Abstract
Aims: To compare the sensitivity and specificity of percutaneous fine needle aspiration (FNA) cytology and needle core biopsy (NCB) in the diagnosis of suspected intra-abdominal tumours.
Methods: One hundred and forty one consecutive patients who underwent radiologically guided combined FNA/NCB of abdominal lesions over a four year period were reviewed. The diagnostic accuracy of both techniques and the value of rapid staining and assessment of cytological preparations were assessed.
Results: FNA cytology and NCB identified 111 of 129 (86%) and 104 of 129 (80.6%) malignant lesions, respectively; in combination, the sensitivity increased to 90.7%. The diagnostic specificity was 100% for both methods, although one case of phaeochromocytoma was misinterpreted as undifferentiated carcinoma on biopsy. More accurate tumour subtying was possible in two cases with FNA and four cases on NCB. The series included 12 benign lesions, of which 11 and nine were accurately identified on FNA and NCB, respectively. Two specific benign diagnoses (Budd-Chiari syndrome and hepatic infarct) were made only on biopsy. The use of rapid assessment cytology preparations ensured that appropriate samples were submitted for microbiology in three liver abscesses, and provided an accurate cytological diagnosis at the time of the procedure in 103 of 141 (73%) cases. None of the patients suffered biopsy related complications.
Conclusions: FNA cytology is more sensitive and accurate than NCB in the diagnosis of abdominal lesions, and also offers more rapid diagnosis. However, the combination of these sampling techniques increases diagnostic sensitivity and occasionally provides more accurate classification of tumours and benign lesions. The techniques should be considered complementary in the investigation of abdominal lesions.
Keywords: fine needle aspiration, cytology, biopsy, abdomen
The management of patients with suspected neoplastic disease involving abdominal sites is dependent on obtaining an accurate tissue diagnosis, usually via percutaneous sampling. Patients with abdominal lesions may present with clinically evident tumour masses, but the increasing use and sensitivity of radiological techniques has also led to the identification of relatively small lesions, which require the use of image guidance for reliable targeting. At present, there are two widely used and accepted methods for obtaining diagnostic material, namely fine needle aspiration (FNA) cytology and needle core biopsy (NCB). FNA specimens are usually acquired using 20–25 gauge needles and generally provide a sample for cytological examination, whereas NCB specimens are obtained using larger 14–18 gauge needles and primarily provide a tissue core for histological assessment. In theory, each sampling method offers different advantages and limitations. Although both techniques are very safe, FNA is often preferred in sampling deeply placed lesions, sites adjacent to major vessels, or in situations in which needles are to be passed through the bowel wall.1 Cytological samples can be rapidly stained and examined, thereby providing immediate assessment of adequacy, and in many cases a provisional diagnosis can be made while the patient remains in the radiology department.1–7 Furthermore, involvement by pathologists on site optimises clinical correlation and ensures that specimens are optimally handled and that appropriate samples are taken as required for ancillary investigations, such as microbiology or molecular studies. The advantages of NCB include the greater familiarity of histological preparations among some pathologists, the preservation of tissue architecture, which may be important in the assessment and subtyping of some tumours, and the relative ease with which histochemical and immunohistochemical techniques can be applied to paraffin wax embedded biopsy material.
“The increasing use and sensitivity of radiological techniques has also led to the identification of relatively small lesions, which require the use of image guidance for reliable targeting”
Therefore, although it might appear that cytological and histological examination would be complementary in the assessment of abdominal lesions, there are conflicting data in the literature regarding the accuracy and usefulness of these techniques. In particular, there are wide variations in the reported diagnostic sensitivities of FNA cytology and NCB,1 to the extent that some authors have suggested that core biopsy alone should be used,8 or that FNA is the preferred technique with biopsy limited to cytologically indeterminate cases.2,9–12 These discrepancies may be partly explained by variations in the types of lesion subject to biopsy, and by the approach to cytological examination (such as the use of rapid staining techniques). Relatively few reports have evaluated FNA cytology and core biopsy obtained in combination in the investigation of patients with abdominal lesions. Therefore, we have compared the sensitivity and specificity of FNA cytology and NCB in a consecutive series of 141 patients undergoing image guided sampling of abdominal masses in our hospital.
