Abstract
Aims: No good predictive marker for the malignant transformation of potentially malignant oral lesions (PMOLs) is currently available. This study re-evaluated the value of p53 immunoexpression to predict malignant transformation of PMOLs after discounting possible confounding factors.
Methods: PMOLs from 18 patients who showed progression to carcinoma, 16 of the respective carcinomas, and PMOLs from 18 matched controls were evaluated by immunohistochemistry (IHC) for p53 expression. A mouse monoclonal antibody that detects wild-type and mutant forms of human p53 was used. The p53 immunostaining pattern was also correlated with the degree of dysplasia.
Results: Suprabasal p53 staining was significantly associated with high grades of dysplasia (p < 0.01). The specificity and positive predictive value (PPV) for malignant transformation of suprabasal p53 staining were superior to the assessment of dysplasia, but sensitivity was inferior. All carcinomas derived from PMOLs with suprabasal p53 showed strong p53 immunostaining. However, the absence of suprabasal p53 staining and/or dysplastic changes did not preclude malignant transformation in a considerable proportion of PMOLs.
Conclusions: This study confirms and extends previous findings that suprabasal p53 immunoexpression has a high PPV for malignant transformation of PMOLs and can be used as a specific marker for lesions that are at high risk for malignant transformation. The absence of suprabasal p53 staining (that is, absence of, or basal, p53 staining) is non-informative for prognostic purposes. Because of its limited sensitivity, p53 IHC is not a substitute for the assessment of dysplasia in the evaluation of PMOLs. Instead, p53 IHC emerges as a clinically useful supplement of histopathological assessment in the prognosis of PMOLs.
Keywords: Keywords potentially malignant oral lesion, p53, prognosis
Oral leukoplakias are a heterogeneous group of lesions unified by their predominantly white aspect and by their recognised, but variable, risk for malignant transformation.1 The range of histopathological features is wide and non-pathognomonic. Epithelial atrophy, hyperplasia with or without hyperkeratosis, and various grades of dysplasia or even carcinoma in situ can be found in lesions clinically classified as leukoplakia. Available figures suggest that approximately 5% of all leukoplakias will transform into cancer during a period of five years.1 Upon prolonged follow up, up to 20% of oral leukoplakias may undergo malignant transformation,2–4 and this figure may even be higher for certain clinical subtypes.5–7
“Approximately 5% of all leukoplakias will transform into cancer during a period of five years”
The need to follow up patients with oral leukoplakia is generally accepted, in view of the established premalignant character of some oral leukoplakias.1 However, there is still no consensus with regard to whether treatment should be mandatory at the preinvasive stage.8–10 This lack of a unifying strategy results mainly from the present unavailability of a good prognostic marker for malignant transformation, because it is generally recognised that early treatment may reduce oral cancer incidence and related morbidity/mortality.
The presence and degree of dysplasia are often used to predict malignant transformation.4,11 However, the assessment of dysplasia can be relatively subjective,12–14 and the predictive values for malignant transformation are far from ideal.3,4,13,15 Relevant biomarkers hold more promise as early prognostic markers.16 Among these, the p53 tumour suppressor gene (TP53) product, p53, deserves particular attention, not only because of its central role in genomic stability and cell cycle regulation,17,18 but also because its function is abrogated in most human cancers19 and, in the case of oral mucosa, also in preinvasive stages.16,20–24
Well documented biopsy material derived from potentially malignant oral lesions (PMOLs) with long term follow up is extremely rare. However, testing putative progression markers requires the analysis of these lesions, because only upon follow up will the true premalignant character of the lesion become apparent by confirmed malignant transformation.
Previously, we analysed p53 immunoexpression in a group of PMOLs with follow up, obtained from a Dutch population.25 Staining patterns were compared with those found in clinically and histologically normal epithelium from healthy individuals and in benign oral lesions, which served as controls.25 In that study, suprabasal p53 staining emerged as highly specific for PMOLs, with a high positive predictive value for malignant transformation.25 The simplicity of the technique allows p53 immunohistochemistry (IHC) to be used in routine/diagnostic laboratories; therefore, we wanted to investigate further suprabasal p53 immunostaining as an early biological marker in oral carcinogenesis.
In our present study, we analysed PMOLs collected from histopathology laboratories in Northern Ireland and compared the predictive values of degree of dysplasia and suprabasal p53 immunoexpression for progression to carcinoma. The aims were twofold, namely: (1) to increase the sample size of rare PMOLs with follow up and validate previous results; and (2) to discount possible confounding factors that were not taken into account in our earlier study.
Our results confirm and extend our previous findings that suprabasal p53 staining is a valuable marker to predict malignant transformation of PMOLs. Suprabasal p53 staining identifies lesions that, if not properly managed, will transform into carcinoma, and therefore p53 IHC provides valuable information for the individual patient. The assessment of dysplasia remains an important tool because it appears to be the most sensitive marker (in terms of the proportion of high risk lesions that it can identify) presently available. Nevertheless, the need to incorporate other markers in the evaluation of PMOLs becomes evident after the demonstration, in the Northern Irish population, of a considerably high proportion of lesions that progressed to carcinoma, despite the absence of both dysplasia and suprabasal p53 staining at the time of the biopsy.
