection in phase 3. Baclofen was not tested in rats with continuous access to a bowl of shortening due to the low 2-h intakes that are generated on that protocol under non-food-deprived conditions [53]. The half-life of baclofen is quite short in the rat (~4 h) with maximal brain concentrations being achieved within 3 h [64]. Therefore, feeding protocols were selected that would reliably induce high intakes in a brief period of time without requiring food-deprivation.
(R)-baclofen (Tocris, Ellisville, MO) was diluted in 0.9% saline, and injected intraperitoneally at a volume of 1 mL/kg at the following dosages: 0 (saline vehicle), 0.3, 0.6, 1.0, and 1.8 mg/kg. These dosages are comparable to those previously reported to reduce nicotine self-administration in rats (26). Higher dosages of the racemic mixture were generally required to reduce drug self-administration and food intake in other studies (see previous citations, above). Rats had to be at least 98% of their last stable weight to receive an injection. If a rat did not meet the body weight criterion, it did not receive an injection, and either the rat was injected at the end of the injection series or the data point was omitted. In phase 1, data for 2 injections and in phase 2 data from 1 injection were not obtained. There were no missing data points during phase 3. At least 48 h intervened between injections. All rats received all dosages, with dosage order assigned to each rat using a uniform Latin Square.
Injections were given 30 min prior to the shortening access period. Chow was removed for the 30-min pretreatment period. Crisco and/or chow were weighed and placed into the cage at the beginning of the 2-h test period, and then re-weighed at the end of the 2-h period. Once the 2-h weights were obtained, the chow was returned to the rats. Thus, the only time that the rats did not have access to food was during the 30-min pretreatment period.
2.4. Statistics
SAS v.8 (SAS institute, Cary, NC) was used to analyze the data. Two-way (group × dosage) ANOVA (proc GLM) was used to analyze 2-h chow (g), 2-h shortening (g), and 2-h total intake (g and kcal). One-way within group ANOVA was used to determine the effect of dosage on each outcome variable within each group. One-way between group ANOVA was used to analyze body weight and cumulative energy intake data. Post hoc comparisons among means were made using Tukey’s HSD. Alpha was set at 0.05 for results to be considered significant. If F was <1, then results were not significant and p-values are not reported.
3. Results
3.1. Body weight and cumulative energy intake
Neither body weight nor cumulative energy intakes differed among the groups when the baclofen testing was initiated [body weight ANOVA F(2,27) <1; cumulative energy intake ANOVA F(2,27) <1]. Body weights (±SE) at the start of testing in phase 1 for the B, FM, and C groups were 418 ± 8, 422 ± 7, 408 ± 11 g, respectively. At the end of the study, there were still no significant differences in body weight or cumulative energy intake among the groups [body weight: ANOVA, F(2,27)=1.34, p >0.2; energy intake: ANOVA, F(2,27) <1].
3.2. Phase 1
3.2.1. 2-h shortening (Fig. 1)
Fig. 1.
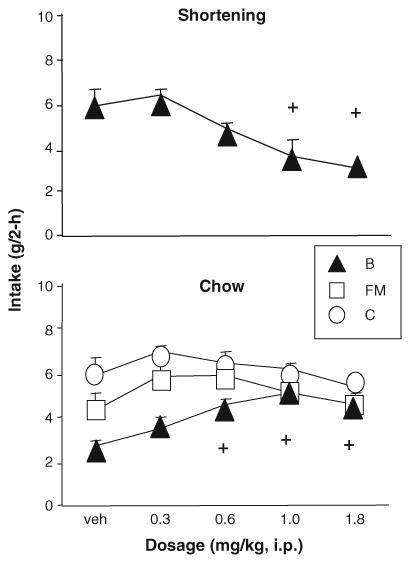
Phase 1: Baclofen significantly reduced shortening intake and stimulated intake of the simultaneously available chow in bingeing rats. + indicates significant difference from vehicle, p <0.05, for group B.
Baclofen (1.0, 1.8 mg/kg) significantly reduced 2-h shortening intake (g) in the B rats [ANOVA, F(4,36)= 12.46, p <0.0001; Tukey’s HSD relative to vehicle, p ≤ 0.05]. The 1.8 mg/kg dosage produced a 48.6% reduction in intake relative to vehicle (Fig. 1).
3.2.2. 2-h chow (Fig. 1)
ANOVA revealed main effects of baclofen dosage [ F(4, 106)=3.02, p < 0.05] and group [ F(2, 27) = 9.62, p < 0.001] on chow intake but no interaction. Individual analyses indicated that baclofen (0.6, 1.0, and 1.8 mg/kg) significantly increased 2-h chow intake (g) in the B rats [ANOVA, F(4,36)=5.70, p <0.01; Tukey’s HSD relative to vehicle, p ≤ 0.05,] but had no effect on intake in the FM [ F(4,35)=1.16, p >0.3.] or C rats [ F(4,35)=1.14, p > 0.3].
3.2.3. 2-h total (data not shown)
Baclofen had no effect on the 2-h total (chow+ shortening) gram intake for group B [ F(4,36)= 1.54, p >0.2] but did significantly reduce the B group’s 2-h total energy intake [ F(4,36)=5.89, p <0.001]. Two-hour total energy intake was significantly lower at 1.0 and 1.8 mg/kg than with vehicle alone in the B group (Tukey’s p ≤ 0.05; data not shown). Since the FM and C groups got only chow but no shortening, the chow data (above) represent the 2-h total for those groups.