PATIENTS AND METHODS
The histopathology and cytopathology databases of the pathology department, Glasgow Royal Infirmary were searched for all patients undergoing combined, image guided percutaneous core biopsy and FNA cytology sampling of abdominal mass lesions during the four year period August 1995 to July 1999. All patients had one or more radiologically detected lesions and the initial clinical suspicion in each case was of neoplastic disease. In total, 141 cases were identified, comprising approximately 25% of all abdominal lesions subject to biopsy in the study period (in most cases FNA or NCB alone was performed). There were 76 men and 65 women with an age range 17–88 years (mean, 65.7). Eighteen patients had a previous history of biopsy confirmed malignancy as follows: carcinomas of the colon (n = 9), stomach (n = 4), breast (n = 2), and pancreas (n = 2), and ocular malignant melanoma (n = 1). Coagulation screen was checked before biopsy in each case. All patients provided written informed consent for the procedure.
The lesions were situated within the liver (n = 105), pancreas (n = 17), kidney (n = 5), retroperitoeum (n = 3), adrenal (n = 2), or miscellaneous abdominal and pelvic sites (n = 9). Sampling was usually performed with ultrasound guidance but computed tomography scanning was used in a few cases. The decision to use FNA and/or core biopsy as a sampling technique was at the discretion of the radiologist performing the procedure. In general, FNA samples were taken first and subject to immediate assessment, as described previously.13 Briefly, the samples were obtained following local anaesthesia using a standard 21 gauge Chiba needle attached to a 20 ml syringe. After localisation, the needle was passed gently through the lesion four to six times with aspiration. The needle was withdrawn and passed to the cytopathologist. Direct smears were prepared from part of the sample and one or more slides were stained using the Diff-Quik method in the scanning room. The smears were examined by the cytopathologist and the radiologist was informed of specimen adequacy. Repeat FNA samples (up to three in total) were taken as required if limited material was obtained. A provisional diagnosis was made whenever possible and in some cases this was included with the radiology report returned to the ward with the patient. After preparing smears the needles were rinsed in normal saline. Cytospin or cell block preparations were made in a few cases for subsequent ancillary investigations, such as histochemistry or immunocytochemistry, most commonly to confirm the epithelial nature of poorly differentiated malignancies or to demonstrate lymphoid or mesenchymal antigens. The needle rinses were also submitted for microbiological study in those cases suspected to be of inflammatory or infective nature on rapid cytological assessment. After the FNA procedure, core biopsy samples were taken using an 18 gauge needle loaded into an automatic biopsy system (Biopty System, Radiplast, Sweden). The adequacy of the specimens was judged visually and up to three separate core samples were taken as required. The core biopsies were fixed in 10% neutral buffered formalin, processed routinely, and stained with haematoxylin and eosin. The specimens were examined and reported as part of the normal diagnostic workload by cytopathology and histopathology staff.
For the purposes of our study, the histological diagnoses from NCB specimens were considered the “gold standard”. In those patients with negative or unsatisfactory core biopsy samples the definitive clinicopathological diagnosis was based on subsequent biopsy or surgical resection specimens, or on clinical follow up data obtained by case record review.
