Abstract
Aims: To determine the prognostic usefulness of the Nottingham histological grade (NHG) and its components in a series of 270 patients with stage pT1N0M0 breast cancer with a median follow up of 12.5 years.
Methods: Microscopic slides were re-examined and the degree of tubule formation, nuclear pleomorphism, and mitotic counts were assessed and scored according to the suggested guidelines. The association with cancer specific survival (CSS) was evaluated by univariate and multivariate analyses.
Results: Whereas tumour size, patient age, menopausal status, type of surgery, or adjuvant treatment were not related to prognosis, histological type (p < 0.01) and NHG (p < 0.005) were associated with CSS. When evaluating the components of NHG separately, survival was not related to the score for pleomorphism, but was significantly better in tumours with score 1 or 2 for tubule formation (p < 0.007) and in those with score 1 for mitotic counts (p < 0.006). The two components retained independent significance in multivariate analysis. When the proposed cut off points for mitotic counts were replaced by lower ones based on tertile values, the mitotic index became the strongest prognostic factor (p = 0.0001) and histological type was the only additional factor of independent prognostic significance.
Conclusions: These findings confirm the prognostic value of NHG in pT1N0M0 breast carcinoma, show that the evaluation of tubule formation and mitotic rate provides independent prognostic information, and suggest that the proposed cut off points for mitotic counts may be too high for this particular group of tumours.
Keywords: breast cancer, grading, mitotic index, prognosis
Despite a considerable body of evidence that the Bloom-Richardson grading system (BRG), which is based on the assessment of tubule formation, nuclear pleomorphism, and mitotic activity, provides important independent prognostic information in patients with breast cancer, this system has not been universally accepted, mainly because of its subjective nature and apparently poor reproducibility. A major improvement has been provided by Elston and Ellis,1 who have clearly defined the criteria, particularly by applying numerical limits to the measurement of tubule formation and mitotic counts. Whereas the relative numbers of both hyperchromatic nuclei and mitotic figures were analysed in the original BRG, only clearly identifiable mitotic figures are evaluated in the new system. In addition, the size of the high power field, which may vary greatly from one microscope to another, is taken into account. This modification of the BRG, now generally known as the Nottingham histological grade (NHG), has gained widespread acceptance during the past decade.
“The Bloom-Richardson grading system has not been universally accepted, mainly because of its subjective nature and apparently poor reproducibility”
The prognostic value of the NHG, used either alone or, along with tumour size and nodal status, as a component of the Nottingham prognostic index, has been confirmed in many large studies of patients with breast carcinoma.2–10 Some of these studies were limited to node negative tumours.3,8 However, to the best of our knowledge, none of them has focused on patients with pathological stage 1 (pT1N0M0) tumours; that is, tumours measuring 2 cm or less, with uninvolved axillary lymph nodes and no distant metastasis. The aim of our present study was to evaluate the long term prognostic value of NHG in this group of patients. In addition, we separately analysed the prognostic impact of each of the three components used in its assessment.
MATERIALS AND METHODS
Patients
Through a review of pathology reports and patient charts we retrospectively identified 339 women with pT1N0M0 breast carcinoma who underwent surgery between January 1983 and December 1987 at the Institute of Oncology, Ljubljana, and in whom follow up data were available. Of the initial set of patients, the following were excluded from further analysis: 18 patients with previous contralateral invasive breast carcinoma; two patients with previous malignancy at another site; three patients who received preoperative chemotherapy; 15 patients with multifocal or multicentric tumours; and 20 patients in whom the original histological slides and/or paraffin wax blocks were unavailable. Histological re-examination revealed seven tumours with largest microscopic diameter exceeding 2 cm, one carcinoma in situ, and 11 microinvasive carcinomas; these were also excluded from our study. Thus, the analyses reported herein are based on the remaining cohort of 270 patients.
The patients ranged in age from 20 to 84 years (median, 54). Of the 260 patients for whom menopausal status was known, 111 (42.7%) were premenopausal.
All patients underwent radical surgery, which consisted of modified radical mastectomy with axillary dissection in 214 and conservative breast surgery with axillary dissection in 56. The number of removed lymph nodes ranged from 1 to 42 (median, 15). In 30 patients (11 with modified radical mastectomy and 19 with conservative breast surgery), surgery was followed by breast irradiation. Thirty eight patients received adjuvant systemic treatment: 34 were treated by chemotherapy, two by hormonal treatment, and two by both.