3.3. Phase 2
3.3.1. 2-h shortening (Fig. 2)
Fig. 2.
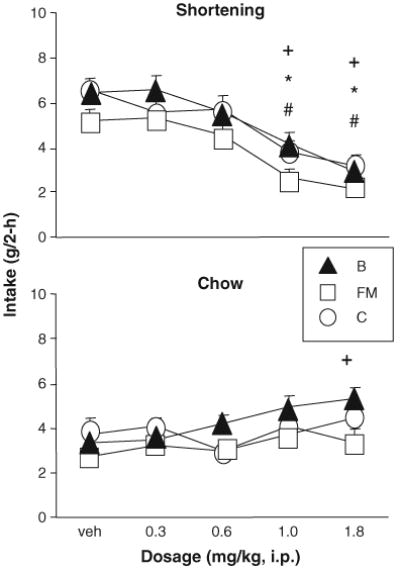
Phase 2: Baclofen significantly reduced shortening intake in rats that had been bingeing for brief (FM, C) as well as extended (B) periods of time. Baclofen stimulated intake of the simultaneously available chow in the B group and had no effect on intake of the simultaneously available chow in the FM and C groups. +, *, # indicate significant difference from vehicle, p <0.05, for B, FM, and C, respectively.
Baclofen (1.0, 1.8 mg/kg) significantly reduced 2-h shortening intake (g) in all three groups [main effect of dosage: F(4,107) = 34.27, p < 0.0001; 1.0, 1.8 p ≤ 0.05 relative to vehicle, Tukey’s HSD]. There was no effect of group, and no interaction. Individual analyses showed the same effect: B [ F(4,36)=15.10, p <0.0001], FM [ANOVA, F(4, 36) = 9.09, p < 0.0001], and C [ F(4, 35) = 12.47, p <0.0001] (1, 1.8 p ≤ 0.05 relative to vehicle, Tukey’s HSD). The 1.8 mg/kg dosage produced 55.0%, 59.4%, and 51.4% reductions in shortening intake relative to vehicle in the B, FM, and C groups, respectively (Fig. 2).
3.3.2. 2-h chow (Fig. 2)
Two-way ANOVA revealed a main effect of baclofen dosage on chow intake [ F(4,107)=4.32, p <0.01], but no effect of group and no interaction. The dosage effect was entirely due to effects in the B group, as baclofen (1.8 mg/ kg) significantly increased 2-h chow intake (g) in group B [ANOVA, F(4,36)= 4.40, p <0.01; Tukey’s HSD p ≤ 0.05] but not group FM [ F(4, 36) = 1.61, p > 0.1] or C [ F(4,35)= 1.23, p >0.3].
3.3.3. 2-h total (data not shown)
Two-way ANOVA revealed a significant effect of baclofen dosage on total 2-h gram intake [ F(4,107) = 141.71, p < 0.0001]. There was also a significant effect of group [ F(2,27)=6.64, p < 0.01] as well as a dosage×group interaction [ F(8,107) = 16.46, p < 0.0001]. These results were obtained because 2-h total gram intakes were significantly less than vehicle after 1 and 1.8 mg/kg baclofen in the C group, but not in the B and FM groups (C group ANOVA F(4,35)= 5.69, p <0.01; 1, 1.8 relative to vehicle p ≤ 0.05, Tukey’s HSD).
Two-hour energy intake, on the other hand, was significantly reduced after baclofen in all three groups [main effect of dosage F(4,107)=4.32, p <0.01; no effect of group, no interaction]. In FM (ANOVA, F(4,36)= 6.18, p <0.001] energy intake was lower than vehicle at 1.8 mg/ kg baclofen, while in groups B [ F(4,36)=9.23, p <0.0001] and C [ F(4, 35)= 11.28, p <0.0001] energy intake was significantly lower than vehicle at 1.0 and 1.8 mg/kg baclofen (Tukey’s HSD p ≤ 0.05).
3.4. Phase 3
3.4.1. 2-h shortening (Fig. 3)
Fig. 3.
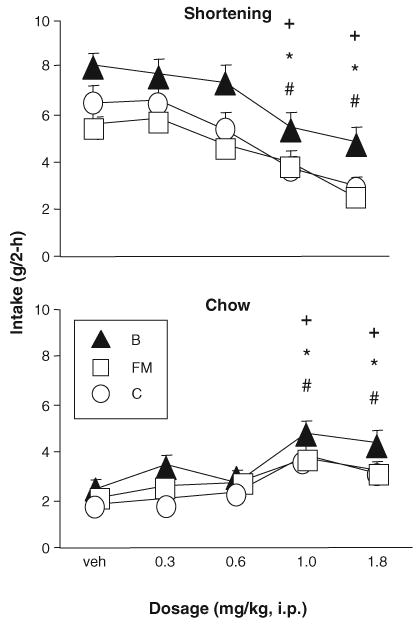
Phase 3: Baclofen significantly reduced shortening intake and stimulated intake of the simultaneously available chow in rats with Monday, Wednesday, and Friday access to shortening (B) as well as rats with daily access to shortening (FM, C).+, *, # indicate significant difference from vehicle, p <0.05, for B, FM, and C, respectively.