RESULTS
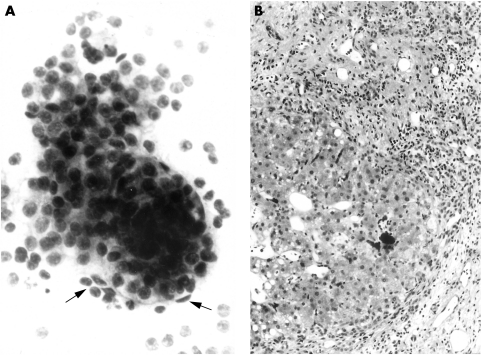
Table 1 outlines the final clinicopathological diagnoses for the 141 patients. One hundred and twenty nine patients had malignant disease (two adrenal phaeochromocytomas are included in the potentially malignant group). The most common diagnosis was metastatic malignancy in the liver (87 cases), comprising 82 metastatic carcinomas, three lymphomas and one case each of melanoma and sarcoma. There were nine cases of primary hepatocellular carcinoma, all of which were diagnosed on FNA; in one case, the NCB showed only cirrhotic liver tissue (fig 1). All pancreatic and renal tumours were primary carcinomas at these sites. The retroperitoneal tumours comprised two leiomyosarcomas and one case of Hodgkin's disease. The miscellaneous abdominal/pelvic tumours comprised seven patients with peritoneal metastatic adenocarcinoma, one case of borderline mucinous tumour of the ovary, and one patient with a malignant gastrointestinal stromal tumour of uncertain origin.
Table 1.
Sensitivity of FNA cytology, NCB, and combined FNA/NCB in 129 malignant cases and 12 benign cases
| Diagnostic sensitivity (%) | |||
| Biopsy site | FNA | NCB | Combined |
| Malignant | |||
| Liver | |||
| Metastatic malignancy (n=87) | 75 (86.2) | 71 (82.8) | 77 (88.5) |
| Hepatocellular carcinoma (n=9) | 9 (100) | 8 (88.9) | 9 (100) |
| Pancreas (n=14) | 11 (78.6) | 10 (71.4) | 13 (92.9) |
| Kidney (n=5) | 5 (100) | 3 (60) | 5 (100) |
| Adrenal (n=2) | 0 (0) | 1 (50) | 1 (50) |
| Retroperitoneum (n=3) | 2 (66.7) | 3 (100) | 3 (100) |
| Abdomen/pelvis, NOS (n=9) | 9 (100) | 8 (88.9) | 9 (100) |
| Total (n=129) | 111 (86) | 104 (80.6) | 117 (90.7) |
| Benign | |||
| Liver (n=9) | 9 (100) | 8 (88.9) | 9 (100) |
| Pancreas (n=3) | 2 (66.7) | 1 (33.3) | 2 (66.7) |
| Total (n=12) | 11 (91.7) | 9 (75) | 11 (91.7) |
FNA, fine needle aspiration; NCB, needle core biopsy, NOS, not otherwise specified.
Figure 1.
Hepatocellular carcinoma. (A) Fine needle aspiration sample includes thickened trabeculae of hepatocytes with peripheral endothelial cells (arrows) and scattered atypical bare nuclei (above). Diff-Quik method, original magnification, ×300. (B) The corresponding core biopsy shows cirrhotic liver but no evidence of malignancy. Haematoxylin and eosin, original magnification, ×120.
In three patients both the FNA samples and the core biopsies were inadequate for assessment (acellular or lacking parenchymal elements); subsequent follow up showed metastatic adenocarcinoma in the liver in two cases and adrenal phaeochromocytoma in one. Three further patients had unsatisfactory NCB specimens but positive cytology, whereas two patients had inadequate FNA but diagnostic core biopsy. Altogether, six NCB and five FNA specimens were inadequate. In the remaining false negative cases, parenchymal tissue was obtained on sampling but later shown to be unrepresentative of the primary lesion. Overall, FNA cytology accurately identified 111 of 129 malignancies (86%), whereas 104 cases (80.6%) were positive on core biopsy. Combining the techniques increased the sensitivity to 90.7% for patients with malignant disease.
There were relatively minor discrepancies between the cytological and histological diagnoses in seven of the malignant cases, all involving liver specimens. In two cases, the FNA samples were reported as consistent with metastatic adenocarcinoma, whereas the corresponding core biopsies showed a large cell carcinoma lacking specific differentiation. Conversely, two large cell carcinomas that were not otherwise specified (NOS) on cytology showed evidence of glandular differentiation on core biopsy. One case was reported as malignant, NOS, on FNA because there was insufficient material for ancillary studies; the corresponding biopsy showed an undifferentiated carcinoma and this was supported by cytokeratin immunoreactivity in the tumour cells. In one case, both the cytology and biopsy showed metastatic adenocarcinoma but the histological pattern was suspicious of prostatic origin and this was confirmed using immunostaining for prostatic acid phosphatase and prostate specific antigen. Finally, one case was reported as favouring primary hepatocellular carcinoma on FNA, whereas metastatic adenocarcinoma was considered more likely on core biopsy; the patient died without resolution of the diagnosis.