METHODS
Patients and tissues
Fifty six formalin fixed, paraffin wax embedded biopsy specimens from patients identified from the records of all histopathology laboratories in Northern Ireland from 1975 to 1994 were evaluated for the expression of the p53 protein. Samples consisted of PMOLs (mainly leukoplakias) from patients for whom clinical and follow up data were available and, in cases progressing to oral squamous cell carcinoma (OSCC), the carcinomas that developed at the same intraoral site. In most of the PMOLs (31 of 36) an incisional biopsy was performed and the patient was followed up clinically. In the remaining five PMOLs an excisional biopsy was performed with the intention to treat the lesion and to prevent the development of OSCC.
Eighteen patients with PMOLs that progressed to OSCC were included in our study. From two of the carcinomas that developed in these patients, material was not available for analysis; the remaining 16 OSCCs that developed at the same site (n = 11) or in direct continuity with the PMOL (n = 5) were analysed. From the group of patients whose lesions did not progress to OSCC we selected, through a minimisation process,26 18 controls matched for variables such as sex, age, and smoking habits, follow up time, and site of lesions. The similarity between cases and controls with regard to most of the variables mentioned can be inferred from table 1.
Table 1.
Characteristics of the two groups of potentially malignant oral lesions analysed
| Cases | Controls | |
| Patients' sex | ||
| Male | 5 | 5 |
| Female | 13 | 13 |
| Patients' age (years) | ||
| Mean | 59.6 | 59.5 |
| SD | 14.3 | 13.5 |
| Range | 27–80 | 34–78 |
| Site of lesion | ||
| Tongue | 8 | 9 |
| Floor of mouth | 2 | 4 |
| Other location within the oral cavity | 8 | 5 |
| Patients' smoking habits* | ||
| Ex-smoker | 1 | 1 |
| Non-smoker | 3 | 4 |
| <10 cigarettes/day | 2 | 2 |
| 10–20 cigarettes/day | 3 | 0 |
| >20 cigarettes/day | 9 | 10 |
| Follow up time (months) | ||
| Mean | 60 | 77 |
| SD | 31 | 42 |
| Range | 6–138 | 24–160 |
| Field | ||
| 1 site (localised lesion) | 7 | 5 |
| >1 site/continuous (diffuse lesion) | 4 | 4 |
| >1 site/independent (multifocal lesions) | 7 | 9 |
Cases consisted of all potentially malignant oral lesions (PMOLs) that progressed to carcinoma for which material was available. Controls were selected from a larger group of PMOLs that did not progress to carcinoma to match the cases in number and in the parameters referred to in the table. Field refers to the number of oral sites occupied by PMOLs and the relative location. When diffuse and multifocal lesions were present in the oral mucosa of the same patient they were entered as multifocal.
*One patient from the cases was a pipe smoker and was not included in the table.
In addition, in four patients we have analysed two different biopsies taken from the PMOL over time (intervals of one to three years).
Immunohistochemical analysis
Sections (4 μm thick) were cut from formalin fixed and paraffin wax embedded tissues for immunohistochemical analysis and for histopathological confirmation of diagnosis.
The immunohistochemical procedure was described in detail previously.25 Briefly, sections were dewaxed in xylol and rehydrated through graded alcohols and water. Antigen retrieval was performed by boiling the sections in citrate solution (pH 6.0) in a microwave oven at 600 W (3 × 5 minutes). Endogenous peroxidase was blocked in 3% hydrogen peroxide in methanol. Non-specific sites of reactivity were blocked by preincubation in normal rabbit serum (15 minutes). The primary antibody used was a mouse monoclonal antibody raised against human p53, which recognises both mutant and wild-type p53 (clone D07; Dako Glostrup, Denmark). Incubation was performed at a 1/500 dilution, overnight, at 4°C. This was followed by a standard streptavidin–biotin complex technique, using diaminobenzydine as chromogen.25 A breast carcinoma with strong p53 expression was included as a positive control for the IHC procedure in each experiment performed. Negative controls were processed in the same manner as the samples, except that phosphate buffered saline was used instead of the primary antibody.
p53 scores
p53 staining profiles were classified according to the relative number of positive cells (nuclei) and the location of positive cells in the different epithelial layers, as described previously.25 Three categories were defined for PMOLs, namely: (1) negative, no nuclear p53 staining detected in any epithelial cell; (2) basal, nuclear p53 staining confined to the basal epithelial layer; (3) suprabasal, nuclear p53 staining in (basal and) suprabasal epithelial layers.