Pathology
All surgical specimens were received fresh and the tumours were immediately incised to obtain material for steroid receptor determination; at least one slice of tumour tissue was immediately fixed in 10% buffered formalin.
Tumour size and histological grade (originally determined according to the BRG by five pathologists for 218 tumours) were retrieved from pathology reports. One of the authors (SFG) re-examined the original haematoxylin and eosin slides and re-determined the histological grade according to the Nottingham scheme.1 This grading method evaluates three parameters and assigns a score of 1 to 3 for each parameter as follows: tubule formation (> 75%, 1; 10–75%, 2; < 10%, 3), nuclear pleomorphism (none, 1; moderate, 2; pronounced, 3), and number of mitoses/10 high power fields (HPF), based on a HPF size of 0.274 mm2 (< 10 mitoses, 1; 10–19 mitoses, 2; ≥ 20 mitoses, 3). The final NHG is based on the sum of the scores of the three parameters: 3, 4, or 5 = grade 1; 6 or 7 = grade 2; and 8 or 9 = grade 3). Because the HPF diameter of the microscope used in our study was 0.5 mm, the cut off points for mitotic score were adjusted to: < 7 mitoses, 1; 7–13 mitoses, 2; ≥ 14 mitoses, 3. In addition to analysis within the NHG, the mitotic index (MI) was analysed separately using the tertile values—that is, 2 and 8 mitoses/10 HPF—as cut off points.
Statistical analysis
Correlations between various clinicopathological features were assessed using the χ2 test.
Survival curves for cancer specific survival (CSS) were constructed using the method of Kaplan and Meier and the differences between groups were assessed with the log rank test; when groups were ordered, the log rank test for trend was used. Cox's regression model was used to evaluate the predictive power of various factors in multivariate analysis. For calculating CSS, the interval between surgery and death caused by or accompanied by clinical evidence of breast cancer was used; patients dying from unrelated causes were censored at the time of their death as were those still alive at the time of their last follow up. The median follow up for censored cases was 12.5 years (range, 7 months to 16 years).
RESULTS
Histological type, grade, and MI
Most patients (n = 212; 78.5%) had invasive ductal carcinoma, followed by invasive lobular carcinoma (n = 30; 11.1%), and other special types of carcinoma (n = 28; 10.4%), of which there were nine mucinous, nine tubular, eight cribriform, and two medullary carcinomas.
Table 1 shows the distribution of grade and scores for the individual components. The distribution of individual scores was rather uneven: approximately two thirds of tumours were assigned score 3 for tubular formation, score 2 for pleomorphism, and score 1 for mitotic counts. Although there was no significant relation between NHG and menopausal status or age, NHG showed a strong relation to tumour type (p = 0.0001) and, to a lesser degree, size (p = 0.02).
Table 1.
Distribution of histological grade and scores for each of its components
| 1 | 2 | 3 | |
| Grade | 104 (38.5%) | 103 (38.1%) | 63 (23.3%) |
| Tubular score | 20 (7.4%) | 69 (25.6%) | 181 (67.0%) |
| Pleomorphism score | 58 (21.5%) | 167 (61.9%) | 45 (16.7%) |
| Mitotic score | 172 (63.7%) | 36 (13.3%) | 62 (23.0%) |
Comparison of the NHG, which was determined on re-examination of the slides, and the BRG, which had been determined originally in 212 tumours, showed complete agreement in 133 cases (61%); in only four cases (1.8%) did the two grades differ by more than one category.
Mitotic counts, which were evaluated in the most mitotically active areas, ranged from 0 to 87/10 HPF and showed a highly skewed distribution, with a mean of 10.5 and median of 4.
Survival analysis
During the follow up period there were 46 cancer related deaths; the five year CSS, 10 year CSS, and 15 year CSS for the whole group were 94.4%, 84.4%, and 76.9%, respectively.
In univariate analysis, CSS was not related to patient age, menopausal status, type of surgery, or adjuvant treatment. Patients with tumours measuring ≤ 1 cm had a slightly better survival, but the difference was not significant (p = 0.2). Histological type was a strong predictor of outcome (p = 0.0096): there were no cancer related deaths among patients with favourable special types of carcinoma, whereas patients with invasive lobular carcinoma had the worst survival.
