Abstract
Monoclonal antibodies have been used in clinical diagnosis for many years but it is only now that these agents are being licensed for clinical treatments. This review will focus on UK licensed monoclonal antibodies highlighting their clinical benefits, limitations, and side effects.
Keywords: monoclonal antibodies, recombinant technology, chimaeric antibodies, tumour necrosis factor α, Crohn's disease, rheumatoid arthritis
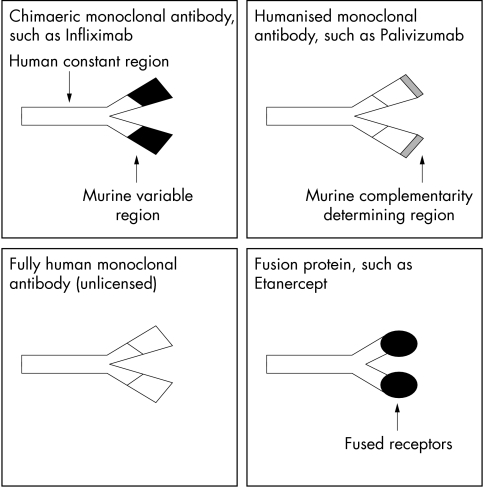
The term monoclonal antibody refers to a single specificity antibody derived from a single B cell clone and initially these were created by fusing B cells (from immunised mice) with lymphoma cells. In clinical practice, however, the administration of murine antibodies induces human antimouse antibodies that may lead to allergic reactions and reduced efficacy. These difficulties have been partially overcome by recombinant technology to develop less immunogenic monoclonal antibodies. Chimaeric antibodies contain only a murine variable fragment whereas humanised antibodies only have a murine complementarity determining region (fig 1).
Figure 1.
Diagram to show different types of monoclonal antibody molecules being developed for treatment.
“The administration of murine antibodies induces human antimouse antibodies that may lead to allergic reactions and reduced efficacy”
GASTROENTEROLOGY
Crohn's disease is a relapsing condition characterised by transmural inflammation in the gastrointestinal tract. The cytokine tumour necrosis factor α (TNF-α) appears to be central to the immunopathogenesis, and during active disease the intestinal mucosa contains increased amounts of TNF-α.1 Conventional treatment options such as azathioprine are limited both by their inability to sustain a clinical remission and their side effects.
Infliximab (Remicade), a human–mouse chimaeric IgG1 antibody that binds to free and membrane bound TNF-α,2 is licensed for use in both resistant and fistularising Crohn's disease. A multicentre, double blind, placebo controlled trial of a single intravenous infusion of Infliximab has shown that 81% of patients given 5 mg/kg of Infliximab had a clinical response at four weeks compared to only 20% of patients on the placebo (p < 0.001). Thirty three per cent of patients given a single dose of Infliximab also achieved clinical remission at four weeks compared with 4% of those on placebo (p = 0.005).3 Eligibility criteria for this study required patients to be on stable doses of drugs, including steroids and azathioprine. An extension of this study giving four doses of 10 mg/kg Infliximab at eight weekly intervals to patients who had initially responded to single dose Infliximab showed that the clinical benefits of Infliximab may be maintained during and eight weeks after repeated doses.4 These clinical improvements were accompanied by considerable healing of endoscopic lesions,5 although healing with stricture formation remains a concern. Histological disease activity was also dramatically reduced, with a decrease in inflammatory cell infiltrate and downregulation of activation markers and adhesion molecules occurring after treatment.5,6
Crohn's disease may be complicated by problematical internal or enterocutaneous fistulae, and a study of 94 patients with draining abdominal or perianal fistulas has shown that 68% of patients receiving three 5 mg/kg Infliximab infusions had a reduction of 50% or more in the number of draining fistulae, compared with 26% on placebo.7 The maximal benefit of Infliximab was in the subgroup of patients who were not taking concurrent immunosuppressant drugs (p = 0.001).
