Abstract
Despite its well known histological and clinical features, Hodgkin's lymphoma (HL) has recently been the object of intense research activity, leading to a better understanding of its phenotype, molecular characteristics, histogenesis, and possible mechanisms of lymphomagenesis. There is complete consensus on the B cell derivation of the tumour in most cases, and on the relevance of Epstein-Barr virus infection and defective cytokinesis in at least a proportion of patients. The REAL/WHO classification recognises a basic distinction between lymphocyte predominance HL (LP-HL) and classic HL (CHL), reflecting the differences in clinical presentation and behaviour, morphology, phenotype, and molecular features. CHL has been classified into four subtypes: lymphocyte rich, nodular sclerosing, with mixed cellularity, and lymphocyte depleted. The borders between CHL and anaplastic large cell lymphoma have become sharper, whereas those between LP-HL and T cell rich B cell lymphoma remain ill defined. Treatments adjusted to the pathobiological characteristics of the tumour in at risk patients have been proposed and are on the way to being applied.
Keywords: Hodgkin's lymphoma, differential diagnosis, subtypes, Epstein-Barr virus
Hodgkin's disease (HD) is a lymphoid tumour that forms less than 1% of all de novo neoplasms occurring every year world wide. Its diagnosis is based on the identification of characteristic multinucleated giant cells within an inflammatory milieu. These cells—termed Reed-Sternberg (RS) or diagnostic cells—represent the body of the tumour: they measure 20–60 μm in diameter and display a large rim of cytoplasm and at least two nuclei with acidophilic or amphophilic nucleoli, covering more than 50% of the nuclear area (fig 1A). The tumoral population also includes a variable number of mononuclear elements—Hodgkin's cells (HCs)—showing similar cytological features to RS cells and neoplastic cell variants, each corresponding to a specific subtype of HD. Molecular studies have recently shown that in most if not all cases RS cells, Hodgkin's cells, and cell variants belong to the same clonal population, which is derived from peripheral B and T cells in about 98% and 2% of cases, respectively.1–8 Accordingly, HD has been included among malignant lymphomas and the term “Hodgkin's lymphoma” (HL) has been proposed.6,9,10
Figure 1.
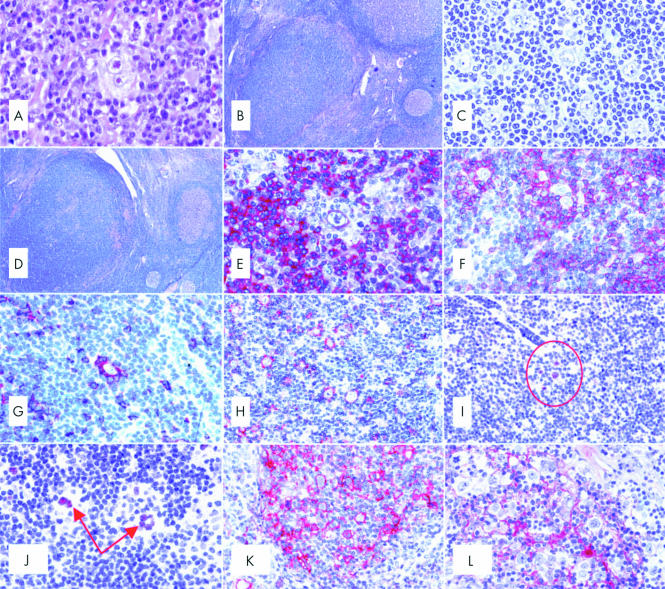
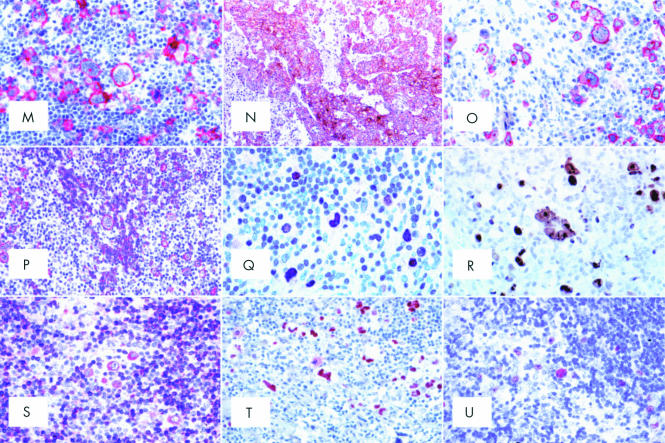
(A) A typical diagnostic Reed-Sternberg (RS) cell within a composite inflammatory milieu (haematoxylin and eosin; original magnification, ×500). (B) Nodular lymphocyte predominant Hodgkin's lymphoma (LP-HL): at low power, a neoplastic nodule can be seen as a densely packed cellular area with a high content of small lymphocytes (Giemsa; original magnification, ×40). (C) Nodular LP-HL: at higher magnification, some popcorn cells and one Reed-Sternberg-like element are detected among small lymphocytes (Giemsa; original magnification, ×450). (D) Nodular LP-HL: at low magnification, a progressively transformed germinal centre looks like a nodule of LP-HL; however, among small lymphocytes there are some centroblasts and centrocytes, but no popcorn cells (Giemsa; original magnification, ×40). (E) Nodular LP-HL: a popcorn cell and most small lymphocytes within a nodule express the B cell marker CD79a (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×500). (F) Nodular LP-HL: popcorn cells are surrounded by rosettes of CD3+ T cells (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×250). (G) Nodular LP-HL: rosetting T cells largely express CD57 (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×250). (H) Nodular LP-HL: popcorn cells express epithelial membrane antigen (EMA) (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×250). (I) Nodular LP-HL: popcorn cells (circled) express the bcl-6 gene product (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×250). (J) Nodular