Abstract
Aims: To evaluate the clinicopathological significance of p16 expression in the surgical management of squamous cell carcinomas of the oral cavity, oropharynx, hypopharynx, and larynx.
Method: p16 expression in 225 head and neck squamous cell carcinomas (HNSCCs) was studied using an immunohistochemical method and paraffin wax embedded tumour tissues. Associations between p16 expression and clinicopathological features were investigated.
Results: Decreased p16 expression was found in 48% of the tumours. There was a higher frequency of decreased p16 expression in tumours of the larynx compared with those from the pharynx and oral cavity. There was a significant correlation between decreased p16 expression and more advanced T stage. There was no significant correlation between p16 expression and sex, age, tumour grade, nodal metastasis, recurrence, or survival.
Conclusion: There was a high frequency of downregulation of p16 expression in HNSCC. Tumours of the larynx had a significantly higher frequency of weak p16 expression compared with tumours of the oral cavity and pharynx. Downregulation of p16 contributed to cellular proliferation, resulting locally in a more advanced tumour. It had no prognostic significance for nodal metastasis and survival.
Keywords: p16, head and neck, squamous cell carcinoma
The p16 gene is a tumour suppressor gene located at chromosome 9p21. The p16 protein blocks cellular proliferation at the G1–S phase by binding to cyclin dependent kinase 4 (CDK4) or CDK6, preventing the formation of the catalytically active cyclin D–CDK4/CDK6 complex for the release of E2F through phosphorylation of the retinoblastoma protein (pRB)–E2F protein.1–3 Functional loss of p16 has been reported for many human cancers.4,5 Hypermethylation, rather than mutation or deletion, is the main cause of p16 dysfunction.6–8
There are only a few small series that have studied the expression of p16 in head and neck squamous cell carcinoma (HNSCC).9,10 The clinicopathological significance of p16 expression has not been reported in HNSCC. Our study aimed to evaluate the clinicopathological significance of p16 expression in the surgical treatment of squamous cell carcinomas of the oral cavity, pharynx, and larynx. For a better analysis of prognosis in relation to surgical treatment, we only recruited patients who met all three criteria: (1) surgical treatment, (2) squamous cell carcinoma, and (3) carcinoma of the oral cavity, oropharynx, hypopharynx, or larynx.
MATERIALS AND METHODS
Archival, formalin fixed tumour specimens from patients with HNSCC who had undergone surgical treatment in the department of surgery, Queen Mary Hospital between 1978 and 2000 were retrieved from the department of pathology for immunohistochemical staining by means of an anti-p16 monoclonal antibody. In total, there were 225 patients—113 of whom received primary surgical treatment and 112 of whom had radiotherapy failure for surgical salvage. We evaluated p16 expression for its clinicopathological significance. There were 184 men and 41 women. The median age was 64 years (range, 16–92). Fifty four tumours were in the oral cavity, 24 the pharynx (12 oropharynx and 12 hypopharynx), and 147 the larynx. The number of patients with tumours at each of these sites reflects the relative proportion of patients who undergo surgical treatment in our department. The pathological stages were 17 stage I, 32 stage II, 49 stage III, and 127 stage IV. The median follow up of those patients who had no tumour at the last follow up was 58 months.
Immunohistochemical staining was carried out by means of an avidin–biotin complex (ABC) immunoperoxidase method. We used similar protocols to those used previously for the study of p16 expression.9–11 The specimens were cut at 4 μm thickness, dewaxed in xylene for 15 minutes, and rehydrated with ethanol. The slides were treated with 3% hydrogen peroxide for 30 minutes at room temperature. After three washes in phosphate buffered saline, antigen retrieval was performed by microwaving in citrate buffer (pH 6) for five minutes. We used a 1/50 dilution of the monoclonal anti-p16 antibody (sc-1661; Santa Cruz Biotechnology, Santa Cruz, California, USA) and incubation was carried out overnight at 4°C. The sections were then incubated with secondary antibody for 30 minutes. Staining was performed using ABC reagents (Dakopatts, Glostrup, Denmark) and 3,3`-diaminobenzidine/hydrogen peroxide as substrate. The sections were counterstained with Mayer's haematoxylin for 30 seconds and blued in Scot's tap water for three minutes. The normal epithelium adjacent to the tumour nests served as an internal positive control. Squamous cell carcinomas known to be positive for p16 were used in each run of the experiment as external positive controls. For negative controls, the antibody was omitted during the immunohistochemical staining.