There was an additional tumour in which the histological diagnoses on core biopsy and on the subsequent resection specimen differed. This was an adrenal mass in which the core biopsy was interpreted as undifferentiated carcinoma but the surgical specimen showed a phaeochromocytoma. There was no evidence of metastatic disease at the time of surgery. The FNA sample was unsatisfactory in this case.
Twelve patients had benign disease including one benign tumour, a serous cystadenoma of the pancreas. The FNA and core biopsy samples were both inadequate for assessment in this case and the diagnosis was made on tumour resection. There were 11 inflammatory/non-neoplastic lesions comprising three liver abscesses, one liver infarct, one case of Budd-Chiari syndrome, four non-specific reactive liver patterns, and two cases of pancreatitis. The FNA samples were adequate in all cases and were reported as consistent with inflammatory/reactive processes. Material from the FNA samples was submitted to microbiology in six cases and Streptococcus milleri was cultured from two of three hepatic abscesses. Core biopsy samples from two of the inflammatory lesions (one pancreatitis, one non-specific liver reaction) were inadequate for diagnosis. The diagnoses of Budd-Chiari syndrome and hepatic infarct were based on the core biopsy findings because the cytology specimens showed non-specific liver tissue.
Overall, therefore, FNA cytology and core biopsy were diagnostic in 122 of 141 (86.5%) and 113 of 141 (80.1%) cases, respectively. A combination of cytology and biopsy improved the diagnostic yield to 90.8% of cases (128 of 141).
A provisional diagnosis was made at the time of the procedure in 103 FNA (73%) specimens (92 malignant cases and 11 reactive/inflammatory lesions). There was no difference between the provisional and final cytology diagnoses in these cases. The definitive cytology report was issued within 24 hours of the procedure in 74% of cases and the mean reporting time was 1.4 days (range, 0–8). The NCB reports took 3.9 days on average to complete (range, 1–12).
None of the patients in the series had serious complications after the biopsy procedures.
DISCUSSION
Needle biopsy of abdominal mass lesions may be used to establish a malignant diagnosis in patients with clinically or radiologically suspected neoplasia or for staging in patients with known tumours at other sites.
The decision to use FNA and/or NCB as sampling techniques depends on many factors including the size and site of the lesion, the suspected likely diagnosis, and the risk of complications. Because most biopsies are performed using image guidance, the experience of individual radiologists is an important factor, and the preferred technique may be influenced by the availability of cytopathologists for on site specimen assessment. The sensitivity and specificity of FNA and NCB should also be considered in choosing the optimal technique.
FNA proved more sensitive than NCB in the diagnosis of the 129 malignant lesions in our series (86% v 80.6%, respectively). Similar findings have been recorded in most previous studies in which cytological and histological sampling of abdominal masses have been directly compared (table 2).10–12,14–19 The sensitivity of FNA was 2–24% greater than that of NCB in these studies, although some reports excluded unsatisfactory specimens in their analysis.14 As with previous reports, we found that the combination of cytology and core biopsy increased the sensitivity of the biopsy procedure. However Nyman and colleagues8 reported only 61.8% sensitivity for FNA compared with 90.1% for NCB in the investigation of 55 patients with malignant liver lesions. The same group also reported broadly similar findings in the diagnosis of paediatric abdominal tumours.20 The authors concluded that NCB should be the preferred sampling technique in patients with abdominal masses and that the combination of FNA and NCB had no additional value. Moulton and Moore17 also found biopsy to be more sensitive than FNA in the assessment of lesions at various anatomical sites (86% v 75%, respectively in abdominal malignancies). However, the false negative rates for FNA in these studies were greater than those in most other combined FNA/NCB series, or in recent reports using FNA cytology alone, in which sensitivities over 90% have been recorded.21–24 It is also pertinent that neither group of authors used immediate assessment of cytology samples which we, and many others,1–7 feel maximise diagnostic yield and accuracy.