LP-HL: positivity of neoplastic elements for the Oct2 gene product (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×300). (K) Nodular LP-HL: popcorn cells strongly express CD40 (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×300). (L) Nodular LP-HL: neoplastic elements are found within a delicate meshwork of CD21+ follicular dendritic cells (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×300). (M) Common Hodgkin's lymphoma (CHL): Hodgkin and Reed-Sternberg (H&RS) cells express the CD30 molecule both at the cytoplasmic membrane and in the Golgi area (dot-like positivity) (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×350). (N) CD30 membrane bound positivity in an example of embryonal carcinoma (immunoperoxidase, SABC technique; Gill's haematoxylin counterstain; original magnification, ×200). (O) CHL: neoplastic cells show membrane bound and dot-like CD15 positivity (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×350). (P) CHL: H&RS cells display variable degrees of CD20 staining (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×200). (Q) CHL: staining for IRF4/MUM1 gene product (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×350). (R) CHL: most neoplastic cells express the Ki-67 molecule as revealed by the Mib-1 monoclonal antibody; some small lymphocytes are also in the cell cycle (immunoperoxidase, SABC technique; Gill's haematoxylin counterstain; original magnification, ×500). (S) CHL: moderate Bcl-2 protein expression by H&RS cells and a ratio of small lymphocytes (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×300). (T) CHL: most neoplastic cells show p53 overexpression (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×300). (U) CHL: H & RS show genomic Epstein-Barr virus integration by in situ hybridisation with fluorescein labelled EBER1/2 probes (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×300).
Although regarded as “diagnostic”, RS cells are not exclusive to HL because similar elements can be seen in reactive lesions (such as infectious mononucleosis), B and T cell lymphomas, carcinomas, melanomas, and sarcomas.11 Thus, the presence of an appropriate cellular background—along with the results of immunophenotyping—is basic for the diagnosis. The reactive milieu, which can form up to 99% of the total population examined, consists of small lymphocytes, histiocytes, epithelioid histiocytes, neutrophils, eosinophils, plasma cells, and fibroblasts in different proportions, depending on the histological subtype of HL. It is sustained by an autocrine and/or paracrine production of cytokines such as interleukin 2 (IL-2), IL-5, IL-6, IL-7, IL-9, IL-10, IL-13, basic fibroblast growth factor, transforming growth factor β1, tumour necrosis factor α (TNF-α), and thymus and activation related chemokine.12–16 The release of these molecules is also responsible for most of the symptoms recorded in patients with HL, in addition to the ability of the neoplastic cells to escape from growth controls and immunosurveillance. More recently, it has been proposed that hepatic growth factor and c-MET might constitute an additional signalling pathway between RS cells and the reactive cellular background, affecting adhesion, proliferation, and the survival of RS cells.16
HISTOPATHOLOGICAL CLASSIFICATION
In 1832, Sir Thomas Hodgkin provided the first macroscopic description of the process in a paper entitled “On some morbid appearances of the absorbent glands and spleen”. In 1898 and 1902, Carl Sternberg and Dorothy Reed independently described the typical “diagnostic” cells. In 1944, Jackson and Parker proposed the first comprehensive classification of the tumour (table 1). However, this classification was subsequently found to be clinically irrelevant, because most patients belonged to the granulomatous subtype and the response to treatment varied greatly from case to case.
Table 1.