The clinical data were unknown to the scorers (PWY and KYL) when the immunohistochemical staining was interpreted. Only those tumour cells with unequivocal brown nuclear staining comparable with the positive controls were regarded as showing positive p16 expression. The percentage of positive tumour cells in each case was counted using a semiquantitative method reported previously.11 Strong p16 expression was defined as more than 50% tumour cells staining positively (fig 1). p16 expression was defined as weak when less than or equal to 50% tumour cells stained positively (fig 2).
Figure 1.
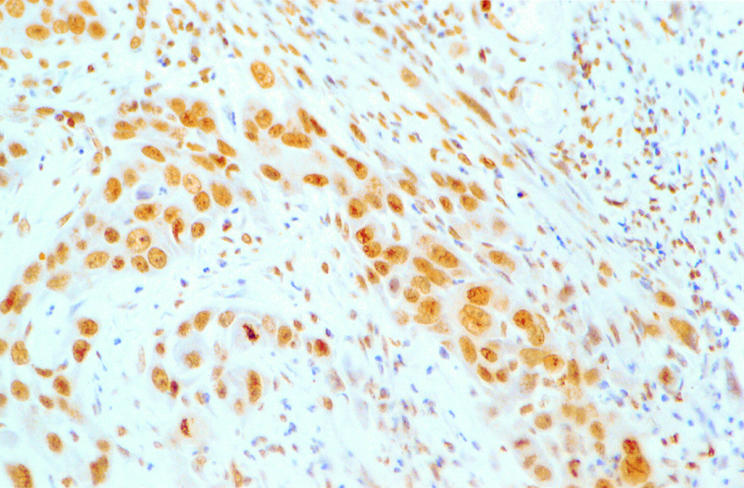
Strong nuclear staining of p16 protein in a squamous cell carcinoma of the oral cavity.
Figure 2.
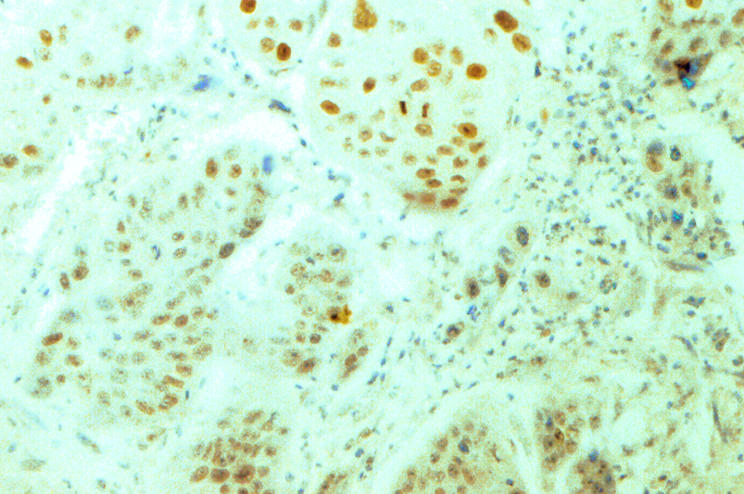
Weak staining of p16 protein in a squamous cell carcinoma of the larynx.
Take home messages.
p16 expression is frequently downregulated in head and neck squamous cell carcinoma
Tumours of the larynx have a significantly higher frequency of weak p16 expression than tumours of the oral cavity and pharynx
Downregulation of p16 contributed to cellular proliferation, resulting locally in a more advanced tumour
p16 expression had no prognostic significance for nodal metastasis or survival
RESULTS
Forty eight percent of the 225 HNSCC samples showed weak expression of p16. Table 1 shows the clinicopathological significance of weak p16 expression. There was a significant difference in the frequency of weak p16 expression of tumours from different anatomical sites including the larynx, oral cavity, and pharynx (oropharynx and hypopharynx). There was also a significant correlation between weak p16 expression and more advanced T stage cancer compared with early cancers. There was also a significantly higher incidence of weak p16 expression in tumours with previous radiotherapy failure. Logistic regression analysis recruiting tumour site, T stage, and previous radiotherapy showed that tumour site was the most important independent factor affecting p16 expression. There was no significant correlation between p16 expression and sex, age, tumour grade, nodal metastasis, or recurrence after surgical treatment.