For carcinomas three categories were defined, namely: (1) negative, no nuclear p53 staining in any tumour cell; (2) +, nuclear p53 staining in less than 25% tumour cells; and (3) ++, nuclear p53 staining in more than 25% tumour cells.27
Grade of dysplasia
The grade of dysplasia was assessed on newly cut consecutive sections stained with haematoxylin and eosin by two oral pathologists (SN and I van der W), without knowledge of the p53 IHC results. Lesions were grouped as “no or mild dysplasia” and “moderate or severe dysplasia” for statistical analysis. This categorisation was performed for two reasons, namely: (1) by creating these two broad groups we attempted to reduce the subjectivity inherent to grading dysplasia25; and (2) lesions with moderate or severe dysplasia carry a higher risk for malignant transformation than mildly or non-dysplastic lesions.10
Value of p53 IHC and assessment of dysplasia as putative prognostic tests
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV)28 of p53 IHC and of assessment of dysplasia were calculated to estimate the clinical value of these parameters as markers of malignant potential. We took two different approaches when estimating the value of the markers (moderate or severe dysplasia and suprabasal p53 staining, respectively) in assessing the prognosis of the PMOL. In the first, we only considered whether the marker was present, regardless of its precise localisation in the histological section. For the second calculation we only included cases in which the marker extended into the margins of the histological section. The rationale for this second approach is that the identification of the marker(s) at the section margins suggests that cells with these alterations have been “left behind” in the patient's oral mucosa, which would have an effect on prognosis.
Statistical analysis
Statistical analysis was performed using SPSS 7.0 software. For those patients who underwent two biopsies in a PMOL over time, we only used the data from the second biopsy in the statistical analysis to prevent biasing the results. The χ2 test with continuity correction and Fisher's exact test were performed, when appropriate. p Values less than 0.05 were considered significant.
RESULTS
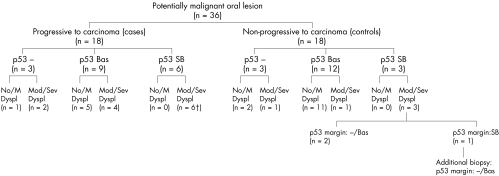
The clinical and demographic data of the patients and the patterns of p53 immunoexpression in the lesions are depicted in table 1A (published as supplementary data in JCP online; www.jclinpath.com). A summary of the natural history of the PMOLs and p53 immunostaining patterns and histopathology in relation to malignant progression is depicted in the flow diagram of fig 1.
Figure 1.
Flow diagram depicting the most relevant characteristics and natural history of the potentially malignant oral lesions analysed. The p53 immunoexpression patterns of the lesions are shown in relation to their histopathological characteristics and behaviour upon follow up. Lesions that did not progress to carcinoma (controls) were selected by minimisation to match the group of lesions that progressed to carcinoma (cases) in variables such as patients' sex, age, and smoking habits, location of the lesion, and time of follow up. p53 immunostaining: −, negative; Bas, basal; SB, suprabasal. No/M Dyspl, no or mild dysplasia; Mod/Sev Dyspl, moderate or severe dysplasia; †suprabasal p53 extended into the margin of the section in five cases; p53 margin, p53 immunostaining at the margin of the section.
Assessment of dysplasia and prognosis of PMOLs
Of the 18 control PMOLs 13 showed mild dysplasia or no signs of dysplasia (biopsies 1, 2, 4–14; table 1A online) and 5 lesions showed moderate or severe dysplasia (biopsies 3 and 15–18; table 1A online). The lesion was completely excised in four of these (biopsies 4 and 15–17; table 1A online), whereas in the remaining 14 non-progressive lesions an incisional biopsy was taken (biopsies 1–3, 5–14, and 18; table 1A online).
Six of the 18 PMOLs from the cases showed mild dysplasia or no signs of dysplasia (cases 21, 24–27, and 30; table 1A online), whereas 12 showed moderate or severe dysplasia (cases 19–20, 22–23, 28–29, and 31–36; table 1A online). Complete excision of the lesion was only performed in one dysplastic lesion from this group (biopsy 31a; table 1A online).
p53 immunoexpression in control lesions
Of the 18 control PMOLs, three showed no p53 staining (biopsies 1–3; table 1A online), 12 showed basal p53 staining (biopsies 4–15; table 1A online), and three showed suprabasal p53 staining (biopsies 16–18; fig 1; table 1A online). For two of these last three lesions, an excisional biopsy was performed; in both of these lesions suprabasal p53 staining was present in the central part of the histological section, but was absent from the margins of the section (biopsies 16 and 17; fig 1; table 1A online). In the remaining lesion showing suprabasal p53 staining an incisional biopsy was taken (biopsies 18a and 18b; table 1A online). In this biopsy, suprabasal staining extended into the margins of the histological section (biopsy 18a; table 1A online). However, suprabasal staining was absent in the margins of the section of a subsequent incisional biopsy, which was performed at the same site three years later (biopsy 18b; figs 1 and 2; table 1A online).
Figure 2.