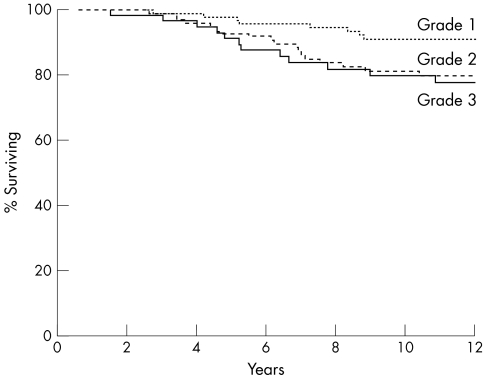
CSS was significantly related to NHG (p = 0.0044). As shown in fig 1, this was mainly the result of the better survival of patients with grade 1 tumours. When analysis of CSS was performed on the subgroup of tumours in which both the BRG and the NHG were determined, the prognostic strength of the NHG (p = 0.0011) was only slightly greater than that of the BRG (p = 0.0057).
Figure 1.
Cancer specific survival relative to Nottingham histological grade (p = 0.0044).
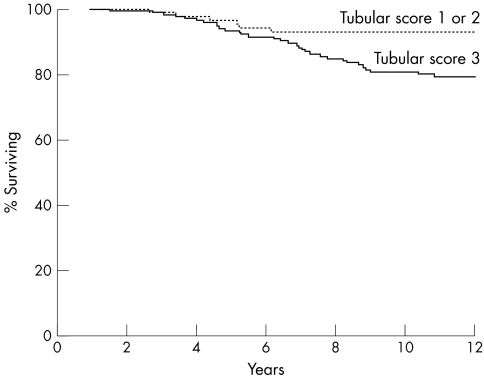
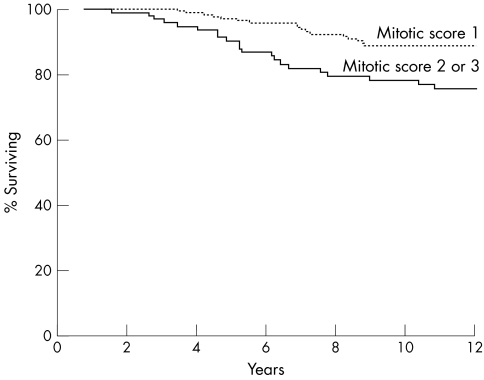
Among the three components of the NHG, tubule formation and mitotic count were significant predictors of CSS, whereas pleomorphism was not. A significantly better CSS was seen in patients with tumours with a tubule formation score 1 or 2 as opposed to those with score 3 (p = 0.0065; fig 2) and in tumours with mitotic score 1 as opposed to those with score 2 or 3 (p = 0.0052; fig 3). When tubule formation and mitotic counts were entered into a Cox's multivariate model, they both retained independent prognostic significance (table 2). However, when histological type was also included in the model, the tubule formation score was no longer an independent predictor of survival.
Figure 2.
Cancer specific survival relative to scores for tubule formation (p = 0.0065).
Figure 3.
Cancer specific survival relative to scores for mitotic counts (p = 0.0052).
Table 2.
Relation of tubule formation and mitotic counts to cancer specific survival in multivariate analysis
| Component of NHG | RR | 95% CI | p Value |
| Tubule formation (score 3 v 1 or 2) | 2.3 | 1 to 5.1 | 0.038 |
| Mitotic counts (score 2 or 3 v 1) | 1.8 | 1 to 3.3 | 0.046 |
CI, confidence interval; NHG, Nottingham histological grade; RR, relative risk.
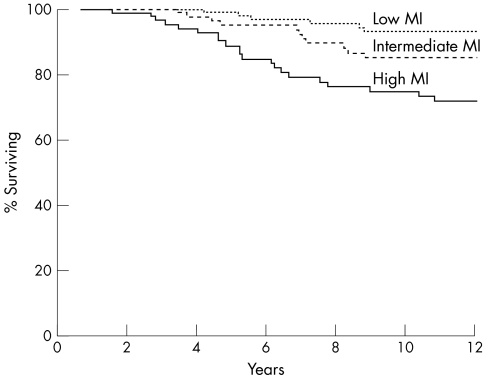
Because almost two thirds of the tumours scored 1 point for mitotic counts, we performed additional analysis after dividing the tumours according to the tertile values into those with low MI (≤ 2 mitoses/10 HPF; n = 100), intermediate MI (3–8 mitoses/10 HPF; n = 84), and high MI (≥ 9 mitoses/10 HPF; n = 86). Defined in this way, MI was a better predictor of CSS than the NHG or any of its components (p = 0.0001; fig 4). When all variables were entered into a Cox's multivariate model, MI was found to be the strongest prognostic factor and histological type was the only additional factor that retained independent prognostic significance.