Table 2.
Review of previous studies comparing FNA cytology and NCB in the diagnosis of malignant abdominal lesions
| Diagnostic sensitivity | |||||
| Reference | No. malignant cases | Site | FNA | NCB | Combined |
| Jacobsen and colleagues10 | 48 | Liver | 100 | 80 | 100 |
| Cochond-Priollet and colleagues14 | 26 | Liver | 81 | 69 | 85 |
| *Livraghi and colleagues12 | 200 | Abdomen | 89 | 83 | 98 |
| Bedenne and colleagues11 | 36 | Liver | 83 | 81 | 92 |
| Lin et al and colleagues15 | 59 | Liver | 95 | 89 | NS |
| Solmi and colleagues16 | 42 | Pancreas | 95 | 71 | 95 |
| †Moulton and Moore17 | 118 | Abdomen | 75 | 86 | 91 |
| ‡Tikkakoski and colleagues18 | 49 | Abdomen | 88 | 73 | NS |
| §Nyman and colleagues8 | 55 | Abdomen | 62 | 91 | 93 |
| Dusenbery and colleagues19 | 40 | Liver | 90 | 85 | 98 |
| Our series | 129 | Abdomen | 86 | 82 | 90 |
*Data expressed as “retrieval rate”; includes benign cases; †data derived from table 1, reference 17; ‡data derived from tables 1 and 2, reference 18; §data expressed as “correct diagnosis”.
FNA, fine needle aspiration; NCB, needle core biopsy; NS, not specified.
In our study, FNA cytology was 100% specific for a malignant diagnosis. Although rare false positive diagnoses have been described with FNA,19,25,26 most studies have confirmed that cytological examination is highly reliable for a malignant diagnosis in abdominal lesions. We also considered NCB to be 100% specific in the current series, although it could be argued that there was one false positive histological diagnosis. This case involved an adrenal mass that was interpreted as undifferentiated carcinoma on core biopsy, but subsequent tumour resection revealed a phaeochromocytoma without evidence of metastatic spread. Although we included the two phaeochromocytomas in our series within the malignant category, most such tumours are clinically benign and the NCB assessment in the above case could therefore be regarded as misleading and inaccurate.
“FNA cytology was 100% specific for a malignant diagnosis”
It is generally accepted that NCB samples provide more accurate subtyping of some tumours than do FNA samples.10,12,16,17,19 This is partly related to the retained architectural pattern in histological specimens and also the ease with which ancillary techniques, such as mucin stains or immunohistochemistry, can be applied to biopsy material. Therefore, core biopsies may be helpful in identifying the probable origin of tumours in patients presenting with metastatic disease. We found that NCB was marginally more successful than FNA in subtyping tumours in our series. Two metastatic liver malignancies reported as undifferentiated carcinoma on FNA were accurately identified as adenocarcinoma on NCB. One liver tumour was reported as malignant NOS cytologically, but histology in conjunction with immunohistochemistry permitted classification of the neoplasm as an undifferentiated carcinoma. Conversely, two undifferentiated carcinomas on histology showed specific glandular differentiation in the cytological preparations. Although such discrepancies were usually of minor clinical relevance, in one further case only the NCB identified the prostatic origin of a metastatic adenocarcinoma in the liver. Core biopsy samples have also been considered more helpful than FNA in the specific diagnosis of benign lesions within the abdomen,16,17 and in other sites such as lung.27 This was true in two of 11 non-neoplastic lesions in our series, where the NCB showed features of Budd-Chiari syndrome and liver infarct, respectively, whereas the cytology preparations showed only non-specific reactive liver changes. However, NCB was unsatisfactory in two further inflammatory cases in which FNA provided diagnostic material. In addition, the identification of three liver abscesses during cytological evaluation ensured that appropriate samples were submitted for microbiology. Thus, the different sampling techniques each proved advantageous in individual cases.