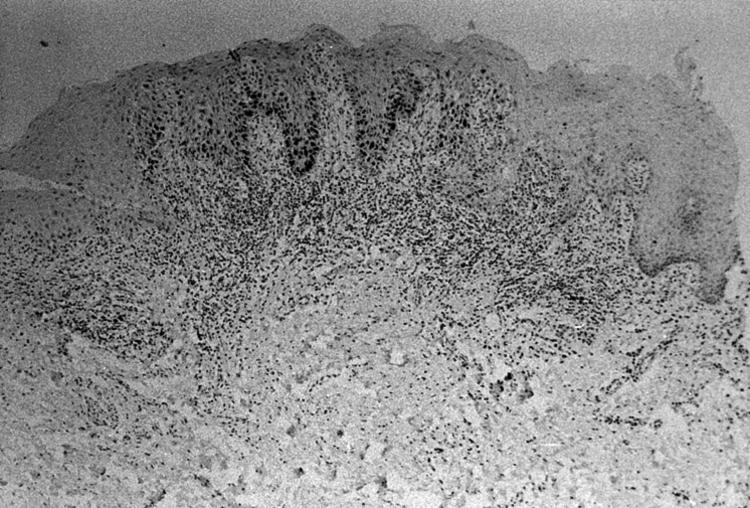
Biopsy of oral leukoplakia. Suprabasal p53 immunoexpression is seen in the centre of the histological section but is absent from its margins. This lesion did not progress to oral squamous cell carcinoma during follow up (p53 immunohistochemistry, counterstained with haematoxylin).
p53 immunoexpression in lesions from cases
Of the 18 PMOLs from the cases, three showed no p53 staining (biopsies 19a–21a; table 1A online), nine showed p53 staining in the basal layer (biopsies 22a–30a; table 1A online), and six lesions showed suprabasal p53 staining (biopsies 31a–36a; fig 1; table 1A online). In five of these cases suprabasal staining extended into at least one of the margins of the histological section (biopsies 31a–35a; figs 1 and 3; table 1A online); these included the only progressive lesion studied in which an excisional biopsy was attempted (biopsy 31a; table 1A online).
Figure 3.
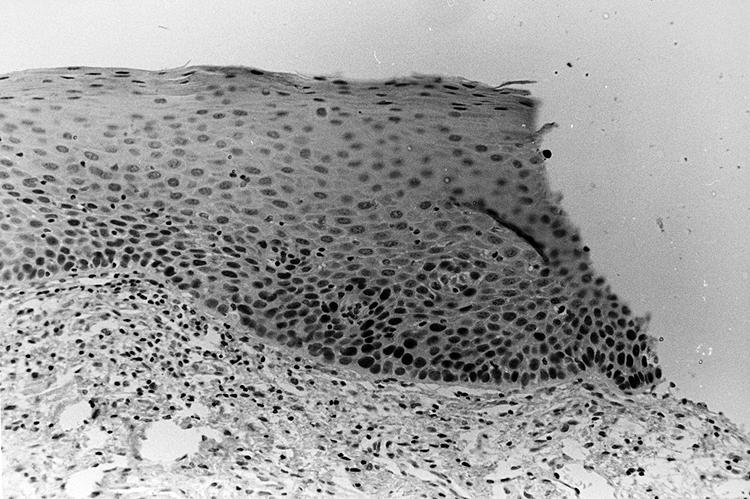
Biopsy of recurrent leukoplakia. Both the primary and recurrent lesions showed suprabasal p53 immunoexpression extending to the margin of the histological section. This lesion progressed to oral squamous cell carcinoma during follow up (p53 immunohistochemistry, counterstained with haematoxylin).
Similarity of (suprabasal) p53 expression in consecutive biopsies of the same lesion
In the four patients (three cases and one control) in whom two consecutive biopsies were analysed over time, both biopsies showed the same p53 immunoexpression pattern and similar dysplastic changes: three patients showed suprabasal staining in both biopsies (biopsies 18a and 18b, 31a and 31b, and 33a and 33b; table 1A online), and one patient showed p53 immunonegativity in both biopsies (biopsies 20a and 20b; table 1A online). Only the data from the second biopsy were included for statistical analysis to prevent biasing the results.
In five of the six cases where the PMOL showed suprabasal p53 staining (biopsies 31a–36a; table 1A online) the respective carcinoma was available for analysis (biopsies 31B–34B and 36B; table 1A online). All these carcinomas stained for p53 in more than 25% of the tumour cells (fig 4).
Figure 4.
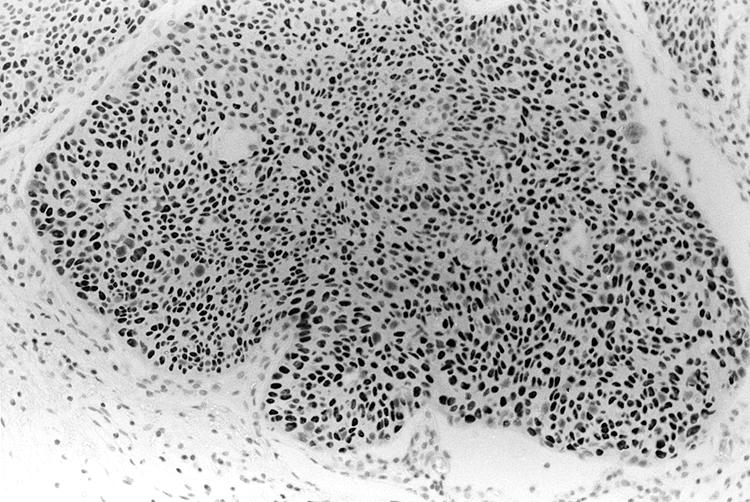
Biopsy of oral squamous cell carcinoma from a patient who presented previously with leukoplakia, in which suprabasal p53 staining was demonstrated, showing p53 immunoexpression in almost all tumour cells (p53 immunohistochemistry, counterstained with haematoxylin).