Figure 4.
Cancer specific survival relative to mitotic index (p = 0.0001). MI, mitotic index.
DISCUSSION
Although patients with pT1N0M0 breast carcinoma have an excellent short term prognosis, more than 20% will eventually develop distant metastases and die of the disease. As already pointed out by others,11,12 and as also shown by our study, late breast carcinoma related deaths are not uncommon in this group of patients, and long term follow up is necessary for the evaluation of possible prognostic factors.
“Although patients with pT1N0M0 breast carcinoma have an excellent short term prognosis, more than 20% will eventually develop distant metastases and die of the disease”
Tumour size has long been regarded as the most important prognostic factor in patients with pT1N0M0 beast carcinoma; therefore, our finding that survival was not significantly better for tumours measuring 1 cm or less (pT1ab) as opposed to larger (pT1c) tumours is rather unexpected. This result may be partly because the proportion of smaller tumours in our series was relatively low. However, it should also be noted that a significant survival difference between pT1ab and pT1c tumours was confirmed in only five published studies11,13–16; in the remaining five,12,17–20 the difference failed to reach significance.
Whereas nuclear grade11,21,22 or histological grade determined according to the BRG12,15 have been shown to be of prognostic value in patients with pT1N0M0 breast carcinoma, we are unaware of any previous studies evaluating the influence of the NHG on long term survival in this group of patients. Although complete agreement between the BRG and NHG was only 61% in our series, they were almost equally good in predicting patient survival. A similar finding was reported by other investigators who compared the two grading systems in large series of lymph node positive tumours7 and stage I–IIA tumours.4
The distribution of grade scores in our series differs significantly from that reported in the original Nottingham series of breast carcinomas, in which almost one half of the tumours were assigned grade 3.1 Because a similar preponderance of grade 3 tumours is also observed in unselected symptomatic breast carcinomas seen at our institution, this probably reflects the selection criteria of our study.
When the three components of the NHG were examined separately, we found that only mitotic rate and tubule formation influenced survival. As shown by multivariate analysis, each one provided independent prognostic information; however, when histological type was also entered in the model, the tubule formation score was no longer significant.
Results of other studies that separately investigated the association between each component of the combined histological grade and prognosis are controversial. Whereas Davis et al found each one to be significantly correlated to outcome,23 only tubule formation and mitotic count,24,25 or only pleomorphism and mitotic count,26–29 were of significance in some studies, and only pleomorphism30,31 or only mitotic count4,32 in others. Comparisons of these results are difficult for several reasons. The studies differed in the number of patients, their stage of disease, and the duration of follow up. With a single exception,4 they did not use the NHG but the original BRG or a modification thereof and, in most studies, grading was limited to invasive ductal carcinomas. Some of these studies were limited to node negative patients,24,27 but only one dealt exclusively with pT1N0M0 tumours,31 whereas one focused on the subgroup of pT1abN0M0 tumours.28 Interestingly, in both of these, pleomorphism was a better predictor of outcome than mitotic counts, whereas the score for tubules was non-contributory.
The prognostic value of MI, but not of other components of histological grade, was also investigated by Joensuu et al in a group of 264 patients with pT1N0M0 tumours who were followed for a median of 17 years, and MI was shown to be a better prognostic variable for CSS than histological grade.12
Although nuclear pleomorphism has been shown to be a strong prognostic factor in some studies of breast carcinoma, this morphological feature is the most difficult to define and measure objectively and therefore its reproducibility is generally the poorest among grade components.33,34 In contrast, MI is, by definition, a quantifiable feature; however, its assessment is hampered by many sources of variation, such as variations in criteria for identifying an acceptable mitotic figure, variations in section thickness and microscopic field size, and intratumoral heterogeneity of mitotic activity. Despite these problems, it has been shown that mitosis counting can be performed in a highly reproducible way if a strict protocol is carefully followed.35
Optimal tissue fixation and preservation are a prerequisite for accurate histological grading and, in particular, mitosis counting. It has been shown that a delay in fixation may substantially decrease the number of detectable mitoses.36 Although this phenomenon was traditionally attributed to the completion of mitosis, inability to recognise mitotic figures in poorly preserved tissue seems a more plausible explanation.37 Because at least one part of each tumour in our series was fixed immediately upon receipt from the operating theatre, the relatively low mitotic counts observed in our cases cannot be attributed to fixation delay.