Separate FNA and NCB specimens were examined in our study, but other investigators have demonstrated the value of deriving both cytological and histological material from either type of sample. The value of cell blocks is well established as an adjunct to cytological assessment in FNA specimens from many anatomical sites, including abdominal lesions.1,26,28–31 Microhistology has been particularly advocated in the diagnosis of primary hepatocellular carcinoma, where assessment of the hepatic architectural pattern and the use of reticulin stains has been shown to be very helpful.32–34 Conversely, cytological preparations can be derived from biopsy material either through imprint/touch preparations or by rinsing core samples in saline before fixation. Hahn et al found that touch preparations from abdominal core biopsies were as accurate as FNA specimens and provided similar rapid assessment of specimen adequacy.35 Zardawi36 also used core imprints to assess specimen adequacy and to minimise the number of biopsy procedures. Tsang and colleagues37 used needle rinses from 18–19 gauge core biopsies to make cytological preparations; the additional cytology preparations lowered the unsatisfactory rate and improved the overall diagnostic sensitivity by 8%.
Key messages.
Fine needle aspiration cytology is more sensitive and accurate than needle core biopsy (NCB) in the diagnosis of abdominal lesions, and also offers more rapid diagnosis
However, NBC can provide specific tumour subtyping in a small number of cases
Therefore, these two techniques should be considered complementary in the investigation of abdominal lesions
One advantage of the immediate assessment of FNA cytology specimens is that a reliable provisional diagnosis can be made in many cases, allowing clinicians to discuss the diagnosis with patients and to instigate further investigation and treatment without delay. In our current series, an immediate diagnosis was possible in 92 of 129 (71.3%) malignant lesions and in 11 of 12 (91.7%) benign lesions while the patient was still in the radiology suite. All provisional diagnoses were confirmed on follow up. The turnaround time of final reports was also significantly shorter with FNA than with NCB (mean values, 1.4 v 3.9 days, respectively) and approximately three quarters of cases were fully reported within 24 hours. Such factors should be considered when the clinical value and cost effectiveness of FNA and immediate cytology assessment are considered.
In conclusion, a direct comparison of FNA and NCB in 141 patients undergoing image guided sampling of abdominal lesions showed that FNA cytology was more sensitive and accurate than biopsy, and that rapid diagnosis was also usually possible with this technique. However, NCB offered the advantage of specific tumour subtyping in a small number of cases. We feel that FNA and NCB should be considered complementary diagnostic techniques and used in combination depending on clinical conditions.
Abbreviations
FNA, fine needle aspiration
NCB, needle core biopsy
NOS, not otherwise specified
REFERENCES
- 1.Orell SR, Sterrett GF, Walters MNI, et al. Manual and atlas of fine needle aspiration cytology, 3rd ed. London: Churchill Livingstone, 1999:267–90.