p53 immunoexpression in relation to dysplastic changes
A significant association was found between p53 immunoexpression and dysplastic changes in the lesions (p < 0.01). All 19 non-dysplastic or mildly dysplastic lesions showed no (biopsies 1, 2, and 21a; table 1A online) or basal p53 (biopsies 4–14, 24a–27a, and 30a; table 1A online) staining (fig 1). Of the 17 lesions with moderately or severely dysplastic changes, nine showed suprabasal staining (biopsies 16, 17, 18a and 18b, 31a and 31b, 32a, 33a and 33b, 34a, 35a, and 36a; table 1A online), five showed p53 staining restricted to the basal cell layer (biopsies 15, 22a, 23a, 28a, and 29a; table 1A online), and three revealed an absence of staining (biopsies 3, 19a, and 20a and 20b; fig 1; table 1A online).
Sensitivity, specificity, PPV, and NPV of p53 IHC and dysplasia in evaluating PMOLs
We estimated the clinical value of p53 IHC for assessing the prognosis of PMOLs and compared it with the assessment of dysplasia (table 2)—the marker that is currently used to predict malignant transformation.
Table 2.
Sensitivity, specificity, positive and negative predictive values of p53 immunohistochemistry (IHC) and assessment of dysplasia in the prognosis of potentially malignant oral lesions
| Marker | Sensitivity | Specificity | Positive predictive value (PPV) | Negative predictive value (NPV) |
| Moderate or severe dysplasia* | 12/18 (67%) | 13/18 (72%) | 12/17 (71%) | 13/19 (68%) |
| Suprabasal p53 immunoexpression* | 6/18 (33%) | 15/18 (83%) | 6/9 (67%) | 15/27 (56%) |
| Moderate or severe dysplasia† | 10/16 (62.5%) | 13/15 (87%) | 11/13 (85%) | 13/19 (68%) |
| Suprabasal p53 immunoexpression† | 5/17 (29%) | 15/15 (100%) | 5/5 (100%) | 15/27 (56%) |
*All lesions entered for analysis, irrespective of presence of the marker at margins of the histological section; †lesions harbouring the marker were only entered for analysis when this extended at least into one margin of the histological section.
Sensitivity: proportion of the “progressive lesions” (cases) that harbour the marker. Specificity: proportion of the “non-progressive lesions” (controls) that do not harbour the marker. PPV: proportion of the lesions harbouring the marker that progressed to carcinoma. NPV: proportion of the lesions not harbouring the marker that did not progress to carcinoma.
When all lesions were considered, independently of the location of the marker within the section, the assessment of dysplasia was more sensitive (67% v 33%) and showed a higher NPV (68% v 56%) than p53 IHC for malignant transformation of the lesions (table 2). Under the same conditions of analysis, the PPV of dysplasia was similar to p53 IHC (71% v 67%), but showed lower specificity (72% v 83%) (table 2). The combination of the assessment of dysplasia with p53 IHC under these conditions of analysis did not show improvement over the assessment of dysplasia alone, because there was a strong association between grade of dysplasia and p53 alterations in this series (all lesions showing suprabasal p53 staining showed moderate or severe dysplasia).
However, when we excluded cases where the marker did not extend into the margins of the histological section, the sensitivity of both markers decreased slightly, their NPVs remained unaltered, and both the specificity and PPV of both markers increased (table 2). The superior value of the p53 marker with regard to these last two parameters became evident—in our present series the specificity and PPV of suprabasal p53 staining for malignant transformation were 100% (table 2). The value of molecular alterations at the resection margins of the lesions is illustrated by case 31, where an excisional biopsy of the PMOL showed suprabasal p53 expression extending into a non-dysplastic or mildly dysplastic margin (biopsy 31a; table 1A online). The lesion recurred and the subsequent biopsy also showed suprabasal p53 staining (biopsy 31b; fig 3; table 1A online). Finally, an OSCC developed from this lesion which showed strong p53 staining (biopsy 31C; table 1A online).
