Abstract
Aims: To review our experience of anti-D immunoglobulin for immune thrombocytopenia (ITP) in patients with primary antibody deficiency.
Methods/patients: A retrospective case notes review of four Rhesus positive patients with ITP and primary antibody deficiency, treated with anti-D. Patients were refractory to steroids and high dose intravenous immunoglobulin (IVIG). Two patients were previously splenectomised.
Results: All patients responded to anti-D immunoglobulin. Improved platelet counts were sustained for at least three months. Side effects included a fall in haemoglobin in all cases; one patient required red blood cell transfusion. Two patients had transient neutropenia (< 1 × 109/litre).
Conclusion: Anti-D immunoglobulin may be an effective treatment for antibody deficiency associated thrombocytopenia, even after splenectomy. Anti-D immunoglobulin may have considerable clinical advantages in this group of patients, where treatments resulting in further immunosuppression are relatively contraindicated.
Keywords: anti-D immunoglobulin, immune thrombocytopenia, primary antibody deficiency, splenectomy
Immune thrombocytopenia (ITP) is a recognised complication of primary immunodeficiency, probably occurring as a result of immune dysregulation.1 Standard treatment is with high dose intravenous immunoglobulin, corticosteroids, and other immunosuppressive agents, or splenectomy in refractory cases.2 Steroids, immunosuppressives, and splenectomy are undesirable in the context of immunodeficiency because they may increase the susceptibility to infection in patients who are already immunocompromised.
Anti-D immunoglobulin has been proposed as an alternative treatment for ITP. Its efficacy in immunocompetent and human immunodeficiency virus (HIV) infected patients is comparable with that of conventional treatments, with response rates of greater than 70%.3 There are important cost benefits.4 The mechanism of action may involve the saturation of macrophage Fc receptors by opsonised red blood cells. Anti-D immunoglobulin is not effective in Rhesus negative individuals or in most immunocompetent patients after splenectomy.3
We report the effect of anti-D treatment on four patients with ITP complicating the primary antibody deficiencies: common variable immunodeficiency (CVID) and IgG subclass with IgA deficiency.
“Steroids, immunosuppressives, and splenectomy are undesirable in the context of immunodeficiency because they may increase the susceptibility to infection in patients who are already immunocompromised”
METHODS AND PATIENTS
We reviewed patients attending our immunodeficiency service who had required anti-D treatment for the management of refractory ITP. Patient characteristics are shown in table 1.
Table 1.
Patient characteristics
| Case | Age (sex) | Duration of ITP | ITP treatment | Antibody deficiency and duration | Alkaline phosphatase (normal range, 25–100 IU/l) | Splenic histology | Anti-D dose |
| 1 | 62 years (female) | 1 year | IVIG, steroids | CVID, 2 years | 170–270 IU/l | NA | 50 μg/kg |
| 2 | 21 years (female) | 15 years | IVIG, steroids, azathioprine, cyclosporin | CVID, 7 years | 150–200 IU/l | Lymphocytosis of red pulp | 50 μg/kg |
| Splenectomy | Focal aggregates of foamy macrophages | ||||||
| 3 | 21 years (female) | 1 year | IVIG, steroids | CVID, 8 years | 68–78 IU/l | NA | 100 μg/kg |
| 4 | 20 years (female) | 5 years | IVIG, steroids, dapsone, cyclosporin, azathioprine, vincristine | IgG2, 4, and IgA deficiency, raised IgM, 15 years | 54–67 IU/l | Follicular hyperplasia | 150 μg/kg |
| Splenectomy | Prominent germinal centres |
CVID, common variable immunodeficiency; ITP, immunec thrombocytopenia; IVIG, intravenous immunoglobulin; NA, not available.