Hodgkin's lymphoma (HL) classification schemes
| 1 | Jackson and Parker classification Paragranuloma |
| 2 | Granuloma |
| 3 | Sarcoma Lukes and Butler classification |
| 1 | Lymphocytic and/or histiocytic, nodular |
| 2 | Lymphocytic and/or histiocytic, diffuse |
| 3 | Nodular sclerosis |
| 4 | Mixed cellularity |
| 5 | Diffuse fibrosis |
| 6 | Reticular Rye conference classification |
| 1 | Lymphocyte predominance |
| 2 | Nodular sclerosis |
| 3 | Mixed cellularity |
| 4 | Lymphocytic depletion Revised European–American lymphoma (REAL) classification |
| 1 | Nodular lymphocyte predominance nodular/diffuse |
| 2 | Classic HL |
| Nodular sclerosis | |
| Mixed cellularity | |
| Lymphocyte depletion | |
| Lymphocyte rich classic HL diffuse (provisional entity) | |
| World Health Organisation scheme | |
| 1 | Nodular lymphocyte predominant HL |
| 2 | Classic HL |
| Nodular sclerosis HL (grades 1 and 2) | |
| Lymphocyte rich classic HL* | |
| Mixed cellularity HL | |
| Lymphocyte depleted HL | |
| Unclassifiable classic HL |
*This includes a nodular (common) and a diffuse (rare) form in contrast to the REAL classification.
In 1956, Smetana and Cohen identified a histopathological variant of granulomatous HD, which had a better prognosis and was characterised by sclerotic changes: this variant was termed “nodular sclerosis HD” in the classification proposed by Lukes, Butler, and Hicks in 1964 (table 1). This last classification, simplified at the Rye conference in 1965 (table 1) has been used routinely over the past 35 years because of the high interpersonal and intrapersonal reproducibility and good clinicopathological correlations.
In 1994, in the light of morphological, phenotypic, genotypic, and clinical findings, HL was listed in the revised European–American lymphoma (REAL) classification17 and subdivided into two main types: lymphocyte predominant (LP-HL) and common HL (CHL). CHL included the following subtypes: nodular sclerosis (NS-CHL), mixed cellularity (MC-CHL), lymphocyte depletion (LD-CHL), and the diffuse form of the lymphocyte rich CHL (LR-CHL) (table 1). This approach has finally been adopted by the recently developed World Health Organisation (WHO) scheme (table 1), which has promoted LR-CHL from a provisional entity to an accepted entity. In this classification, the nodular form of LR-CHL has been included, as proposed by the European lymphoma task force.6,18,19
It is noteworthy that HL subtyping should be performed only in lymph node biopsies at the onset of the disease: in fact, chemotherapy and/or radiotherapy modify the histopathological picture by inducing a lymphocyte depleted pattern.
LP-HL
LP-HL represents 4–5% of all HL cases,20 and differs greatly from the common type in terms of morphology, phenotype, genotype, and clinical behaviour (table 2). The only feature shared by LP-HL and CHL is the low number of neoplastic cells. For a long time, after the adoption of the Lukes classification, the tumour was also called “nodular paragranuloma”, a designation coined by the Kiel group,21 based on the term “paragranuloma” introduced much earlier by Jackson and Parker. This designation intended to underline the differences existing between this type of HL and the remaining ones.
Table 2.
Differential diagnosis between T cell rich, large B cell lymphoma (TCRBCL), lymphocyte predominant Hodgkin's lymphoma (LP-HL), and common Hodgkin's lymphoma (CHL)
| Diagnostic criteria | TCRBCL | LP-HL Nodular/diffuse | CHL |
| Neoplastic component | |||
| Cell distribution | Dispersed | Within the nodules | Dispersed |
| L&H/L&H-like cells | −/+ | +/− | − |
| RS/RS-like cells | −/+ | −/+ | − |
| CD45 expression | + | + | − |
| CD30 expression | −* | −* | + |
| CD15 expression | − | − | +/− |
| CD79a expression | +/− | −/+ | −/+ Variable |
| CD20 expression | +† | +† | −/+ Variable |
| BOB.1 expression | + | + | − |
| Oct2 expression | +‡ | +‡ | Rare |
| CD3 expression | − | − | −/+ |
| EMA expression | +/− | +/− | Rare |
| J chain expression | +/− | +/− | − |
| MIB-1/Ki-67 expression | High | High | High |
| EBV | − | − | + Variable |
| Ig gene rearrangement | + | + | +§ |
| Reactive component | |||
| T cells | Numerous | Moderate | Variable |
| T cells with irregular nuclear profile | +/− | − | −/+ |
| Bcl-6+/CD57+ rosettes | − | Numerous | − |
| Amount of TIA-1+ cells | Very high | Low | High |
| Amount of CD20+ small lymphocytes | Low | High | Low |
| Histiocytes | Variable | Some | Variable |
| FDC | − | + | + |
| Clinical findings | |||
| Stage | III/IV | I/II | I/III |
| Bone marrow involvement | +/− | − | − |
| Orderly progression in the spread | − | − | + |
EMA, epithelial membrane antigen; FDC, follicular dendritic cells; L/H, lymphocytic/histiocytic (popcorn); RS, Reed Sternberg.