DISCUSSION
In this study we have tested, retrospectively, the value of p53 IHC to predict the clinical behaviour of PMOLs, which were collected in Northern Ireland from 1975 to 1994. Because sensitive antibodies presently available recognise both wild-type and mutant forms of p53, we used suprabasal p53 immunoexpression as a surrogate of abnormal/non-physiological p53 expression, based on our previous study on a Dutch population, which suggested that suprabasal p53 immunostaining is a highly specific marker for premalignant lesions with high PPV for malignant transformation.25 The following factors could explain the high PPV of suprabasal p53 staining for malignant transformation found previously25 and form the rationale of the study: (1) the loss of tumour suppressor function is often the result of mutation of the TP53 gene19,29 and p53 mutant protein often shows an increased half life30; (2) absence of p53 tumour suppressor function allows the accumulation of genetic damage,17,18 which makes this gene and its product “key” elements in cancer pathways; and (3) in head and neck premalignancy inactivation of p53 is often preceded by at least two other genetic events (loss of heterozygosity (LOH) at 3p and 9p).24,31 In summary, alterations of this crucial gene, and/or its product, not only are commonly preceded by other genetic alterations, but facilitate the accumulation of further genetic alterations needed for the multistep process of carcinoma development.31
We found a predominance of non-dysplastic or mildly dysplastic lesions in the control group, whereas most dysplastic lesions from this group were totally excised and, therefore, were not expected to progress. This supports the current notion that histopathology is of value for the evaluation of PMOLs. Nevertheless, about a third of mildly dysplastic or non-dysplastic lesions progressed to carcinoma in our present series. In addition, two moderately/severely dysplastic lesions failed to show malignant progression (in spite of not being completely removed), and one such lesion recurred and progressed to carcinoma, despite an apparent total excision. Similar findings have been reported by others, reinforcing the idea that the value of histopathology to predict malignant transformation of a PMOL is still limited for the individual patient.
All lesions showing suprabasal p53 staining at the margins of the histological section eventually developed into OSCC, whereas in three of the four cases where these alterations were absent from the margins the lesion did not recur or progress to OSCC. This suggests that the presence of suprabasal p53 staining, in addition to being a marker of malignant potential, may help to assess the adequacy of excision. One patient in our series particularly illustrates the potential value of p53 suprabasal staining at the resection margins (patient 31; table 1A online). Although apparently totally excised, the PMOL showed suprabasal p53 staining at a resection margin categorised as “no or mild dysplasia” and this patient developed a recurrence that later transformed into an OSCC. Both the recurrence and OSCC lesion overexpressed p53, suggesting that cells with p53 alterations left at the margin of the biopsy might have been responsible for the malignant transformation.
Interestingly, one patient in our study presented suprabasal p53 staining in the central part of the histological section, and developed carcinoma at the same intra-oral site, despite apparently p53 immunonegative margins. The fact that this particular carcinoma was strongly p53 immunopositive suggests that we might have missed an important area of the biopsy margin, which obviously cannot be analysed entirely in one histological section.
The prognostic value of p53 immunoexpression has been investigated in several studies but conflicting data have been reported.32–34 Some have found no evidence for the clinical usefulness of p53 IHC, and a possible reason for such negative findings is the lack of use of a cut off value for normal p53 immunoexpression. In line with our results, Ball et al found an increased odds ratio to develop local recurrence when at least one tumour surgical margin was p53 immunopositive,33 and Brennan et al confirmed these findings by showing that TP53 mutations specific for the primary tumour were present at the tumour resection margins and that these were predictive of local recurrence.34 This indicates that biological markers at the resection margins of lesions (in particular p53 alterations) might be more sensitive in the prediction of local recurrence than histopathology alone.
The maintenance of suprabasal p53 staining in consecutive PMOL biopsies performed over time, and overexpression of p53 in the respective carcinomas that developed in those lesions (as shown in this and our previous studies)25,27 suggest that suprabasal p53 staining reflects a permanent genetic alteration rather than a temporary stabilisation of the p53 protein by an epigenetic event. Although we did not perform mutational analysis in these cases, mainly because of the limited material available, several molecular studies support this hypothesis by demonstrating identical LOHs in consecutive biopsies of the same lesion; namely, at the TP53 locus.31,35 In particular, Koch et al demonstrated the same TP53 mutation in an OSCC, its histological normal margin, and its lymph node metastasis.36 Altogether, these data suggest that an early TP53 mutation is maintained during all subsequent stages in the process of oral carcinogenesis.
Suprabasal p53 staining was significantly associated with the presence of moderate or severe dysplasia. This association was previously found in non-invasive margins immediately adjacent to OSCCs,27 but not in our series of PMOLs from Dutch patients, where a considerable proportion of lesions classified as no or mild dysplasia showed suprabasal p53 staining.25 In our present study, suprabasal p53 staining was seen in the section margin of lesions categorised as no or mild dysplasia in which the central part of the section revealed moderate to severe dysplasia, supporting our previous findings that this alteration can occur before the development of high grade dysplasia.
The specificity and PPV of p53 suprabasal staining were high, reaching 100% when this staining pattern extended into the margins of the histological section. The latter criteria might be important because when the entire “clone” of p53 altered cells is excised it will be unable to influence further progression. However, the sensitivity and NPV of p53 IHC remained rather low to be useful for clinical purposes. This was expected because several forms of p53 abrogation do not result in IHC detectable p53 (previously discussed by Cruz and colleagues25). Therefore, p53 IHC cannot be used as a single marker to rule out malignant potential, and negative or basal p53 staining should be considered as non-informative for prognostic purposes. It is still unclear whether these lesions harbour p53 alterations that cannot be recognised by the methodology used, whether p53 abrogation occurs later in the progression of the lesion, or whether the malignant transformation of these lesions follows different carcinogenic pathways in which p53 abrogation does not play a role.
Take home messages.