Human anti-chimaeric antibodies occur in about 3–15% of patients treated with Infliximab,4,7 and acute allergic reactions are seen in approximately 5% of infusions.8 Delayed hypersensitivity reactions with myalgia, polyarthralgia, rash, and fever have been reported in 25% of patients after re-exposure to Infliximab after a two to four year interval.9 Anti-double stranded DNA (dsDNA) antibodies have been observed during treatment, although this has only rarely been associated with clinical lupus.4 There have been several cases of lymphoproliferative disease (B cell non-Hodgkins lymphoma and nodular sclerosing Hodgkin's disease) occurring in the nine months after Infliximab infusions. It is unclear whether this is a drug or disease related phenomenon.10 The chief medical officer has recently warned of a possible association between Infliximab and extrapulmonary tuberculosis. Trials with other agents are also in progress. CDP571, a humanised IgG4 anti-TNF monoclonal antibody, also appears to be effective in moderate to severe Crohn's disease.11
RHEUMATOLOGY
TNF-α is produced in the rheumatoid synovium and through its numerous proinflammatory actions is central to the pathological process. Infliximab (in combination with methotrexate) is licensed in the UK for treating refractory rheumatoid arthritis (RA).
In 1994, a double blind, placebo controlled trial demonstrated the benefits of intravenous infusions of Infliximab in active RA.12 Study patients had their disease modifying drugs discontinued for at least four weeks before receiving a single infusion of Infliximab (1 or 10 mg/kg) and were assessed at four weeks using a clinical scoring system including the erythrocyte sedimentation rate (Paulus scale). At four weeks, significantly more patients on Infliximab reached a 20% Paulus response compared with those on placebo (p < 0.0001 and p = 0.0083 for high and low dose Infliximab, respectively). Over half the patients on high dose Infliximab also reached the more stringent 50% Paulus criteria.
“In 1994, a double blind, placebo controlled trial demonstrated the benefits of intravenous infusions of infliximab in active rheumatoid arthritis”
A subsequent double blind, placebo controlled trial of 428 patients investigated repeat Infliximab infusions in combination with methrotrexte versus methotrexate alone in patients with active disease despite treatment with methotrexate.13 In this study, 61% of patients were also concurrently taking up to 10 mg/kg prednisone or equivalent. Treatment with Infliximab together with methotrexate resulted in significantly more patients reaching both the American College of Rheumatology's 20% and 50% improvement criteria at 30 weeks (p < 0.001 for both criteria, compared with methotrexate alone). Higher dose Infliximab regimens were associated with significantly higher rates of infection, although serious infections were not more frequent. Sixteen per cent of patients on Infliximab developed detectable anti-dsDNA antibodies, with a single patient developing drug induced lupus (anti-nuclear antibody positive, anti-dsDNA negative). Four malignancies were also reported in three patients on Infliximab.
Etanercept is another anti-TNF agent licensed and effective for the treatment of refractory RA.14 Etanercept is a recombinant IgG1 Fc fragment fused to two p75 TNF receptors, as opposed to a monoclonal antibody. Etanercept has also been used as monotherapy in early RA, where it has comparable efficacy to methotrexate alone.15 It is given as a twice weekly subcutaneous injection.
Both Etanercept and Infliximab appear to reduce radiographical joint disease progression.15,16 The role of these biological agents in the treatment hierarchy still needs to be established, and currently they are likely to be used only for patients who have active disease despite previous use of at least two conventional disease modifying drugs. Etanercept has also been studied in psoriatic arthritis, where improvements in both joint pain and swelling and skin lesions have been demonstrated.17
Monoclonal antibodies to interleukin 12 (IL-12), and antagonists to IL-1 and costimulatory molecules are under development for arthritis.18–20
INFECTION
Anti-endotoxin and anti-TNF-α monoclonal antibodies proved disappointing in sepsis trials, 21,22 but monoclonal antibodies are currently being developed against certain infectious agents, including cytomegalovirus and human immunodeficiency virus.
Palivizumab (Synagis) is licensed for respiratory syncitial virus (RSV) prophylaxis. RSV is the most important respiratory pathogen in infants and approximately 1% of infections require hospitalisation. Prematurity, chronic heart and lung disease, and immunodeficiency all predispose to more severe infection. Treatment is mainly supportive, often with assisted ventilation. The role of antiviral drugs such as ribavarin remains controversial.23 Palivizumab is a humanised IgG1 monoclonal antibody that binds to the F protein of RSV subtypes A and B. A single intravenous infusion of Palivizumab significantly reduces the RSV concentration in tracheal secretions for two days in children mechanically ventilated for RSV.24 However, this reduction was not translated into clinical/cost benefit because no reductions in days in hospital or days of mechanical ventilation were noted.