The diagnosis of ITP was made on the basis of thrombocytopenia, blood film, and bone marrow features, and the exclusion of other diagnoses. The diagnosis of primary antibody deficiency was on the basis of a history of repeated bacterial infections (before immunosuppressive treatment), low antibody concentrations (assessed when off immunomodulatory treatment), and the absence of factors known to be associated with secondary immunodeficiency. All patients had thrombocytopenia refractory to steroids and high dose IVIG (1 g/kg for at least two doses). Two patients had relapsed after splenectomy. At the time of the anti-D infusion, three patients were on replacement doses (0.4 g/kg/3 weeks) of IVIG to prevent infection. One patient (patient 4) was taking cyclosporin (100 mg twice daily) at the time of her first set of anti-D treatments and vincristine (2 mg weekly for four doses) and dexamethasone (40 mg daily for four days, monthly) at the time of her second set of anti-D treatments, having failed to respond to dexamethasone alone. Anti-D immunoglobulin was given intravenously over 30 minutes. If the initial dose produced inadequate response, a higher dose was given subsequently. To minimise the risk of viral transmission, we used a detergent treated anti-D product (Win-Rho; Octapharma, Vienna, Austria) and saw no evidence of hepatitis C transmission.
RESULTS
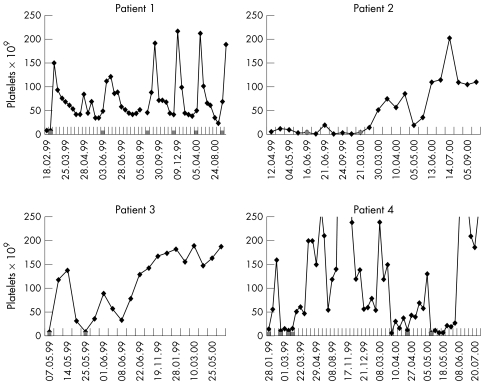
All patients responded to anti-D (fig 1). Three of our patients had a biphasic response with an early small peak and a later prolonged peak. The duration of the response was 125–390 days, and this was not reduced by previous splenectomy. Repeat infusions have also been effective. All patients are currently in remission and off immunosuppressive treatment.
Figure 1.
Platelet response to anti-D immunoglobulin. Anti-D infusions are indicated by the shaded squares along the x axis. Patient 1 has responded repeatedly to anti-D infusions (50 μg/kg). Patient 2 had a suboptimal response to 40 μg/kg anti-D immunoglobulin. This reduced dose was chosen because of previous haemolytic anaemia. She was re-treated nine months later with 50 μg/kg. Patient 3 had a transient response to 50 μg/kg anti-D immunoglobulin and was re-treated after 18 days with 100 μg/kg. Patient 4 had a transient response to doses of 50 and 100 μg/kg anti-D immunoglobulin three days apart. She was re-treated 21 days later with doses of 100, 100 and 150 μg/kg at 10 and five day intervals, respectively. Relapse one year later was treated with two doses of 150 μg/kg at an eight day interval.
All patients had a fall in haemoglobin (of 13–62 g/litre); one required red blood cell transfusion. Transient neutropenia occurred in two patients (nadirs 0.6 and 0.8 × 109/litre, respectively).
Headache, fever, and malaise were treated symptomatically and could be prevented by premedication with an antihistamine and paracetamol.
DISCUSSION
Anti-D immunoglobulin is a well established treatment for ITP in a variety of settings, including HIV associated secondary immunodeficiency.3 Most series report that anti-D immunoglobulin is ineffective after splenectomy.3,5 Our experience suggests that anti-D immunoglobulin may be effective in primary antibody deficiency, even after splenectomy.