Suprabasal p53 immunoexpression is highly predictive for malignant transformation of potentially malignant oral lesions (PMOLs) and can be used as a specific marker for lesions that are at high risk for malignant transformation
The absence of suprabasal p53 staining cannot be used for prognostic purposes
p53 staining is a clinically useful supplement in the histopathological assessment of the prognosis of PMOLs
“This was expected because several forms of p53 abrogation do not result in IHC detectable p53”
It is beyond the scope of this paper to discuss the advantages and disadvantages of mutational analysis versus IHC. Among these, the costs, labour, and expertise involved, the sensitivity and specificity of the methods, and the possibility of preserving tissue morphology to localise p53 alterations in the margin would certainly be worth considering.
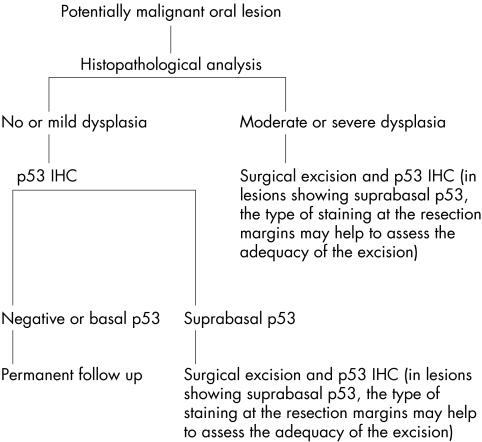
CONCLUSION
This study confirms and extends our previous findings that suprabasal p53 immunostaining is predictive for the malignant transformation of PMOLs. Based on these findings we propose a protocol for the evaluation of PMOLs (fig 5). We believe that by combining histopathological analysis with p53 IHC the evaluation of PMOLs will be improved. This hypothesis is supported by the study of Lee et al,37 who found the strongest predictive values for the development of carcinoma in PMOLs, by combining histopathology with three other biomarkers, which included p53 protein (over)expression.
Figure 5.
Protocol proposed for the evaluation of potentially malignant oral lesions. ICH, immunohistochemistry.
Supplementary Material
Abbreviations
IHC, immunohistochemistry
NPV, negative predictive value
OSCC, oral squamous cell carcinoma
PMOL, potentially malignant oral lesion
PPV, positive predictive value
References
- 1.Axéll T, Pindborgg JJ, Smith CJ, et al. Oral white lesions with special reference to precancerous and tobacco-related lesions: conclusions of an international symposium held in Uppsala, Sweden, May 18–21, 1994. J Oral Pathol Med 1996;25:49–54. [DOI] [PubMed] [Google Scholar]
- 2.Leonardelli GB, Talamazzi F. Leucoplasie del caro orale e precancerosi, Nota I. Archivo Italiano di Otologia Rinologia e Laringologia 1950;61:107–14. [PubMed] [Google Scholar]
- 3.Schepman KP, van der Meij EH, Smeele LE, et al. Malignant transformation of oral leukoplakia: a follow-up study of a hospital-based population of 166 patients with oral leukoplakia from The Netherlands. Oral Oncol 1998;34:270–5. [PubMed] [Google Scholar]
- 4.Silverman S, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer 1984;53:563–8. [DOI] [PubMed] [Google Scholar]
- 5.Hansen LS, Olson JA, Silverman S, Jr. Proliferative verrucous leukoplakia: a long-term study of 30 patients. Oral Surg Oral Med Oral Pathol 1985;60:285–98. [DOI] [PubMed] [Google Scholar]
- 6.Silverman S, Jr, Gorsky M. Proliferative verrucous leukoplakia: a follow-up of 54 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;84:154– 7. [DOI] [PubMed] [Google Scholar]
- 7.Suarez P, Batsakis JG, El-Naggar AK. Leukoplakia: still a gallimaufry or is progress being made? A review. Adv Anat Pathol 1998;5:137–55. [PubMed] [Google Scholar]
- 8.Marley JJ, Cowan CG, Lamey PJ, et al. Management of potentially malignant oral mucosal lesions by consultant UK oral and maxillofacial surgeons. Br J Oral Maxillofac Surg 1996;34:28–36. [DOI] [PubMed] [Google Scholar]
- 9.Tradati N, Grigolat R, Calabrese L, et al. Oral leukoplakias: to treat or not? Oral Oncol 1997;33:317–21. [DOI] [PubMed] [Google Scholar]
- 10.Van der Waal I, Schepman KP, van der Meij EH, et al. Oral leukoplakia: a clinicopathological review. Oral Oncol 1997;33:291–301. [DOI] [PubMed] [Google Scholar]
- 11.Burkhardt A. Advanced methods in the evaluation of premalignant lesions and carcinomas of the oral mucosa. J Oral Pathol 1985;14:751–78. [DOI] [PubMed] [Google Scholar]
- 12.Karabulut A, Reibel J, Therkildsen MH, et al. Observer variability in the histologic assessment of oral premalignant lesions. J Oral Pathol Med 1995;24:198–200. [DOI] [PubMed] [Google Scholar]
- 13.Lumerman H, Freedman P, Kerpel S. Oral epithelial dysplasia and the development of invasive squamous cell carcinoma. Oral Surg Oral Med Oral Pathol 1995;79:321–9. [DOI] [PubMed] [Google Scholar]