Baclofen (1.0, 1.8 mg/kg) significantly reduced 2-h shortening intake (g) in all three groups [main effect of dosage F(4,108)=28.51, p <0.0001; 1.0, 1.8 mg/kg relative to vehicle p ≤ 0.05, Tukey’s HSD]. There was also a main effect of group [ F(2,27)=5.67, p <0.01; B>FM p ≤ 0.05, Tukey’s HSD] but no interaction. Individual analyses confirmed that 1.0 and 1.8 mg/kg reduced shortening intake in all three groups [B: F(4,36)= 9.03, p <0.0001, FM: F(4, 36) = 12.20, p < 0.0001, and C: F(4, 36) = 9.64, p <0.0001; 1.0, 1.8 relative to vehicle p ≤ 0.05, Tukey’s HSD]. The 1.8 mg/kg dosage produced 43.9%, 54.9%, and 53.4% reductions in shortening intake relative to vehicle in the B, FM, and C groups, respectively (Fig. 3).
3.4.2. 2-h chow (Fig. 3)
In contrast to the effects on shortening, baclofen (1.0, 1.8 mg/kg) significantly increased 2-h chow intake (g) in all three groups [main effect of dosage F(4, 108) = 19.92, p <0.0001; 1.0, 1.8 mg/kg relative to vehicle p ≤ 0.05, Tukey’s HSD]. There was also a main effect of group [ F(2,27)=4.84, p <0.05, B>C p ≤ 0.05 Tukey’s HSD], but no interaction. Individual analyses confirmed that 1.0 and 1.8 mg/kg stimulated chow intake in all three groups [B: F(4,36)= 6.80, p <0.001, FM: F(4, 36)= 6.69, p <0.001, and C: F(4,36)= 7.65, p <0.001; 1.0, 1.8 mg/kg relative to vehicle p ≤ 0.05, Tukey’s HSD).
3.4.3. 2-h total (data not shown)
There was a main effect of baclofen dosage [ F(4,108)= 8.08, p < 0.0001] and of group [ F(2,27)=8.93, p <0.01] on 2-h total gram intake. Baclofen (1.8 mg/kg) significantly reduced 2-h total intake (g) in group C [ANOVA, F(4, 36) = 4.23, p < 0.01; p ≤ 0.05 relative to vehicle, Tukey’s HSD] but not groups FM [ F(4, 36) = 2.32, p >0.07] or B [4,36] =2.35, p >0.07].
Baclofen did, however, reduce 2-h total energy intake in all three groups [main effects of dosage: F(4,108)=19.17, p < 0.0001, and group: F(2,17)=5.91, p <0.01; no interaction]. In groups B [ F(4,36)=6.46, p < 0.001] and C [ F(4,36)=7.94, p <0.001], 2-h energy intake was significantly lower than vehicle at 1.0 and 1.8 mg/kg ( p ≤ 0.05, Tukey’s HSD). In group FM [ANOVA, F(4,36)=5.65, p < 0.01], energy intake was lower after 1.8 mg/kg than after vehicle [ p ≤ 0.05, Tukey’s HSD].
4. Discussion
Baclofen (1, 1.8 mg/kg) significantly reduced 2-h shortening intake, while simultaneously having no effect on or increasing 2-h chow intake, under limited access binge-type conditions. Baclofen was effective in all three conditions tested, i.e. when rats had extensive experience with shortening, when rats were first exposed to shortening, and when shortening was provided under relatively frequent (daily) or infrequent (MWF) access schedules.
The fact that baclofen reduced intake of shortening without reducing intake of the continuously available chow demonstrates that the baclofen-induced attenuation of shortening intake was not due to a generalized disruption of behavior. Indeed, in most cases, baclofen appears to have ‘‘normalized’’ the eating behavior of the rats by reducing consumption of the binge food (shortening) and increasing consumption of the simultaneously available non-binge food (chow). This selective reduction in intake was not due to differences between the baseline shortening and chow intakes, since the amount of shortening consumed by the B group (6.09 g) was comparable to the amount of chow consumed by the C group (5.88 g) during the 2-h test period in phase 1. In addition, the total amount of fat consumed was not critical to the effects. This was clearly demonstrated in phase 1, as the B and FM groups consumed the same cumulative amount of fat (and other nutrients), but baclofen only reduced intake in the B group. This would suggest that the manner in which the fat is consumed is more important to the effects of baclofen than the total amount consumed. Finally, results obtained in the FM and C groups in phase 2 demonstrate that the reductions in shortening intake were not simply due to the stimulation of chow intake. In these two groups, baclofen significantly reduced shortening intake while having no effect on intake of the simultaneously available chow.
The effects of baclofen on chow intake in this study are similar to effects reported by other groups, i.e. baclofen generally either has no effect on or increases food intake [21,22,36–50]. However, the effects on shortening intake are new. Only a few studies have shown baclofen-induced reductions in food intake. Two of these studies required an operant response (lever press or nose poke). In one study, baclofen reduced lever pressing for nicotine at lower dosages than were required to reduce responding for 45 mg food pellets [26]. This suggests that the reductions in food-maintained responding may have been due to non-specific effects at the higher baclofen dosages. In the other study, baclofen reduced lever pressing maintained by sucrose. However, the rats had a history of ethanol self-administration [65], and a crossover design was not used. There is evidence that even a single exposure to ethanol can produce long lasting potentiation of GABA synapses in the VTA, and that this potentiation may involve GABA-B receptors [66]. Therefore, it is not known if the prior ethanol exposure affected the sucrose results in the Anstrom et al. study [65]. In other studies, relatively large dosages of baclofen were administered [32,67,68]. Since baclofen can have muscle relaxant effects at higher dosages, it is likely that the reductions in food intake in those studies simply reflected an overall non-specific disruption of behavior.