Subsequent investigations have indicated that the main role of Palivizumab is in the prophylaxis of RSV infection (presently no vaccination is available for RSV). Intravenous polyclonal RSV specific immunoglobulin (RSV IgG) does, however, reduce RSV hospitalisation when given prophylactically to premature infants and infants with bronchopulmonary dysplasia.25,26
A large placebo controlled study has demonstrated benefit with five monthly injections of intramuscular Palivizumab given prophylactically during the RSV season to children less than 6 months of age born < 35 weeks gestation, and children under 2 years with bronchopulmonary dysplasia.27 Hospitalisation with RSV during 150 days follow up from first dose was decreased from 8.1% to 1.8% (p < 0.001) for the premature birth group and from 12.8% to 7.9% (p = 0.038) for those with bronchopulmonary dysplasia. Palivizumab also produced a decrease in the number of days hospitalised and supplementary oxygen administration, and a reduction in the frequency of intensive care admissions as a result of RSV. Pavilizumab was well tolerated with infrequent mild injection site reactions.
Prophylactic Pavilizumab has not been compared directly with RSV IgG. Intramuscular Pavilizumab has the potential to be administered out of hospital and avoids intravenous cannulation and potential fluid load. Pavilizumab may also be more advantageous than RSV IgG in terms of cost effectiveness.28
CARDIOLOGY
Platelet activation at the site of damaged coronary vessels produces a prothrombotic state during acute coronary syndromes and after percutaneous coronary revascularisation. Abciximab (ReoPro) is a Fab human murine chimaeric monoclonal antibody, which blocks the platelet membrane glycoprotein IIb/IIIa receptor. This interaction antagonises receptor binding to circulating fibrinogen/VonWillebrand factor, inhibiting platelet crosslinking and aggregation.
The EPILOG trial demonstrated the benefit of Abciximab for patients undergoing elective or urgent percutaneous transluminal coronary angioplasty (PTCA) who had not had acute infarction or unstable angina within the preceding 24 hours. The 30 day composite endpoint of death, infarction, or myocardial ischaemia resulting in urgent revascularisation was significantly decreased in the Abciximab group (p < 0.0001 compared with PTCA and placebo).29 This was maintained during the one year follow up, although there was no overall reduction in the need for surgical or repeat percutaneous revascularisation procedures.30
Abciximab may also be beneficial in the treatment of refractory unstable angina, where plaque rupture and subsequent platelet aggregation and intracoronary thrombosis may result in infarction. The CAPTURE study demonstrated that in patients with refractory unstable angina undergoing PTCA, Abciximab significantly reduced the combined rate of death, infarction, and urgent intervention for recurrent ischaemia at 30 days (p = 0.012).31 Much of this benefit could be attributed to the reduction of myocardial infarction before and within 24 hours after PTCA (p values, 0.029 and 0.009, respectively). However, follow up at six months showed Abciximab to have no significant benefit in terms of death or myocardial infarction.
“Abciximab may also be beneficial in the treatment of refractory unstable angina”
The current UK licence for Abciximab is restricted to a single infusion before or during high risk percutaneous intervention. Abciximab appears to be generally well tolerated. The initial problems with major haemorrhage have been limited by diligent heparin administration. Thrombocytopenia can occur with Abciximab but is usually transient and often asymptomatic.31 The relative merits of Abciximab versus non-antibody glycoprotein IIb/IIIa inhibitors, such as Tirofiban, also need to be established.
HAEMATOLOGY/ONCOLOGY
Appropriately selected monoclonal antibodies may have antitumour effects via direct antibody mediated cell death or inhibition of cell growth. The conjugation of drugs, toxins, or radioisotopes to tumour specific monoclonal antibodies can also be of therapeutic benefit .
Rituximab is licensed for use in chemotherapy resistant advanced B cell follicular lymphoma. Most patients with low grade or follicular lymphoma relapse with current treatments.32 Rituximab is a mouse human chimaeric IgG1 antibody that recognises the transmembrane phosphoprotein CD20 expressed by B cells and their related lymphomas. An infusion of Rituximab dramatically reduces circulating B cell numbers,33 through antibody dependent cellular cytoxicity or apoptosis.34,35 Normal CD20 positive B cells are regenerated from early pre-B or stem cells (CD20 negative) in contradistinction to tumour cells, which have no equivalent counterpart.
In 1998, a multicentre study examined the use of four weekly infusions of Rituximab in 166 adults with low grade or follicular CD20 positive B cell lymphoma, who had relapsed or not responded to primary treatment.36 Six per cent of patients achieved a complete response, defined as the resolution of all signs and symptoms of lymphoma with a clear bone marrow for at least 28 days. Furthermore, 42% of patients had a partial response with a greater than 50% reduction in the sum of measurable lesions. These response rates are comparable with those of standard single chemotherapy agents. The projected median time to progression was 13 months.