- 2.Pitman MB. Fine needle aspiration biopsy of the liver. Principal diagnostic challenges. Clin Lab Med 1998;18:483–506. [PubMed] [Google Scholar]
- 3.Bottles K, Cohen MB. An approach to fine-needle aspiration biopsy diagnosis of hepatic masses. Diagn Cytopathol 1991;7:204–10. [DOI] [PubMed] [Google Scholar]
- 4.Dodd LG, Mooney EE, Layfield LJ, et al. Fine-needle aspiration of the liver and pancreas: a cytology primer for radiologists. Radiology 1997;203:1–9. [DOI] [PubMed] [Google Scholar]
- 5.Tao LC, Sanders DE, Weisbrod GL, et al. Value and limitations of transthoracic and transabdominal fine-needle aspiration cytology in clinical practice. Diagn Cytopathol 1986;2:271–6. [DOI] [PubMed] [Google Scholar]
- 6.Silverman JF, Finley JL, O'Brien KF, et al. Diagnostic accuracy and role of immediate interpretation of fine needle aspiration biopsy specimens from various sites. Acta Cytol 1989;33:791–6. [PubMed] [Google Scholar]
- 7.Logrono R, Kurtycz DFI, Sproat IA et al. Multidisciplinary approach to deep-seated lesions requiring radiologically-guided fine-needle aspiration. Diagn Cytopathol 1998;18:338–42. [DOI] [PubMed] [Google Scholar]
- 8.Nyman RS, Cappelen-Smith J, Brismar J, et al. Yield and complications in ultrasound guided biopsy of abdominal lesions. Comparison of fine needle aspiration biopsy and 1.2-mm needle core biopsy using an automated gun. Acta Radiol 1995;36:485–90. [PubMed] [Google Scholar]
- 9.Fornari F, Civardi G, Cavanna L, et al. Ultrasonically guided fine-needle aspiration biopsy: a highly diagnostic procedure for hepatic tumors. Am J Gastroenterol 1990;85:1009–13. [PubMed] [Google Scholar]
- 10.Jacobsen GK, Gammelgaard J, Fuglo M. Coarse needle biopsy versus fine needle aspiration biopsy in the diagnosis of focal lesions of the liver. Ultrasonically guided needle biopsy in suspected hepatic malignancy. Acta Cytol 1983;27:152–6. [PubMed] [Google Scholar]
- 11.Bedenne L, Mottot C, Courtois B, et al. L'aiguille tru-cut est-elle plus efficace que l'aiguille fine dans le diagnostic des lesions hepatiques? Gastroenterol Clin Biol 1990;14:62–6. [PubMed] [Google Scholar]
- 12.Livraghi T, Sangalli G, Giordano, et al. Fine needle aspiration versus fine cutting needle, and comparison between smear cytology, inclusion cytology and microhistology in abdominal lesions. Tumori 1988;74:361–4. [DOI] [PubMed] [Google Scholar]
- 13.Stewart CJR, Stewart IS. Immediate assessment of fine needle aspiration cytology of lung. J Clin Pathol 1996;49:839–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochond-Priollet B, Chagnon S, Ferrand J, et al. Comparison of cytologic examination of smears and histologic examination of tissue cores obtained by fine needle aspiration biopsy of the liver. Acta Cytol 1987;31:476–80. [PubMed] [Google Scholar]
- 15.Lin BPC, Chu JMG, Rose RA. Ultrasound guided fine needle biopsy of the liver for cytology and histology. Australas Radiol 1991;35:33–7. [DOI] [PubMed] [Google Scholar]
- 16.Solmi L, Muratori R, Bacchini P, et al. Comparison between echo-guided fine-needle aspiration cytology and microhistology in diagnosing pancreatic masses. Surg Endosc 1992;6:222–4. [DOI] [PubMed] [Google Scholar]
- 17.Moulton JS, Moore PT. Coaxial percutaneous biopsy technique with automated biopsy devices: value in improving accuracy and negative predictive value. Radiology 1993;186:515–22. [DOI] [PubMed] [Google Scholar]
- 18.Tikkakoski T, Paivansalo M, Siniluoto T, et al. Percutaneous ultrasound-guided biopsy. Fine needle biopsy, cutting needle biopsy, or both? Acta Radiol 1993;34:30–4. [PubMed] [Google Scholar]
- 19.Dusenbery D, Ferris JV, Thaete FL, et al. Percutaneous ultasound-guided needle biopsy of hepatic mass lesions using a cytohistologic approach. Comparison of two needle types. Am J Clin Pathol 1995;104:583–7. [DOI] [PubMed] [Google Scholar]