The results obtained in this study are similar to those that have been reported for drug self-administration, i.e. baclofen reduced consumption of a preferred substance. In other studies, for instance, baclofen selectively reduced cocaine and ethanol self-administration at dosages that did not reduce food-maintained responding [21,22,69–72]. This suggests that binge-type eating, as induced by limited access protocols, may be similar in some ways to drug self-administration. This would be of interest given the clinical co-morbidity that exists between binge-eating and substance abuse/dependence [7–18].
The present study also showed that baclofen would reduce shortening intake in rats with a history of limited access as well as in rats that had just started the limited access protocol. Specifically, in phase 2, baclofen (1.0, 1.8 mg/kg) reduced 2-h shortening intake in B, which had an established pattern of limited-access induced bingeing, but also reduced 2-h shortening intake in FM and C, which were just acquiring the limited access-induced behavior. These results show that baclofen is equally effective at reducing 2-h shortening intake in both the acquisition and maintenance stages of bingeing induced by limited access, while having no effect on, or stimulating, chow intake. Others have shown that baclofen can reduce cocaine and ethanol self-administration during the early period when the behavior is being acquired [73,74]. Thus, repeatedly engaging in self-administration is not required for baclofen to be effective. This is interesting not only mechanistically, but also therapeutically, as therapy could be implemented during the early stages of the addictive process. The present results are consistent with these previous findings, and strengthen the proposition that bingeing on fat, as induced by the limited access protocol, may be similar in some ways to self-administration of ethanol and cocaine.
Baclofen also reduced shortening intake in rats maintained on daily access to shortening. On the daily access schedule, intakes during the limited access period were smaller relative to intakes on the MWF access schedule, as previously reported (51–54). The fact that baclofen was equally effective under both access schedules provides additional evidence that the effects were not baseline dependent. In addition, this result indicates that limited access protocols are responsive to GABA-B activation whether those protocols promote larger (MWF) or smaller (Daily) intakes in brief periods of time. Whether the results reported here are specific to fat or will generalize to other palatable foods under limited access conditions remains to be determined. Others have reported minimal effects of baclofen on intake of and responding for palatable foods such as sucrose and milk, suggesting that palatability in-and-of itself cannot explain our results [36,65,69–71,75]. Rather, the manner in which a palatable food is consumed and/or the fat content of the food may be critical.
Baclofen reduced shortening intake under the present limited access binge-type conditions. Previous reports have shown no effect of two peptides thought to be involved in the regulation of fat intake (galanin and enterostatin) using similar protocols [76–78]. Taken together, these findings indicate that food intake induced by limited access is different from food intake induced by other protocols. The mechanisms that account for the food intake reducing effect of baclofen in the present report are not known, and may or may not involve alterations in GABA-B receptors. It is possible, for instance, that bingeing on fat could result in differential up- or down-regulation of pre- and post-synaptic GABA-B receptors. Fat has been shown to increase brain GABA [79], and others have reported increased GABA release coupled with down-regulation of pre- but not post-synaptic GABA-B receptors after a regimen of intermittent cocaine in rats [80]. Intermittent episodes of fat consumption might produce a similar profile of events. This would enhance baclofen’s already strong post-synaptic inhibitory actions in regions of the brain relevant to food intake and drug abuse, such as the ventral tegmental area [81–84], while at the same time reducing its pre-synaptic actions [85]. The net result could be increased sensitivity to the effects of baclofen, i.e. a shift to the left in the baclofen dose-effect function. Alternatively, GABA-B receptor activation might be enhanced, not because of direct effects on the GABA-B receptor itself, but indirectly because of alterations in the actions of other neural substrates that interact with GABA-B receptors. Further experimentation will be needed in order to determine the mechanisms by which baclofen exerts its effects under the present conditions, as well as to determine the specific contributions of the physical/chemical characteristics of the shortening (e.g. texture, fatty acid composition), palatability, access conditions, etc. to these effects.
In conclusion, the present results demonstrate that baclofen can reduce fat intake when access is limited to brief periods of time under non-food-deprived conditions, i.e. baclofen can reduce the size of a fat binge as induced by the limited access protocol. The effects of baclofen reported here are similar to the effects of baclofen on drug self-administration reported by others. The present results suggest that repeated intermittent excessive behavior directed toward obtaining and consuming commodities for which access is limited (such as drugs of abuse, or ‘‘forbidden’’ foods) may involve GABA-B receptors and may be amenable to treatment with GABA-B agonists. Indeed, recent clinical trials indicate the potential promise of this approach in the treatment of some of the chemical addictions [29–35].
Acknowledgments
Support provided by the National Institute of Mental Health (MH067943), and the Penn State University College of Health and Human Development.