Adverse events were common with more than 60% of patients having a reaction to the first infusion characterised by fever, nausea, angio-oedema, and bronchospasm; however, prophylactic antihistamines and analgesics can reduce the impact of this. Cytopenias occurred in 14% of patients during treatment and a single case of red cell aplasia occurred within the one year follow up.
Rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) in patients with newly diagnosed, or relapsed low grade or follicular B cell lymphoma, has revealed that this combined regimen may be superior to standard CHOP.37 Rituximab is also emerging as a potential treatment for B lymphoproliferative post-transplant disorder, which can follow both solid organ and bone marrow transplantation.38,39 Such uses are presently outwith its licence.
Targeting protooncogene products offers an alternative antiproliferative strategy, and potential targets include the HER2 gene, the overexpression of which predicts a worse prognosis in breast cancer.40 Trastuzumab (Herceptin) is a humanised IgG1 antibody that targets the extracellular domain of the HER2 growth receptor that has intrinsic tyrosine kinase activity. Trastuzumab has recently been licensed in the UK for patients with relapsed HER2 highly expressing breast malignancies. A non-placebo designed study of 222 patients with HER2 positive breast cancer who had progressed after chemotherapy examined the use of weekly infusions of Trastuzumab.41 Four per cent of patients achieved a complete response (disappearance of radiographical, palpable, and/or visually apparent tumour for at least four weeks) and 11% a partial response, with a greater than 50% reduction in measurable lesions. In responding patients, the median time to treatment failure was 11 months, compared with a median of 5.4 months after their earlier cytotoxic chemotherapy. However, treatment related cardiac dysfunction is a concern.41
TRANSPLANTATION
Successful solid organ transplantation requires selective immunosuppression to prevent organ rejection while avoiding infection. The past two decades have seen the introduction of selective immunosuppressants including cyclosporine, tacrolimus, and mycophenolate mofetil, which have contributed to reduced rates of early rejection.42–44 Early rejection may still occur in 35% of renal transplants,45 and although this rejection is frequently steroid responsive, early rejection appears to be a risk factor for chronic rejection.46 Both monoclonal and polyclonal antibody (OKT3 and antithymocyte globulin, respectively) treatments have been used in transplantation for many years.
OKT3 is a murine monoclonal antibody used particularly in renal transplantation, which binds to the pan-T cell marker CD3, and therefore exerts its action on both resting and activated T cells. Disadvantages of OKT3 include the cytokine release syndrome comprising fever, bronchospasm, and pulmonary oedema and an increased susceptibility to infections and malignancy.47
Two new monoclonal antibodies have recently been licensed in the UK for the prophylaxis of acute rejection in allogeneic renal transplantation in conjunction with steroids and cyclosporine. Basiliximab (Simulect) is an IgG1κ chimaeric antibody given as two infusions and Daclizumab (Zenapex) is a humanised IgG1 construct administered in five doses over eight weeks. Both these antibodies target CD25 (IL-2 receptor α chain), the expression of which increases in early T cell activation. Interference with the association of the α chain with the β and γ chains of the IL-2 receptor prevents assembly of the functional receptor.
In 1997, a double blind, placebo controlled trial assessed the use of Basiliximab, plus steroids and cyclosporine, in primary cadaveric renal transplantation.48 Basiliximab treated patients had a significantly lower incidence of biopsy confirmed acute rejection at six months (29.8%) compared with the placebo group (44%; p = 0.012). A further study involving 348 patients, receiving cadaveric or living transplants, confirmed this benefit by demonstrating reduced rejection rates on Basiliximab at both six and 12 months.49
A further study demonstrated a significantly better one year patient survival rate with Daclizumab (99%) compared with placebo (95%), plus cyclosporin and steroids (p = 0.01).50 However, this may reflect significantly more patients in the placebo group developing steroid resistant rejection requiring rescue with OKT3 or polyclonal antilymphocyte globulin, with their attendant mortality. Daclizumab may also have beneficial effects in cardiac transplantation and liver transplantation, but the drug combinations have yet to be resolved.51–53
Daclizumab and Basiliximab appear to have favourable safety profiles and do not induce cytokine release syndromes. Rates of infection and early malignancy appear to be similar to conventional drug regimens, although careful analysis of concurrent drug use is required.
Campath is a monoclonal antibody recognising CD52, a highly glycosylated molecule on lymphocytes and monocytes. Although initially explored in RA and multiple sclerosis,54,55 Campath now appears to be emerging as a successful treatment for some B cell chronic lymphatic leukaemias, in addition having to a role in haemopoietic transplantation.56–58 Campath has been shown to reduce dramatically graft versus host disease after bone marrow and stem cell transplantation. Furthermore, this benefit may not occur at the expense of an increased relapse of leukaemia.57 Campath currently remains unlicensed in the UK.