Table 1.
Clinicopathological significance of p16 expression
| Factors | Weak p16 expression (%) | p Value |
| Sex | ||
| Male | 48% | χ2 test: p=0.604 |
| Female | 44% | |
| Age | ||
| Mean age weak p16 expression: 64 years | t test: p=0.236 | |
| Mean age strong p16 expression: 62 years | ||
| Grade | ||
| Well | 50% | Spearman: p=0.501 |
| Moderate | 47% | |
| Poor | 42% | |
| Sites | ||
| Oral cavity | 15% | χ2 test: p<0.001 |
| Pharynx | 25% | |
| Larynx | 63% | |
| Stage | ||
| I | 35% | Spearman: p=0.210 |
| II | 47% | |
| III | 43% | |
| IV | 51% | |
| T stage | ||
| 1 | 34% | Spearman: p=0.043 |
| 2 | 35% | |
| 3 | 55% | |
| 4 | 52% | |
| N stage | ||
| 0 | 52% | Spearman: p=0.213 |
| 1 | 21% | |
| 2 | 54% | |
| 3 | 33% | |
| Previous radiotherapy | ||
| No | 35% | χ2 test: p<0.001 |
| Yes | 61% | |
| Recurrence | ||
| No | 41% | χ2 test: p=0.706 |
| Yes | 46% | |
At the last follow up, 100 patients had died of tumour or were alive with tumour. The five year actuarial disease free survival rate was 56% for patients with weak expression of p16 and 47% for those with strong expression (life table method; Wilcoxon statistics, p = 0.522).
DISCUSSION
We have shown a high frequency (48%) of reduced p16 expression in HNSCC. The reported frequencies of negative expression (the definitions of negative expression were not mentioned in the reports) of p16 in HNSCC were 83% in 29 tumours (sites not mentioned) by Reed and colleagues9 and 67% in 27 maxillofacial squamous cell carcinomas by Zhao et al.10 However, the clinicopathological significance was not analysed in these two previous reports. Because we were interested to know the clinicopathological significance in relation to surgical management, we excluded patients without surgical treatment.
p16 expression was not significantly related to sex, age of patients, or grade of tumours. However, decreased expression of p16 was found more frequently in carcinomas of the larynx compared with the pharynx (the oropharynx and hypopharynx had similar incidences) and the oral cavity. There are significant differences in the clinical features, risk of nodal metastasis, and prognosis for squamous cell carcinoma of the oral cavity, pharynx, and larynx, despite their similar histological features.12–17 Different p16 expression patterns in distinct sites in the head and neck region may be one of the genetic abnormalities that have contributed to their differences in clinical behaviour.
Weak expression of p16 was also found more frequently in advanced T stages. In HNSCC, the larger the tumour size, the higher the T stage. Because the p16 protein is an important cell cycle regulatory protein, the underexpression of this protein will allow cancer cells to proliferate without control. In HNSCC, it might indicate that weak expression of p16 contributes to a more proliferative cancer behaviour so that tumours with weak p16 expression would tend to be of a larger size and higher T stage.
The most common treatment failure in HNSCC is nodal metastasis.12–16 Although downregulation of p16 expression contributed significantly to tumour proliferation and tumour size, it did not significantly affect nodal metastasis. p16 gene expression is unrelated to metastasis phenotype. However, p16 expression in HNSCC had no prognostic significance for survival in patients who were treated by surgery. The result of prognosis of surgical patients cannot be projected for those patients who are treated by primary radiotherapy and or chemotherapy.
In conclusion, downregulation of p16 expression was seen frequently in HNSCC. Tumours of the larynx, pharynx, and oral cavity had significantly different incidences of decreased expression of p16. Downregulation of p16 significantly contributed to cellular proliferation and tumour size. However, it has no prognostic significance for nodal metastasis and survival.