References
- 1.Plotnick GD, Corretti MC, Vogel RA. Effect of antioxidant vitamins on the transient impairment of endothelium-dependent brachial artery vasoactivity following a single high-fat meal. JAMA. 1997;278:1682–6. [PubMed] [Google Scholar]
- 2.Beiles CB, Rogers G, Upjohn J, Wise AG. Gastric dilatation and necrosis in bulimia: a case report. Australas Radiol. 1992;36:75 – 6. doi: 10.1111/j.1440-1673.1992.tb03083.x. [DOI] [PubMed] [Google Scholar]
- 3.Abdu RA, Garritano D, Culver O. Acute gastric necrosis in anorexia nervosa and bulimia. Two case reports. Arch Surg. 1987;122:830– 2. doi: 10.1001/archsurg.1987.01400190096021. [DOI] [PubMed] [Google Scholar]
- 4.Marcus M. Binge eating in obesity. In: Fairburn CG, Wilson GT, editors. Binge eating: nature, assessment, and treatment. New York: The Guilford Press; 1993. pp. 77–96. [Google Scholar]
- 5.Safer DL, Lively TJ, Telch CF, Agras WS. Predictors of relapse following successful dialectical behavior therapy for binge eating disorder. IJED. 2002;32:155–63. doi: 10.1002/eat.10080. [DOI] [PubMed] [Google Scholar]
- 6.Halmi KA, Agras WS, Mitchell J, Wilson GT, Crow S, Bryson SW, et al. Relapse predictors of patients with bulimia nervosa who achieved abstinence through cognitive behavioral therapy. Arch Gen Psychiatry. 2002;59:1105– 9. doi: 10.1001/archpsyc.59.12.1105. [DOI] [PubMed] [Google Scholar]
- 7.Barry DT, Grilo CM, Masheb RM. Gender differences in patients with binge eating disorder. Int J Eat Disord. 2002;31:63–70. doi: 10.1002/eat.1112. [DOI] [PubMed] [Google Scholar]
- 8.Braun DL, Sunday SR, Halmi KA. Psychiatric comorbidity in patients with eating disorders. Psychol Med. 1994;24:859– 67. doi: 10.1017/s0033291700028956. [DOI] [PubMed] [Google Scholar]
- 9.Brewerton TD, Lydiard RB, Herzog DB, Brotman AW, O’neill PM, Ballenger JC. Comorbidity of axis I psychiatric disorders in bulimia nervosa. J Clin Psychiatry. 1995;56:77– 80. [PubMed] [Google Scholar]
- 10.Bulik CM, Sullivan PF, Kendler KS. Medical and psychiatric morbidity in obese women with and without binge eating. IJED. 2002;32:72 – 8. doi: 10.1002/eat.10072. [DOI] [PubMed] [Google Scholar]
- 11.Bushnell JA, Wells JE, McKenzie JM, Hornblow AR, Oakley-Browne MA, Joyce PR. Bulimia comorbidity in the general population and in the clinic. Psychol Med. 1994;24:605– 11. doi: 10.1017/s0033291700027756. [DOI] [PubMed] [Google Scholar]
- 12.Carlat DJ, Camargo CA, Jr, Herzog DB. Eating disorders in males: a report on 135 patients. Am J Psychiatry. 1997;154:1127– 32. doi: 10.1176/ajp.154.8.1127. [DOI] [PubMed] [Google Scholar]
- 13.Dohm FA, Striegel-Moore RH, Wilfley DE, Pike KM, Hook J, Fairburn CG. Self-harm and substance use in a community sample of black and white women with binge eating disorder or bulimia nervosa. IJED. 2002;32:389– 400. doi: 10.1002/eat.10104. [DOI] [PubMed] [Google Scholar]
- 14.Grilo CM, Levy KN, Becker DF, Edell WS, McGlashan TH. Eating disorders in female inpatients with versus without substance use disorders. Addict Behav. 1995;20:255– 60. doi: 10.1016/0306-4603(94)00065-4. [DOI] [PubMed] [Google Scholar]
- 15.Jackson TD, Grilo CM. Weight and eating concerns in outpatient men and women being treated for substance abuse. Eat Weight Disord. 2002;7:276–83. doi: 10.1007/BF03324973. [DOI] [PubMed] [Google Scholar]
- 16.O’brien KM, Vincent NK. Psychiatric comorbidity in anorexia and bulimia nervosa: nature, prevalence, and causal relationships. Clin Psychol Rev. 2003;23:57–74. doi: 10.1016/s0272-7358(02)00201-5. [DOI] [PubMed] [Google Scholar]
- 17.Stice E, Burton EM, Shaw H. Prospective relations between bulimic pathology, depression, and substance abuse: unpacking comorbidity in adolescent girls. J Consult Clin Psychol. 2004;72:62– 71. doi: 10.1037/0022-006X.72.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiederman MW, Pryor T. Substance use among women with eating disorders. Int J Eat Disord. 1996;20:163–8. doi: 10.1002/(SICI)1098-108X(199609)20:2<163::AID-EAT6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 19.Brebner K, Ahn S, Phillips AG. () Attenuation of d-amphetamine self-administration by baclofen in the rat: behavioral and neurochemical correlates. Psychopharmacology (Berl) 2005;177:409– 17. doi: 10.1007/s00213-004-1968-6. [DOI] [PubMed] [Google Scholar]
- 20.Brebner K, Childress AR, Roberts DC. A potential role for GABA(B) agonists in the treatment of psychostimulant addiction. Alcohol Alcohol. 2002;37:478– 84. doi: 10.1093/alcalc/37.5.478. [DOI] [PubMed] [Google Scholar]
- 21.Brebner K, Phelan R, Roberts DCS. Intra-VTA baclofen attenuates cocaine self-administration on a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 2000;66:857– 62. doi: 10.1016/s0091-3057(00)00286-0. [DOI] [PubMed] [Google Scholar]