CONCLUSION
The clinical benefits of monoclonal antibodies have been demonstrated, in particular, in patients with more severe disease, and often as an adjunct to standard treatments. In many instances, the regimens for these licensed monoclonal antibodies still need to be refined. The costs of monoclonal antibody treatment remain high but must be weighed against the potential gains in reducing disability, hospitalisation, and mortality.
“Anti-IgE and anti-IL-5 monoclonal antibodies have potential for the treatment of allergic asthma and other allergic diseases”
We have focused on licensed monoclonal antibodies (table 1); however, many other agents are emerging for use in a wide range of medical settings. For example anti-IgE and anti-IL-5 monoclonal antibodies have potential for the treatment of allergic asthma and other allergic diseases.59,60 Monoclonal antibody treatment may also extend into novel areas, such as xenotransplantation and masking major histocompatibility complex–peptide complexes involved in the initiation of autoimmune disease.61,62 Crucial insight into the immunopathogenesis of many diseases may also be gleaned from these treatments.
Table 1.
UK licensed monoclonal antibodies
| Name | Type of antibody | Target | Licensed indication |
| Infliximab (Remicade) | Human–mouse chimaera IgG1 | TNF-α | Refractory Crohn's, Crohn's fistulas, refractory rheumatoid arthritis |
| Palivizumab (Synagis) | Humanised IgG1 | F protein on RSV | Prophylaxis, RSV in premature infants or brochopulmonary dysplasia |
| Abciximab (ReoPro) | Human–mouse chimaera | Platelet glycoprotein IIb/IIIa | High risk coronary intervention |
| Rituximab (MabThera) | Human–mouse chimaera IgG1 | CD20 | Refractory low grade or follicular B cell lymphoma |
| Basiliximab (Simulect) | Human–mouse chimaera IgG1K | IL-2 receptor α chain | Prophylaxis of acute rejection in allogeneic renal transplantation |
| Daclizumab (Zenapax) | Humanised IgG1 | IL-2 receptor α | As Basiliximab |
| Trastuzumab (Herceptin) | Humanised IgG1 | HER 2 growth receptor | Relapsed HER2 (high) breast malignancy |
IL-2, interleukin 2; TNF-α, tumour necrosis factor α; RSV, respiratory syncitial virus.
Take home messages.
Several different monoclonal antibodies have been licensed for the treatment of a variety of diseases, and have proved to be useful in patients with more severe disease, and often as an adjunct to standard treatments, although they are not without side effects
Infliximab (directed against tumour necrosis factor α) has shown efficacy in Crohn's disease and rheumatoid arthritis
Palivizumab (directed against respiratory syncitial virus) may be useful prophylactically in premature infants and young children with bronchopulmonary dysplasia
Abciximab (directed against a platelet glycoprotein receptor) may be beneficial in the treatment of refractory unstable angina, during acute coronary syndromes, and after coronary revascularisation
Rituximab (which recognises CD20 on B cells) may be useful in chemotherapy resistant follicular CD20 positive B cell lymphoma and Trastuzumab (which targets the HER2 protooncogene) has proved useful in breast cancer
Two new antibodies, Basiliximab and Daclizumab, which target CD25 (the interleukin 2 receptor α chain), have been shown to reduce graft rejection in some instances
Genetic engineering will allow further manipulation of monoclonal antibodies and, because antibodies can penetrate living cells, the scope of potential targets is considerable. Trials with monoclonal antibodies derived entirely from human DNA, such as the anti-TNF agent D2E7, are in progress.63 Furthermore, the place of monoclonal antibodies in medicine will be challenged by other recombinant immunotherapies, such as chemokine receptors and anti-apoptotic, adhesion, and angiogenic constructs.