- 20.Hugosson CO, Nyman RS, Cappelen-Smith JM, et al. Ultrasound-guided biopsy of abdominal and pelvic lesions in children. A comparison between fine-needle aspiration and 1.2 mm-needle core biopsy. Pediatr Radiol 1999;29:31–6. [DOI] [PubMed] [Google Scholar]
- 21.David O, Green L, Reddy V, et al. Pancreatic masses: a multi-institutional study of 364 fine-needle aspiration biopsies with histopathologic correlation. Diagn Cytopathol 1998;19:423–7. [DOI] [PubMed] [Google Scholar]
- 22.Nobrega J, dos Santos G. Aspirative cytology with fine-needle in the abdomen, retroperitoneum and pelvic cavity: a seven year experience of the Portugese Institute of Oncology, Center of Porto. Eur J Surg Oncol 1994;20:37–42. [PubMed] [Google Scholar]
- 23.Civardi G, Fornari F, Cavanna L, et al. Value of rapid staining and assessment of ultrasound-guided fine needle aspiration biopsies. Acta Cytol 1988;32:552–4. [PubMed] [Google Scholar]
- 24.Tsou MH, Lin YM, Lin KJ, et al. Fine needle aspiration cytodiagnosis of liver tumors. Results obtained with Riu's stain. Acta Cytol 1998;42:1359–64. [DOI] [PubMed] [Google Scholar]
- 25.Sautereau D, Vire O, Cazes PY, et al. Value of sonographically guided fine needle aspiration biopsy in evaluating the liver with sonographic abnormalities. Gastroenterology 1987;93:715–18. [DOI] [PubMed] [Google Scholar]
- 26.Schwerk WB, Durr HK, Schmitz-Moormann P. Ultrasound guided fine-needle biopsies in pancreatic and hepatic neoplasms. Gastrointest Radiol 1983;8:219–25. [DOI] [PubMed] [Google Scholar]
- 27.Greif J, Marmor S, Schwarz Y, et al. Percutaneous core needle biopsy vs. fine needle aspiration in diagnosing benign lung lesions. Acta Cytol 1999;43:756–60. [DOI] [PubMed] [Google Scholar]
- 28.Chiu KW, Chang-Chien CS, Chen L, et al. Ultrasonically-guided needle aspiration with preparation of cell blocks in the diagnosis of liver tumors. Hepatogastroenterology 1994;41:30–3. [PubMed] [Google Scholar]
- 29.Kern WH, Haber H. Fine needle aspiration minibiopsies. Acta Cytol 1986;30:403–8. [PubMed] [Google Scholar]
- 30.Bell DA, Carr CP, Szyfelbein WM. Fine needle aspiration cytology of focal liver lesions. Results obtained with examination of both cytologic and histologic preparations. Acta Cytol 1986;30:397–402. [PubMed] [Google Scholar]
- 31.Axe SR, Erozan YS, Ermatinger SV. Fine-needle aspiration of the liver. A comparison of smear and rinse preparations in the detection of cancer. Am J Clin Pathol 1986;86:281–5. [DOI] [PubMed] [Google Scholar]
- 32.Guy CD, Ballo MS. Fine needle aspiration biopsy of the liver. Adv Anat Pathol 1999;6:303–16. [DOI] [PubMed] [Google Scholar]
- 33.Kung ITM, Chan SK, Fung KH. Fine-needle aspiration in hepatocellular carcinoma: combined cytologic and histologic approach. Cancer 1991;67:673–80. [DOI] [PubMed] [Google Scholar]
- 34.De Boer WB, Segal A, Frost FA, et al. Cytodiagnosis of well differentiated hepatocellular carcinoma: can indeterminate diagnoses be reduced? Cancer 1999;87:270–7. [DOI] [PubMed] [Google Scholar]
- 35.Hahn PF, Eisenberg PJ, Pitman MB, et al. Cytopathologic touch preparations (imprints) from core needle biopsies: accuracy compared with that of fine-needle aspirates. AJR Am J Roentgenol 1995;165:1277–9. [DOI] [PubMed] [Google Scholar]
- 36.Zardawi IM. Fine needle aspiration cytology vs. core biopsy in a rural setting. Acta Cytol 1998;42:883–7. [DOI] [PubMed] [Google Scholar]
- 37.Tsang P, Greenebaum E, Starr G, et al. Image-directed percutaneous biopsy with large-core needles. Comparison of cytologic and histologic findings. Acta Cytol 1995;39:753–8. [PubMed] [Google Scholar]