- 22.Brebner K, Phelan R, Roberts DCS. Effect of baclofen on cocaine self administration in rats reinforced under fixed-ratio 1 and progressive ratio schedules. Psychopharmacology. 2000;148:314–21. doi: 10.1007/s002130050056. [DOI] [PubMed] [Google Scholar]
- 23.Corrigall WA, Coen KM, Adamson KL, Chow BLC, Zhang J. Response of nicotine self-administration in the rat to manipulations of mu-opioid and g-aminobutyric acid receptors in the ventral tegmentum area. Psychopharmacology. 2000;149:107– 14. doi: 10.1007/s002139900355. [DOI] [PubMed] [Google Scholar]
- 24.Cousins MS, Roberts DC, de Wit H. GABA(B) receptor agonists for the treatment of drug addiction: a review of recent findings. Drug Alcohol Depend. 2002;65:209– 20. doi: 10.1016/s0376-8716(01)00163-6. [DOI] [PubMed] [Google Scholar]
- 25.Di Ciano P, Everitt BJ. The GABA(B) receptor agonist baclofen attenuates cocaine- and heroin-seeking behavior by rats. Neuro-psychopharmacology. 2003;28:510– 8. doi: 10.1038/sj.npp.1300088. [DOI] [PubMed] [Google Scholar]
- 26.Paterson NE, Froestl W, Markou A. The GABA-B receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology. 2004;72:179–86. doi: 10.1007/s00213-003-1637-1. [DOI] [PubMed] [Google Scholar]
- 27.Ranaldi R, Poeggel K. Baclofen decreases methamphetamine self-administration in rats. Neuroreport. 2002;13:1107– 10. doi: 10.1097/00001756-200207020-00007. [DOI] [PubMed] [Google Scholar]
- 28.Stromberg MF. The effect of baclofen alone and in combination with naltrexone on ethanol consumption in the rat. Pharmacol Biochem Behav. 2004;78:743– 50. doi: 10.1016/j.pbb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Addolorato G, Caputo F, Capristo E, Domenicali M, Bernard M, Janiri L, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double blind randomized controlled study. Alcohol Alcohol. 2002;37:504– 8. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- 30.Assadi SM, Radgoodarzi R, Ahmadi-Abhari SA. Baclofen for maintenance treatment of opioid dependence: a randomized double-blind placebo-controlled clinical trial. BMC Psychiatry. 2003;3:16. doi: 10.1186/1471-244X-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cousins MS, Stamat HM, deWit H. Effects of a single dosage of baclofen on self-reported subjective effects and tobacco smoking. Nicotine Tob Res. 2001;3:123 – 9. doi: 10.1080/14622200110042624. [DOI] [PubMed] [Google Scholar]
- 32.Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37:504–8. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- 33.Flannery BA, Garbutt JC, Cody MW, Renn W, Grace K, Osborne M, et al. Baclofen for alcohol dependence: a preliminary open-label study. Alcohol Clin Exp Res. 2004;28:1517– 23. doi: 10.1097/01.alc.0000141640.48924.14. [DOI] [PubMed] [Google Scholar]
- 34.Ling W, Shoptaw S. Baclofen as a cocaine anti-craving medication: a preliminary clinical study. Neuropsychopharmacology. 1998;18:403–4. doi: 10.1016/S0893-133X(97)00128-0. [DOI] [PubMed] [Google Scholar]
- 35.Shoptaw A, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charuvastra VC, et al. Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry. 2003;64:1440–8. doi: 10.4088/jcp.v64n1207. [DOI] [PubMed] [Google Scholar]
- 36.Ebenezer IS. Intraperitoneal administration of baclofen increases consumption of both solid and liquid diets in rats. Eur J Pharmacol. 1995;273:183– 5. doi: 10.1016/0014-2999(94)00707-e. [DOI] [PubMed] [Google Scholar]
- 37.Ebenezer IS, Patel SM. Effects of the GABAB receptor agonists baclofen and 3-aminopropylphosphinic acid (3-APA) on food intake in rats. Methods Find Exp Clin Pharmacol. 2004;26:627–30. doi: 10.1358/mf.2004.26.8.863728. [DOI] [PubMed] [Google Scholar]
- 38.Ebenezer IS, Pringle AK. The effect of systemic administration of baclofen on food intake in rats. Neuropharmacology. 1992;31:39– 42. doi: 10.1016/0028-3908(92)90158-l. [DOI] [PubMed] [Google Scholar]
- 39.Ebenezer IS. The effect of intracerebroventricular administration of baclofen on food intake in rats. Neuroreport. 1990;1:73–6. doi: 10.1097/00001756-199009000-00020. [DOI] [PubMed] [Google Scholar]
- 40.Ebenezer IS. Baclofen pretreatment attenuates the suppressant effect of intraperitoneal administration of cholecystokinin (CCK) on food intake in rats. Brain Res Bull. 1996;41:269– 71. doi: 10.1016/s0361-9230(96)00188-8. [DOI] [PubMed] [Google Scholar]
- 41.Echo JA, Lamonte N, Ackerman TF, Bodnar RJ. Alterations in food intake elicited by GABA and opioid agonists and antagonists administered into the ventral tegmental region of rats. Physiol Behav. 2002;76:107–16. doi: 10.1016/s0031-9384(02)00690-x. [DOI] [PubMed] [Google Scholar]