Although anti-D immunoglobulin is generally well tolerated, a mean fall in haemoglobin of 8 g/litre is expected and severe haemolysis may occur.3,6 All our patients had a fall in haemoglobin of greater than 10 g/litre and one required red blood cell transfusion. Neutropenia, which occurred in two of our patients, has not been described previously. This limited experience suggests that adverse events may be more common in antibody deficient patients. Whether the presence of pre-existing CVID associated autoimmune haemolytic anaemia and neutropenia increases the risk of cytopenia after anti-D immunoglobulin is unknown.1
The duration of response in our patients with immunodeficiency was longer than that reported in other series, where the median duration of response was 21 days.3
These observations suggest underlying differences in pathogenesis and are not compatible with the proposed mechanism of action of anti-D immunoglobulin. It is possible that, in our patients, the initial competitive saturation is followed by longer lasting changes in phagocyte Fc receptor expression. There have been isolated cases of responses to anti-D immunoglobulin even after splenectomy in immunocompetent patients. These have been attributed to increased platelet uptake by hepatic macrophages after splenectomy.7,8 The granulomatous variant of CVID is associated with thrombocytopenia, probably as a result of increased platelet destruction by activated phagocytes.9,10 Two of our patients (patients 1 and 2) had persistently raised alkaline phosphatase concentrations, although no granulomata were seen on splenic histology of the two patients (patients 2 and 4) who underwent splenectomy. We speculate that our patients may have had activated hepatic macrophages or granulomata, resulting in relapse of thrombocytopenia after splenectomy and explaining the unexpectedly high success rate of anti-D immunoglobulin.
Take home messages.
Anti-D immunoglobulin appeared to be an effective treatment for antibody deficiency associated thrombocytopenia, even after splenectomy
Anti-D immunoglobulin has considerable clinical advantages compared with conventional immunosuppressive treatments in the treatment of immunosuppressed patients
Although anti-D immunoglobulin is generally well tolerated, a fall in haemoglobin is expected and severe haemolysis may occur
Thus, anti-D immunoglobulin should be administered with caution because of the risk of neutropenia and red blood cell destruction. However, it is a valuable non-immunosuppressive, long acting alternative to conventional ITP treatments in primary antibody deficiency, even after splenectomy.
Abbreviations
CVID, common variable immunodeficiency
ITP, immune thrombocytopenia
IVIG, intravenous immunoglobulin
NA, not applicable
REFERENCES
- 1.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol 1999;92:34–48. [DOI] [PubMed] [Google Scholar]
- 2.George JN, Woolf SH, Raskob GE, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood 1996;88:3–40. [PubMed] [Google Scholar]
- 3.Scaradavou A, Woo B, Woloski BMR, et al. Intravenous anti-D treatment of immune thrombocytopenic purpura: experience in 272 patients. Blood 1997;89:2689–700. [PubMed] [Google Scholar]
- 4.Simpson KN, Coughlin CM, Eron J, et al. Idiopathic thrombocytopenic purpura: treatment patterns and an analysis of cost associated with intravenous immunoglobulin and anti-D therapy. Semin Hematol 1998;35:58–64. [PubMed] [Google Scholar]
- 5.Bussel, JB, Graziano, JN, Kimberly RP, et al. Intravenous anti-D treatment of immune thrombocytopenic purpura: analysis of efficacy, toxicity and mechanism of effect. Blood 1991;77:1884–93. [PubMed] [Google Scholar]
- 6.Gaines AR. Acute onset hemoglobinuria and sequalae following Rh(D) immune globulin intravenous administration in immune thrombocytopenic purpura patients. Blood 2000;95:2523–9. [PubMed] [Google Scholar]
- 7.Boughton BJ, Chakraverty R, Baglin TP, et al. The treatment of chronic idiopathic thrombocytopenia with anti-D (Rho) immunoglobulin: its effectiveness, safety and mechanism of action. Clin Lab Haematol 1988;10:275–84. [DOI] [PubMed] [Google Scholar]
- 8.Shibuya A, Danya N, Shinozawa T. Successful anti-D immunoglobulin therapy in refractory chronic idiopathic thrombocytopenic purpura showing reduction in thrombocytes even after splenectomy. Acta Paediatr Jpn 1994;36:297–300. [DOI] [PubMed] [Google Scholar]
- 9.Fasano MB, Sullivan KE, Sarpong SB, et al. Sarcoidosis and common variable immunodeficiency: report of 8 cases and review of the literature. Medicine 1996;75:251–61. [DOI] [PubMed] [Google Scholar]
- 10.Spickett GP, Zhang J, Green T, et al. Granulomatous disease in common variable immunodeficiency: effect on immunoglobulin replacement therapy and response to steroids and splenectomy. J Clin Pathol 1996;49:431–4. [DOI] [PMC free article] [PubMed] [Google Scholar]