Abbreviations
CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone
dsDNA, double stranded DNA
IL, interleukin
PTCA, percutaneous transluminal coronary angioplasty
RA, rheumatoid arthritis
RSV, respiratory syncitial virus
TNF-α
tumour necrosis factor α
REFERENCES
- 1.Reimund JM, Wittersheim C, Dumont S, et al. Mucosal inflammatory cytokine production by intestinal biopsies in patients with ulcerative colitis and Crohn's disease. J Clin Immunol 1996;16:144–50. [DOI] [PubMed] [Google Scholar]
- 2.Scallon BJ, Moore MA, Trinh H, et al. Chimeric anti-TNFα monoclonal antibody cA2 binds recombinant transmembrane TNFα and activates immune effector functions. Cytokine 1995;7:251–9. [DOI] [PubMed] [Google Scholar]
- 3.Targan SR, Hanauer SB, Van Deventer SJH, et al. A short term study of chimeric monoclonal antibody cA2 to TNFα for Crohns disease. N Engl J Med 1997;337:1029–35. [DOI] [PubMed] [Google Scholar]
- 4.Rutgeerts P, D'Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-TNF antibody (Infliximab) to maintain remission of Crohns disease. Gastroenterology 1999;117:761–9. [DOI] [PubMed] [Google Scholar]
- 5.D'Haens G, Van Deventer S, Van Hogezand R, et al. Endoscopic and histological healing with Infliximab anti-TNF antibodies in Crohns disease. Gastroenterology 1999;116:1029–34. [DOI] [PubMed] [Google Scholar]
- 6.Baert FJ, D'Haens G, Peeters M, et al. TNFα antibody (Infliximab) therapy profoundly downregulates the inflammation in Crohns ileocolitis. Gastroenterology 1999;116:22–8. [DOI] [PubMed] [Google Scholar]
- 7.Present DH, Rutgeerts MD, Targan MD, et al. Infliximab for the treatment of fistulas in patients with Crohns disease. N Engl J Med 1999;340:1398–405. [DOI] [PubMed] [Google Scholar]
- 8.Bell S, Kamm MA. Antibodies to TNFα as treatment for Crohns disease. Lancet 2000;355:858–60. [DOI] [PubMed] [Google Scholar]
- 9.Hanauer SB, Rutgeerts, D'Haens G, et al. Delayed hypersensitivity to infliximab (Remicade) re-infusion after a 2–4 year interval without treatment. Gastroenterology 1999;116:A731. [Google Scholar]
- 10.Bickston SJ, Lichtenstein GR, Arseneau KO, et al. The relationship between treatment and lymphoma in Crohns disease. Gastroenterology 1999;117:1433–7. [DOI] [PubMed] [Google Scholar]
- 11.Sandborn WJ, Feagon BG, Hanauer SB, et al. An engineered antibody to TNF (CDP571) for active Crohns disease. Gastroenterology 2001;120:1330–8. [DOI] [PubMed] [Google Scholar]
- 12.Elliott MJ, Maini RN, Feldmann, et al. Randomised double-blind comparison of chimeric monoclonal antibody to TNFα (cA2) versus placebo in rheumatoid arthritis. Lancet 1994;344:1105–10. [DOI] [PubMed] [Google Scholar]
- 13.Maini R, St Clair EW, Breedveld F, et al. Infliximab versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet 1999;354:1932–9. [DOI] [PubMed] [Google Scholar]
- 14.Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of Etanercept, a recombinant TNF receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999;340:253–9. [DOI] [PubMed] [Google Scholar]
- 15.Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 2000;343:1586–93. [DOI] [PubMed] [Google Scholar]
- 16.Lipsky P, van der Heijde D, St Clair W, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med 2000;343:1594–602. [DOI] [PubMed] [Google Scholar]
- 17.Mease PJ, Goffe BS, Metz J, et al. Etanercept in the treatment of psoriatic arthritis and psoriasis. Lancet 2000;356:385–90. [DOI] [PubMed] [Google Scholar]
- 18.Butler DM, Malfait AM, Maini RN, et al. Anti-IL 12 and anti-TNF antibodies synergistically suppress the progression of murine collagen-induced arthritis. Eur J Immunol 1999;29:2205–12. [DOI] [PubMed] [Google Scholar]
- 19.Bresnihan B, Newmark RD, Robbins S, et al. Anakinra reduces the rate of joint destruction after 1 year of treatment in a randomised controlled cohort of patients with rheumatoid arthritis. Arthritis Rheum 2000;43(suppl):S289. [Google Scholar]