- 42.Higgs S, Barber DJ. Effects of baclofen on feeding behavior examined in the runway. Prog Neuro-Psychopharmacol Biol Psychiatry. 2004;28:405– 8. doi: 10.1016/j.pnpbp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Jonaidi H, Babapour V, Denbow DM. GABAergic control of food intake in the meat-type chickens. Physiol Behav. 2002;76:465–8. doi: 10.1016/s0031-9384(02)00692-3. [DOI] [PubMed] [Google Scholar]
- 44.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655– 63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minano FJ, Meneres Sancho MS, Sancibrian M, Salinas P, Myers RD. GABAA receptors in the amygdala: role in feeding in fasted and satiated rats. Brain Res. 1992;586:104–10. doi: 10.1016/0006-8993(92)91377-q. [DOI] [PubMed] [Google Scholar]
- 46.Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–40. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoaib M, Swanner LS, Beyer CE, Goldberg SR, Schindler CW. The GabaB agonist baclofen modifies cocaine self-administration in rats. Behav Pharmacol. 1998;9:195 –206. [PubMed] [Google Scholar]
- 48.Ward BO, Somerville EM, Clifton PG. Intraaccumbens baclofen selectively enhances feeding behavior in the rat. Physiol Behav. 2000;68:463– 8. doi: 10.1016/s0031-9384(99)00197-3. [DOI] [PubMed] [Google Scholar]
- 49.Wirtshafter D, Straford TR, Pitzer MR. Studies on the behavioral activation produced by stimulation of GABA-B receptors in the median raphe nucleus. Behav Brain Res. 1993;59:83 – 93. doi: 10.1016/0166-4328(93)90154-i. [DOI] [PubMed] [Google Scholar]
- 50.Znamensky V, Echo JA, Lamonte N, Christian G, Ragnauth A, Bodnar RJ. Gamma-aminobutyric acid receptor subtype antagonists differentially alter opioid-induced feeding in the shell region of the nucleus accumbens in rats. Brain Res. 2001;906:84–91. doi: 10.1016/s0006-8993(01)02558-6. [DOI] [PubMed] [Google Scholar]
- 51.Corwin RL, Wojnicki FHE, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–53. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 52.Corwin RL. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite. 2004;42:139– 42. doi: 10.1016/j.appet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 53.Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. IJED. 2000;28:436–45. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 54.Thomas MA, Rice HB, Weinstock D, Corwin RL. Effects of aging on food intake and body composition in rats. Physiol Behav. 2002;76:487– 500. doi: 10.1016/s0031-9384(02)00800-4. [DOI] [PubMed] [Google Scholar]
- 55.Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–30. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 56.Covasa M, Grahn J, Ritter RC. High fat maintenance diet attenuates hindbrain neuronal response to CCK. Regul Pept. 2000;86:83– 8. doi: 10.1016/s0167-0115(99)00084-1. [DOI] [PubMed] [Google Scholar]
- 57.Covasa M, Grahn J, Ritter RC. Reduced hindbrain and enteric neuronal response to intestinal oleate in rats maintained on a high fat diet. Auton Neurosci. 2000;84:8– 18. doi: 10.1016/S1566-0702(00)00176-4. [DOI] [PubMed] [Google Scholar]
- 58.Horn CC, Friedman MI. Metabolic inhibition increases feeding and brain Fos-like immunoreactivity as a function of diet. Am J Physiol. 1998;275:R448 – 59. doi: 10.1152/ajpregu.1998.275.2.R448. [DOI] [PubMed] [Google Scholar]
- 59.Kelly JF, Joseph JA, Denisova NA, Erat S, Mason RP, Roth GS. Dissociation of striatal GTPase and dopamine release responses to muscarinic cholinergic agonists in F344 rats: influence of age and dietary manipulation. J Neurochem. 1995;64:2755– 64. doi: 10.1046/j.1471-4159.1995.64062755.x. [DOI] [PubMed] [Google Scholar]
- 60.Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–14. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 61.Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high fat diet on synaptic and behavioral plasticity associated to the action of brain derived neurotrophic factor. Neuroscience. 2004;123:429– 40. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 62.Wu A, Ying Z, Gomez-Pinilla F. A saturated-fat diet aggravates the outcome of traumatic brain injury on hippocampal plasticity and cognitive function by reducing brain derived neurotrophic factor. Neuroscience. 2003;119:365– 75. doi: 10.1016/s0306-4522(03)00154-4. [DOI] [PubMed] [Google Scholar]
- 63.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci. 2004;19:1699– 707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- 64.Popova ED, Puzin MN, Kolyvanov GB, Litvin AA. Baclofen pharmacokinetics in rats. Eksp Klin Farmakol. 1995;58:53– 4. [PubMed] [Google Scholar]