In the few studies that provided data regarding the distribution of mitotic scores within the NHG and in which tumours of various sizes were included, the prevalence of scores was also very uneven and most tumours were assigned the lowest mitotic score.4,9,33 Because it has been shown that there is a significant correlation between tumour size and proliferative activity,38 it is not surprising that in our series, which was limited to pT1N0M0 cases, almost two thirds of the tumours were assigned score 1 for mitotic counts. However, with such an unbalanced distribution, the statistical power for assessing the prognostic effect of mitotic counts may be greatly reduced.
Our findings suggest that the prognostic value of MI might be improved with the use of cut off points that are lower than those proposed for scoring mitotic counts within the NHG. Some published data also seem to support this notion. Thus, in the study of 825 pT12N01M0 breast carcinomas by Genestie et al,4 lowering of cut off points resulted in a stronger prognostic value of the MI. Similarly, Simpson et al,9 who recently studied 560 node positive patients, further divided the tumours in the lowest mitotic group (< 10 mitoses/10 HPF) into two categories. In their study, MI was found to be the most significant prognostic factor; its effect was mainly a result of the difference between the group with < 3 mitoses/10 HPF and those with ≥ 3 mitoses/10 HPF.
Take home messages.
The Nottingham histological grade (NHG) is of prognostic value in pT1N0M0 breast carcinoma
Among the components of the NHG, tubule formation and mitotic counts were independent predictors of survival, whereas pleomorphism was not
The proposed cut off points for mitotic counts may be too high for this particular group of tumours
In our study, MI was a stronger predictor of outcome than the NHG. However, this finding should be interpreted with caution. Whereas with regard to NHG our study was a confirmatory validation study, because NHG was determined according to previously proposed criteria, the prognostic value of MI was analysed on the same set of data from which the cut off points were derived. Although we did not use the much criticised “optimal cut off point” approach, which generally leads to a considerable overestimation of the effect of the prognostic factor,39,40 but rather chose the tertile values as cut off points, our results could still be too optimistic and should be validated in independent sets of patients. Nevertheless, the findings of our study suggest that in early stage breast carcinoma the prognostic value of MI may be concealed to some extent when using the NHG criteria for MI grouping.
Acknowledgments
This work was supported in part by grant J3-2411-0302-00 from the Slovenian Ministry of Science and Technology.
Abbreviations
BRG, Bloom-Richardson grading system
CI, confidence interval
CSS, cancer specific survival
HPF, high power fields
MI, mitotic index
NHG, Nottingham histological grade
RR, relative risk
This paper was presented in part at the 17th European Congress of Pathology, Barcelona, September 1999
REFERENCES
- 1.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403–10. [DOI] [PubMed] [Google Scholar]
- 2.Balslev I, Axelsson CK, Zedeler K, et al. The Nottingham prognostic index applied to 9,149 patients from the studies of the Danish breast cancer cooperative group (DBCG). Breast Cancer Res Treat 1994;32:281–90. [DOI] [PubMed] [Google Scholar]
- 3.Barbareschi M, Caffo O, Veronese S, et al. Bcl-2 and p53 expression in node-negative breast carcinoma: a study with long-term follow-up. Hum Pathol 1996;27:1149–55. [DOI] [PubMed] [Google Scholar]
- 4.Genestie C, Zafrani B, Asselain B, et al. Comparison of the prognostic value of Scarff-Bloom-Richardson and Nottingham histological grades in a series of 825 cases of breast cancer: major importance of the mitotic count as a component of both grading systems. Anticancer Res 1998;18:571–6. [PubMed] [Google Scholar]