- 14.Pindborg JJ, Reibel J, Holmstrup P. Subjectivity in evaluating oral epithelial dysplasia, carcinoma in situ and invasive carcinoma. J Oral Pathol 1985;14:698–708. [DOI] [PubMed] [Google Scholar]
- 15.Waldron CA, Schaffer WG. Leukoplakia revisited: a clinicopathologic study of 3256 oral leukoplakias. Cancer 1975;36:1386–92. [DOI] [PubMed] [Google Scholar]
- 16.Mao L. Can molecular assessment improve classification of head and neck premaligancy? Clin Cancer Res 2000;6:321–2. [PubMed] [Google Scholar]
- 17.Lane DP. p53, guardian of the genome. Nature 1992;358:15–16. [DOI] [PubMed] [Google Scholar]
- 18.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell 1997;88:323–31. [DOI] [PubMed] [Google Scholar]
- 19.Greenblatt MS, Bennet WP, Hollstein M, et al. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res 1994;54:4855–78. [PubMed] [Google Scholar]
- 20.Kashiwazaki H, Tonoki H, Tada M, et al. High frequency of p53 mutations in human oral epithelial dysplasia and primary squamous cell carcinoma detected by yeast functional assay. Oncogene 1997;15:2667–74. [DOI] [PubMed] [Google Scholar]
- 21.Kusama K, Okutsu S, Takeda A, et al. p53 gene alterations and p53 protein in oral epithelial dysplasia and squamous cell carcinoma. J Pathol 1996;178:415–21. [DOI] [PubMed] [Google Scholar]
- 22.Lazarus P, Garewall H, Sciubba J, et al. A low incidence of p53 mutations in pre-malignant lesions of the oral cavity from non-tobacco users. Int J Cancer 1995;60:458–63. [DOI] [PubMed] [Google Scholar]
- 23.Quin G-Z, Park JY, Chen S-Y, et al. A high prevalence of p53 mutations in pre-malignant oral erythroplakia. Int J Cancer 1999;80:345–8. [DOI] [PubMed] [Google Scholar]
- 24.Califano J, van der Riet P, Westra W, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res 1996;56:2488–92. [PubMed] [Google Scholar]
- 25.Cruz IB, Snijders PJ, Meijer CJ, et al. p53 expression above the basal cell layer is an early event of malignant transformation and has predictive value for developing squamous cell carcinoma. J Pathol 1998;184:360–8. [DOI] [PubMed] [Google Scholar]
- 26.Altman DG. Designing research. In: Altman DG, ed. Practical statistics for medical research. London: Chapman & Hall, 1995:74–106.
- 27.Cruz IB, Meijer CJLM, Snijders PJF, et al. p53 immunoexpression in non-malignant mucosa adjacent to oral squamous cell carcinoma: potential consequences for clinical management. J Pathol 2000;191:132–7. [DOI] [PubMed] [Google Scholar]
- 28.Altman DG. Some common problems in medical research. In: Altman DG, ed. Practical statistics for medical research. London: Chapman & Hall, 1995:396–439.
- 29.Hollstein M, Shomer B, Greenblatt M, et al. Somatic point mutations in the p53 gene of human tumors and cell lines: updated compilation. Nucleic Acids Res 1996;24:141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quartin RS, Levine AJ. A comparison of the properties of human p53 mutant alleles. In: Yang SS, Warner HR, eds. The underlying molecular, cellular, and immunological factors in cancer and aging. New York: Plenum Press, 1993:55–65. [DOI] [PubMed]
- 31.Rosin MP, Cheng X, Poh C, et al. Use of allelic loss to predict malignant risk for low-grade oral epithelial dysplasia. Clin Cancer Res 2000;6:357–62. [PubMed] [Google Scholar]
- 32.Ogden GR, Chisholm DM, Morris AM, et al. Overexpression of p53 in normal oral mucosa of oral cancer patients does not necessarily predict further malignant disease. J Pathol 1997;182:180–4. [DOI] [PubMed] [Google Scholar]
- 33.Ball VA, Righi PD, Tejada E, et al. p53 immunostaining of surgical margins as a predictor of local recurrence in squamous cell carcinoma of the oral cavity and oropharynx. Ear Nose Throat J 1997;76:818–23. [PubMed] [Google Scholar]
- 34.Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med 1995;332:429–35. [DOI] [PubMed] [Google Scholar]
- 35.Califano J, Westra WH, Meininger G, et al. Genetic progression and clonal relationship of recurrent premalignant head and neck lesions. Clin Cancer Res 2000;6:347–52. [PubMed] [Google Scholar]
- 36.Koch WM, Boyle JO, Mao L, et al. p53 gene mutations as markers of tumor spread in synchronous oral cancers. Arch Otolaryngol Head Neck Surg 1994;120:943–7. [DOI] [PubMed] [Google Scholar]
- 37.Lee JJ, Hong WK, Hittelman WN, et al. Predicting cancer development in oral leukoplakia: ten years of translational research. Clin Cancer Res 2000;6:1702–10. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.