- 65.Anstrom KK, Cromwell HC, Markowski T, Woodward DJ. Effect of baclofen on alcohol and sucrose self-administration in rats. Alcohol Clin Exp Res. 2003;27:900–8. doi: 10.1097/01.ALC.0000071744.78580.78. [DOI] [PubMed] [Google Scholar]
- 66.Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074– 82. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bungo T, Izumi T, Kawamura K, Takagi T, Ueda H, Furose M. Intracerebroventricular injection of muscimol, baclofen, or nipecotic acid stimulates food intake in layer-type, but not meat-type, chicks. Brain Res. 2003;993:235– 8. doi: 10.1016/j.brainres.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 68.Zarrindast MR, Hosseini-Nia T, Allah-Maddadi S. Food intake suppressant effect of baclofen in rats. Gen Pharmacol. 1989;20:701– 3. doi: 10.1016/0306-3623(89)90110-9. [DOI] [PubMed] [Google Scholar]
- 69.Janak PH, Michael Gill T. Comparison of the effects of allopreg-nanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30:1–7. doi: 10.1016/s0741-8329(03)00068-5. [DOI] [PubMed] [Google Scholar]
- 70.Petry NM, Heyman GM. Bidirectional modulation of sweet and bitter taste by chlordiazepoxide and Ro 15– 4513: lack of effect with GABA drugs. Physiol Behav. 1997;61:119–26. doi: 10.1016/s0031-9384(96)00351-4. [DOI] [PubMed] [Google Scholar]
- 71.Petry NM. Benzodiazepine-GABA modulation of concurrent ethanol and sucrose reinforcement in the rat. Exp Clin Psychopharmacol. 1997;5:183–94. [PubMed] [Google Scholar]
- 72.Roberts DC, Andrews MM, Vickers GJ. Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology. 1996;15:417–23. doi: 10.1016/0893-133X(96)00002-4. [DOI] [PubMed] [Google Scholar]
- 73.Colombo G, Serra S, Brunetti G, Atzori G, Pani M, Vacca G, et al. The GABA(B) receptor agonists baclofen and CGP 44532 prevent acquisition of alcohol drinking behaviour in alcohol-preferring rats. Alcohol Alcohol. 2002;37:499–503. doi: 10.1093/alcalc/37.5.499. [DOI] [PubMed] [Google Scholar]
- 74.Campbell UC, Morgan AD, Carroll ME. Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self administration in rats. Drug Alcohol Depend. 2002;66:61–9. doi: 10.1016/s0376-8716(01)00185-5. [DOI] [PubMed] [Google Scholar]
- 75.Columbo G, Serra S, Brunetti G, Vacca G, Carai MA, Gessa GL. Suppression by baclofen of alcohol deprivation effect in sardinian alcohol-preferring rats. Drug Alcohol Depend. 2003;70:105–8. doi: 10.1016/s0376-8716(02)00333-2. [DOI] [PubMed] [Google Scholar]
- 76.Corwin RL, Rowe PM, Crawley JN. Galanin and the galanin antagonist M40 do not change fat intake in a fat-chow choice paradigm in rats. Am J Physiol. 1995;269:R511 –18. doi: 10.1152/ajpregu.1995.269.3.R511. [Regulatory Integrative and Comp. Physiol. 38] [DOI] [PubMed] [Google Scholar]
- 77.Rice HB, Corwin RL. Effects of enterostatin on consumption of optional foods in non-food deprived rats. Obes Res. 1998;6:54– 61. doi: 10.1002/j.1550-8528.1998.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 78.Corwin RL, Rice HB. Effects of enterostatin on optional oil or sucrose consumption in non-food deprived rats. Physiol Behav. 1998;65:1– 10. doi: 10.1016/s0031-9384(98)00078-x. [DOI] [PubMed] [Google Scholar]
- 79.Fisler JS, Shimizu H, Bray GA. Brain 3-hydroxybutyrate, glutamate, and GABA in a rat model of dietary obesity. Physiol Behav. 1989;45:571– 7. doi: 10.1016/0031-9384(89)90075-9. [DOI] [PubMed] [Google Scholar]
- 80.Shoji S, Simms D, McDaniel WC, Gallagher JP. Chronic cocaine enhances γ-aminobutyric acid and glutamate release by altering presynaptic and not postsynaptic γ-aminobutyric acid B receptors within the rat dorsolateral septal nucleus. J Pharmacol Exp Ther. 1997;280:129–37. [PubMed] [Google Scholar]
- 81.Klitenick MA, DeWitte P, Kalivas PW. Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. J Neurosci. 1992;12:2623– 32. doi: 10.1523/JNEUROSCI.12-07-02623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Westerink BH, Kwint HF, deVries JB. Eating-induced dopamine release from mesolimibic neurons is mediated by NMDA receptors in the ventral tegmental area: a dual probe microdialysis study. J Neurochem. 1997;69:662–8. doi: 10.1046/j.1471-4159.1997.69020662.x. [DOI] [PubMed] [Google Scholar]
- 83.Westerink BH, Enrico P, Feiman J, deVries JB. The pharmacology of mesocortical dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and prefrontal cortex of the rat brain. J Pharmacol Exp Ther. 1998;285:143– 54. [PubMed] [Google Scholar]
- 84.Xi ZX, Stein EA. Baclofen inhibits heroin self-administration behavior and mesolimbic dopamine release. J Pharmacol Exp Ther. 1999;290:1369–74. [PubMed] [Google Scholar]
- 85.Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19:3723– 30. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