- 5.Kollias J, Murphy CA, Elston CW, et al. The prognosis of small primary breast cancers. Eur J Cancer 1999;35:908–12. [DOI] [PubMed] [Google Scholar]
- 6.Nixon AJ, Schnitt SJ, Gelman R, et al. Relationship of tumor grade to other pathologic features and to treatment outcome of patients with early stage breast carcinoma treated with breast-conserving therapy. Cancer 1996;78:1426–31. [DOI] [PubMed] [Google Scholar]
- 7.Pinder SE, Murray S, Ellis IO, et al. The importance of the histologic grade of invasive breast carcinoma and response to chemotherapy. Cancer 1998;83:1529–39. [PubMed] [Google Scholar]
- 8.Reed W, Hannisdal E, Boehler PJ, et al. The prognostic value of p53 and c-erb B-2 immunostaining is overrated for patients with lymph node negative breast carcinoma: a multivariate analysis of prognostic factors in 613 patients with a follow-up of 14–30 years. Cancer 2000;88:804–13. [DOI] [PubMed] [Google Scholar]
- 9.Simpson JF, Gray R, Dressler LG, et al. Prognostic value of histologic grade and proliferative activity in axillary node-positive breast cancer: results from the Eastern cooperative oncology group companion study, EST 4189. J Clin Oncol 2000;18:2059–69. [DOI] [PubMed] [Google Scholar]
- 10.Sundquist M, Thorstenson S, Brudin L, et al. Applying the Nottingham prognostic index to a Swedish breast cancer population. South East Swedish breast cancer study group. Breast Cancer Res Treat 1999;53:1–8. [DOI] [PubMed] [Google Scholar]
- 11.Rosen PP, Groshen S. Factors influencing survival and prognosis in early breast carcinoma (T1N0M0–T1N1M0). Assessment of 644 patients with median follow-up of 18 years. Surg Clin North Am 1990;70:937–62. [DOI] [PubMed] [Google Scholar]
- 12.Joensuu H, Pylkkänen L, Toikkanen S. Late mortality from pT1N0M0 breast carcinoma. Cancer 1999;85:2183–9. [DOI] [PubMed] [Google Scholar]
- 13.Abner AL, Collins L, Peiro G, et al. Correlation of tumor size and axillary lymph node involvement with prognosis in patients with T1 breast carcinoma. Cancer 1998;83:2502–8. [PubMed] [Google Scholar]
- 14.Moon TE, Jones SE, Bonadonna G, et al. Development and use of a natural history data base of breast cancer studies. Am J Clin Oncol 1987;10:396–403. [DOI] [PubMed] [Google Scholar]
- 15.Rauschecker HF, Sauerbrei W, Gatzemeier W, et al. Eight-year results of a prospective non-randomised study on therapy of small breast cancer. The German breast cancer study group (GBSG). Eur J Cancer 1998;34:315–23. [DOI] [PubMed] [Google Scholar]
- 16.Sosa JA, Diener-West M, Gusev Y, et al. Association between extent of axillary lymph node dissection and survival in patients with stage I breast cancer. Ann Surg Oncol 1998;5:140–9. [DOI] [PubMed] [Google Scholar]
- 17.Chaitchik S, Kabakow B, De Chabon A, et al. Prognostic factors in breast cancer—a pathological and immunological study of patients with stage 1 breast cancer. Eur J Surg Oncol 1987;13:499–504. [PubMed] [Google Scholar]
- 18.Quiet CA, Ferguson DJ, Weichselbaum RR, et al. Natural history of node-negative breast cancer: a study of 826 patients with long-term follow-up. J Clin Oncol 1995;13:1144–51. [DOI] [PubMed] [Google Scholar]
- 19.Stæl O, Dufmats M, Hatschek T, et al. S-phase fraction is a prognostic factor in stage I breast carcinoma. J Clin Oncol 1993;11:1717–22. [DOI] [PubMed] [Google Scholar]
- 20.Stenmark-Askmalm M, Stæl O, Olsen K, et al. p53 as a prognostic factor in stage I breast cancer. South-East Sweden breast cancer group. Br J Cancer 1995;72:715–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilik R, Mor C, Wolloch Y, et al. Histopathologic high risk factors influencing the prognosis of patients with early breast cancer (T1N0M0). Am J Surg 1986;151:460–4. [DOI] [PubMed] [Google Scholar]