Acknowledgments
This study was supported by the Kadoorie Cancer Research Fund and a research grant of The University of Hong Kong.
Abbreviations
CDK, cyclin dependent kinase
HNSCC, head and neck squamous cell carcinomas
pRB, retinoblastoma protein
REFERENCES
- 1.Stone S, Jiang P, Dayananth P, et al. Complex structure and regulation of the p16 (MTS1) locus. Cancer Res 1995;55:2988–94. [PubMed] [Google Scholar]
- 2.Shapiro GI, Edwards CD, Kobzik L, et al. Reciprocal Rb inactivation and p16 expression in primary lung cancers and cell lines. Cancer Res 1995;55:505–9. [PubMed] [Google Scholar]
- 3.Merlo A, Herman JG, Li M, et al. 5` CpG island methylation is associated with transcriptional silencing of the tumor suppressor p16/CDKN2/MTS1 in human cancers. Nat Med 1995;1:686–92. [DOI] [PubMed] [Google Scholar]
- 4.Liggett WH, Sidransky D. Role of the p16 tumor suppressor gene in cancer. J Clin Oncol 1998;16:1197–206. [DOI] [PubMed] [Google Scholar]
- 5.Herman JG, Civin CI, Issa JPJ, et al. Distinct patterns of inactivation of p15 and p16 characterize the major types of haematological malignancies. Cancer Res 1997;57:837–41. [PubMed] [Google Scholar]
- 6.Gonzalez MV, Pello MF, Lopez-Larrea C, et al. Deletion and methylation of the tumor suppressor gene p16/CDKN2 in primary head and neck squamous cell carcinoma. J Clin Pathol 1997;50:509–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waber P, Dlugosz S, Cheng QC, et al. Genetic alterations of chromosome band 9p21–22 in head and neck cancer are not restricted to p16Ink4a. Oncogene 1997;15:1699–704. [DOI] [PubMed] [Google Scholar]
- 8.Lang JC, Tobin EJ, Knobloch TJ, et al. Frequent mutation of p16 in squamous cell carcinoma of head and neck. Laryngoscope 1998;108:923–8. [DOI] [PubMed] [Google Scholar]
- 9.Reed AL, Califano J, Cairns P, et al. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res 1996;56:3630–3. [PubMed] [Google Scholar]
- 10.Zhao Y, Zhang S, Fu B, et al. Abnormalities of tumor suppressor genes p16 and p15 in primary maxillofacial squamous cell carcinomas. Cancer Genet Cytogenet 1999;112:26–33. [DOI] [PubMed] [Google Scholar]
- 11.Fujita M, Enomoto T, Haba T, et al. Alteration of p16 and p15 genes in common epithelial ovarian tumors. Int J Cancer 1997;74:148–55. [DOI] [PubMed] [Google Scholar]
- 12.Yuen APW, Lam KY, Chan CLA, et al. Clinicopathological analysis of elective neck dissection for N0 neck of early oral tongue carcinoma. Am J Surg 1999;177:90–2. [DOI] [PubMed] [Google Scholar]
- 13.Yuen APW, Wei WI, Wong YM, et al. Elective neck dissection versus observation in the surgical treatment of early oral tongue carcinoma. Head Neck 1997;19:583–8. [DOI] [PubMed] [Google Scholar]
- 14.Ho CM, Lam KH, Wei WI, et al. Squamous cell carcinoma of the hypopharynx—analysis of treatment results. Head Neck 1993;15:405–12. [DOI] [PubMed] [Google Scholar]
- 15.Yuen APW, Ho CM, Wei WI, et al. Analysis of recurrence after surgical treatment of advanced laryngeal carcinoma. J Laryngol Otol 1995;109:1063–7. [DOI] [PubMed] [Google Scholar]
- 16.Li XM, Wei WI, Guo XF, et al. Cervical lymph node metastasis patterns of squamous carcinomas in the upper aerodigestive tract. J Laryngol Otol 1996;110:937–41. [DOI] [PubMed] [Google Scholar]
- 17.Yuen APW, Ho CM, Wei WI, et al. Prognosis of recurrent laryngeal carcinoma after laryngectomy. Head Neck 1995;17:526–30. [DOI] [PubMed] [Google Scholar]