- 20.Quattrocchi E, Dallman MJ, Feldmann M. Adenovirus-mediated gene transfer of CTLA-4Ig fusion protein in the suppression of experimental autoimmune arthritis. Arthritis Rheum 2000;43:1688–97. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler EJ, Fisher JC, Jr, Sprung CL, et al. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. N Engl J Med 1991;324:429–36. [DOI] [PubMed] [Google Scholar]
- 22.Abraham E, Anzueto A, Guillermo G, et al. Double blind randomised controlled trial of monoclonal antibody to human TNF in treatment of septic shock. Lancet 1998;351:929–33. [PubMed] [Google Scholar]
- 23.Moler FW, Steinhary CM, Ohmit SE, et al. Effectiveness of ribavarin in otherwise well infants with RSV associated respiratory failure. J Pediatr 1996;128:422–8. [DOI] [PubMed] [Google Scholar]
- 24.Malley R, DeVincenzo J, Ramilo O, et al. Reduction of RSV in tracheal aspirates in intubated infants by use of humanised monoclonal antibody to RSV F protein. J Infect Dis 1998;178:1555–61. [DOI] [PubMed] [Google Scholar]
- 25.Groothuis JR, Simoes EAF, Levin MJ, et al. Prophylactic administration of RSV immune globulin to high risk infants and young children. N Engl J Med 1993;329;1524–30. [DOI] [PubMed] [Google Scholar]
- 26.The PREVENT Study Group. Reduction of RSV hospitalization among premature infants and infants with bronchopulmonary dysplasia using RSV immune globulin prophylaxis. Pediatrics 1997;99:93–9. [DOI] [PubMed] [Google Scholar]
- 27.The Impact-RSV Study Group. Palivizumab, a humanised RSV monoclonal antibody, reduces hospitalization from RSV infection in high risk infants. Pediatrics 1998;102:531–7. [PubMed] [Google Scholar]
- 28.Joffe S, Ray GT, Escobar GJ, et al. Cost effectiveness of RSV prophylaxis among preterm infants. Pediatrics 1999;104:419–27. [DOI] [PubMed] [Google Scholar]
- 29.The EPILOG Investigators. Platelet glycoprotein IIb/IIIa receptor blockade and low dose heparin during percutaneous coronary revascularization. N Engl J Med 1997;336:1689–96. [DOI] [PubMed] [Google Scholar]
- 30.Lincoff AM, Tcheng JE, Califf RM, et al. Sustained suppression of ischemic complications of coronary intervention by platelet GP IIb/IIIa blockade with abciximab. Circulation 1999;99:1951–8. [DOI] [PubMed] [Google Scholar]
- 31.The CAPTURE Investigators. Randomised placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina. Lancet 1997;349:1429–35. [PubMed] [Google Scholar]
- 32.Gallagher CJ, Gregory WM, Jones AE, et al. Follicular lymphoma: prognostic factors for response and survival. J Clin Oncol 1986;4:1470–80. [DOI] [PubMed] [Google Scholar]
- 33.Maloney DG, Liles TM, Czerwinski C, et al. Phase I clinical trials using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody in patients with recurrent B-cell lymphoma. Blood 1994;84:2457–66. [PubMed] [Google Scholar]
- 34.Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994;83:435–45. [PubMed] [Google Scholar]
- 35.Maloney DG, Smith B, Appelbaum FR. The anti-tumour effect of monoclonal antibody anti-CD20 antibody therapy includes direct anti-proliferative activity and induction of apoptosis in CD20 positive NHL cell lines. Blood 1996;88(suppl):2535. [Google Scholar]
- 36.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapse indolent lymphoma. J Clin Oncol 1998;16:2825–33. [DOI] [PubMed] [Google Scholar]
- 37.Czucman MS, Grillo-Lopez AJ, White CA, et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol 1999;17:268–76. [DOI] [PubMed] [Google Scholar]
- 38.Oertal SH, Anagnostopoulus I, Bechstein WO, et al. Treatment of posttransplant lymphoproliferative disorder with the anti-CD20 monoclonal antibody rituximab alone in an adult after liver transplantation. Transplantation 2000;69:430–2. [DOI] [PubMed] [Google Scholar]
- 39.Kuehnle I, Huls MH, Liu Z, et al. CD20 monoclonal antibody (rituximab) for therapy of EBV lymphoma after hemopoietic stem-cell transplantation. Blood 2000;95;1502–5. [PubMed] [Google Scholar]
- 40.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;385:177–81. [DOI] [PubMed] [Google Scholar]
- 41.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanised anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999;17:2639–48. [DOI] [PubMed] [Google Scholar]