- 22.Nealon TFJ, Nkongho A, Grossi C, et al. Pathologic identification of poor prognosis stage I (T1N0M0) cancer of the breast. Ann Surg 1979;190:129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis BW, Gelber RD, Goldhirsch A, et al. Prognostic significance of tumor grade in clinical trials of adjuvant therapy for breast cancer with axillary lymph node metastasis. Cancer 1986;58:2662–70. [DOI] [PubMed] [Google Scholar]
- 24.Parl FF, Dupont WD. A retrospective cohort study of histologic risk factors in breast cancer patients. Cancer 1982;50:2410–16. [DOI] [PubMed] [Google Scholar]
- 25.Zedeler K. Assessment and presentation of survival experience in the Danish breast cancer cooperative group. Acta Oncol 1988;27:649–62. [DOI] [PubMed] [Google Scholar]
- 26.Le Doussal V, Tubiana-Hulin M, Friedman S, et al. Prognostic value of histologic grade nuclear components of Scarff-Bloom-Richardson (SBR). An improved score modification based on a multivariate analysis of 1262 invasive ductal breast carcinomas. Cancer 1989;64:1914–21. [DOI] [PubMed] [Google Scholar]
- 27.Le Doussal V, Tubiana-Hulin M, Hacene K, et al. Nuclear characteristics as indicators of prognosis in node negative breast cancer patients. Breast Cancer Res Treat 1989;14:207–16. [DOI] [PubMed] [Google Scholar]
- 28.Stierer M, Rosen H, Weber R. Nuclear pleomorphism, a strong prognostic factor in axillary node-negative small invasive breast cancer. Breast Cancer Res Treat 1992;20:109–16. [DOI] [PubMed] [Google Scholar]
- 29.Stierer M, Rosen HR, Weber R, et al. Long term analysis of factors influencing the outcome in carcinoma of the breast smaller than one centimeter. Surg Gynecol Obstet 1992;175:151–60. [PubMed] [Google Scholar]
- 30.Rank F, Dombernowsky P, Jespersen NC, et al. Histologic malignancy grading of invasive ductal breast carcinoma. A regression analysis of prognostic factors in low-risk carcinomas from a multicenter trial. Cancer 1987;60:1299–305. [DOI] [PubMed] [Google Scholar]
- 31.Schumacher M, Schmoor C, Sauerbrei W, et al. The prognostic effect of histological tumor grade in node-negative breast cancer patients. Breast Cancer Res Treat 1993;25:235–45. [DOI] [PubMed] [Google Scholar]
- 32.Parham DM, Hagen N, Brown RA. Simplified method of grading primary carcinomas of the breast. J Clin Pathol 1992;45:517–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boiesen P, Bendahl PO, Anagnostaki L, et al. Histologic grading in breast cancer—reproducibility between seven pathologic departments. South Sweden breast cancer group. Acta Oncol 2000;39:41–5. [DOI] [PubMed] [Google Scholar]
- 34.Frierson HFJ, Wolber RA, Berean KW, et al. Interobserver reproducibility of the Nottingham modification of the Bloom and Richardson histologic grading scheme for infiltrating ductal carcinoma. Am J Clin Pathol 1995;103:195–8. [DOI] [PubMed] [Google Scholar]
- 35.van Diest PJ, Baak JP, Matze-Cok P, et al. Reproducibility of mitosis counting in 2,469 breast cancer specimens: results from the multicenter morphometric mammary carcinoma project. Hum Pathol 1992;23:603–7. [DOI] [PubMed] [Google Scholar]
- 36.Start RD, Flynn MS, Cross SS, et al. Is the grading of breast carcinomas affected by a delay in fixation? Virchows Arch A Pathol Anat Histopathol 1991;419:475–7. [DOI] [PubMed] [Google Scholar]
- 37.Bergers E, Jannink I, van Diest PI, et al. The influence of fixation delay on mitotic activity and flow cytometric cell cycle variables. Hum Pathol 1997;28:95–100. [DOI] [PubMed] [Google Scholar]
- 38.Moezzi M, Melamed J, Vamvakas E, et al. Morphological and biological characteristics of mammogram-detected invasive breast cancer. Hum Pathol 1996;27:944–8. [DOI] [PubMed] [Google Scholar]
- 39.Hilsenbeck SG, Clark GM, McGuire WL. Why do so many prognostic factors fail to pan out? Breast Cancer Res Treat 1992;22:197–206. [DOI] [PubMed] [Google Scholar]
- 40.Altman DG, Lausen B, Sauerbrei W, et al. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994;86:829–35. [DOI] [PubMed] [Google Scholar]