- 42.The Canadian Multicentre Transplant Study Group. A randomised clinical trial of cyclosporin in cadaveric renal transplanation. N Engl J Med 1986;314:1219–25. [DOI] [PubMed] [Google Scholar]
- 43.Vincenti F, Laskow DA, Neylan JF, et al. One year follow up of an open label trial of FK506 for primary kidney transplanation. Transplantation 1996;61:1576–81. [DOI] [PubMed] [Google Scholar]
- 44.Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplanation 1995;60:225–32. [DOI] [PubMed] [Google Scholar]
- 45.Vincenti F, Kirkman R, Light S, et al. IL-2R blockade with daclizumab to prevent acute rejection in renal transplantation. N Engl J Med 1998;338:161–5. [DOI] [PubMed] [Google Scholar]
- 46.Almond S, Matas A, Gillingham K, et al. Risk factors for chronic rejection in renal allograft recipients. Transplanation 1993;55:752–7. [DOI] [PubMed] [Google Scholar]
- 47.Jeyarajah DR, Thistlewaite JR, Jr. General aspects of cytokine release syndrome. Transplant Proc 1993;25(suppl 2):16–20. [PubMed] [Google Scholar]
- 48.Nashan B, Moore R, Amlot P, et al. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. Lancet 1997;350:1193–8. [DOI] [PubMed] [Google Scholar]
- 49.Kahan BD, Rajagopalan PR, Hall M, et al. Reduction of the occurrence of acute cellular rejection among renal allograft recipients treated with basiliximab, a chimeric anti-IL-2 receptor monoclonal antibody. Transplantation 1999;67:276–84. [DOI] [PubMed] [Google Scholar]
- 50.Nashan B, Light S, Hardie IR, et al. Reduction of acute renal allograft rejection by Daclizumab. Transplantation 1999;67:110–15. [DOI] [PubMed] [Google Scholar]
- 51.Beniaminovitz A, Itescu S, Lietz K, et al. Prevention of rejection in cardiac transplantation by blockade of the IL-2 receptor with a monoclonal antibody. N Engl J Med 2000;342:613–19. [DOI] [PubMed] [Google Scholar]
- 52.Eckhoff DE, McGuire B, Sellers M, et al. The safety and efficacy of a two dose daclizumab (Zenapax) induction therapy in liver transplant recipients. Transplantation 2000;69:1867–72. [DOI] [PubMed] [Google Scholar]
- 53.Hirose R, Roberts JP, Quan D, et al. Experience with Daclizumab in liver transplantation. Transplantation 2000;69:307–11. [DOI] [PubMed] [Google Scholar]
- 54.Isaacs JD, Watts RA, Hazleman BL, et al. Humanised monoclonal antibody therapy for rheumatoid arthritis. Lancet 1992;340:748–52. [DOI] [PubMed] [Google Scholar]
- 55.Moreau T, Thorpe J, Miller D, et al. Preliminary evidence from magnetic resonance imaging for reduction in disease activity after lymphocyte depletion in multiple sclerosis. Lancet 1994;344:298–301. [DOI] [PubMed] [Google Scholar]
- 56.Osterburg A, Dyer MJS, Bunjes D, et al. Phase II multicentre study of human CD52 antibody in previously treated chronic lymphocytic leukaemia. J Clin Oncol 1997;15:1567–74. [DOI] [PubMed] [Google Scholar]
- 57.Novitzky N, Thomas V, Hale G, et al. Ex vivo depletion of T cells from bone marrow grafts with CAMPATH-1 in acute leukaemia. Transplantation 1999;67:620–6.10071037 [Google Scholar]
- 58.Hale G, Jacobs P, Wood L, et al. CD52 antibodies for prevention of graft-versus-host disease and graft rejection following transplantation of allogeneic peripheral blood stem cells. Bone Marrow Transplant 2000;26:69–76. [DOI] [PubMed] [Google Scholar]
- 59.Milgrom H, Fick RB, Su JQ, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. N Engl J Med 1999;341:2006–8. [DOI] [PubMed] [Google Scholar]
- 60.Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 2000;356:2114–18. [DOI] [PubMed] [Google Scholar]
- 61.Wang H, Rollins SA, Gao Z, et al. Complement inhibition with an anti-C5 monoclonal antibody prevents hyperacute rejection in a xenograft heart transplantation model. Transplantation 1999;68:1643–51. [DOI] [PubMed] [Google Scholar]
- 62.Enberg J, Krogsgaard M, Fugger L. Recombinant antibodies with antigen-specific, MHC restricted specificty of T cells. Immunotechnology 1999;4:273–8. [DOI] [PubMed] [Google Scholar]
- 63.Van de Putte LBA, Rau R, Breedveld FC, et al. One year efficacy of the fully humanised anti-TNF antibody D2E7 in rheumatoid arthritis. Arthritis Rheum 2000;43(suppl):S269. [Google Scholar]