*Weakly positive in some instances; †negative in rare instances; ‡usually overexpressed; §in 1–2% of the cases T cell receptor gene rearrangement.
Clinical findings
LP-HL displays features that are not generally encountered in CHL, which makes its clinical picture closer to that of “indolent” B cell lymphoma.22 First, it has a unimodal age distribution, with a single peak in the 4th decade, which contrasts with the two peaks of CHL, one in the 3rd and the other in the 7th decade.22 The disease usually affects single cervical, axillary, or inguinal nodes rather than groups of nodes. Bone marrow involvement is found only occasionally during staging procedures in patients whose disease appears to be limited to a single node22; this pattern of spread differs from the orderly progression classically seen in CHL.23 Involvement of the thymus is most unusual, unlike the other types of HL.22 The tumour has a very indolent course, with prolonged disease free intervals, despite a high rate of late relapses, which usually respond well to treatment.22,24 In addition, it can be associated with a diffuse large B cell lymphoma (DLBCL), which has a more favourable outcome than de novo large B cell lymphomas.22,25 In general, the prognosis is good and specific treatment protocols are now beginning to be used; it has even been questioned whether a patient whose disease is restricted to a single lymph node needs further treatment.22 These clinical findings alone justify a clear cut distinction between LP-HL and CHL, a concept that is supported by morphological and phenotypic data (see below).
Morphological findings
In most instances, the growth is—at least in part—nodular (fig 1B), with the occurrence of a diffuse variant of the process being very rare.22,26 The neoplastic population consists of large elements, called L&H (lymphocytic/histiocytic) or popcorn cells.22 The former term has almost completely been abandoned in the light of the confirmed lymphoid derivation of the tumour.2–7 Popcorn cells show nuclei resembling those of centroblasts, with a polylobular profile, finely dispersed chromatin, and small nucleoli, which are often adjacent to the nuclear membrane (fig 1C).22 Their cytoplasmic rim is narrow and basophilic when stained with Giemsa. Occasionally, neoplastic elements display the features of RS cells and/or of lacunar cells of NS-CHL and are associated with minimal sclerosis22; under these circumstances, immunophenotyping plays a fundamental role in the differential diagnosis between LP-HL and LR-CHL or NS-CHL (table 2). The reactive milieu consists of small lymphocytes with some plasma cells and epithelioid elements, which at times become so numerous as to mimic a histiocyte rich, large B cell lymphoma (HCRBCL).27
Progressively transformed germinal centres
Progressively transformed germinal centres (PTGCs)—first described by Lennert in collaboration with Müller-Hermelink in 197828—are a peculiar form of follicular hyperplasia, which can be confused with LP-HL.
PTGCs occur in children and young adults, and these individuals reveal a slightly higher risk of developing LP-HL than the average population. PTGCs can precede, concur with, or follow LP-HL.21
On morphological grounds, PTGCs are two to three times larger than reactive follicles and predominantly consist of small lymphocytes, mainly mantle cells, intermingled with some centroblasts and follicular dendritic cells (FDCs) (fig 1D). PTGCs can be differentiated from LP-HLs because of the lack of popcorn elements and their cytological composition: they are composed of a mixture of B (CD20+) and T (CD3+) cells, histiocytes, and FDCs, which overall produce a “motheaten” appearance.28,29
Phenotypic findings
The neoplastic cells have a characteristic profile, which differs greatly from that of CHL.6,17,19,22 In particular, they are CD45+, CD19+, CD20+, CD22+, CD79a+, J chain+/−, epithelial membrane antigen (EMA)+/−, and CD15−. CD30 positivity is rare and, when detected, weak. Interestingly, a certain number of extrafollicular reactive blasts (smaller than the popcorn cells) are detected by the anti-CD30 antibodies: in the past, they have been misinterpreted as tumoral elements.19 Popcorn cells regularly express Oct2 and BOB.1 (fig 1E–J).30 The transcription factor Oct2 and its coactivator BOB.1 play a basic role in immunoglobulin synthesis by triggering the specific gene promoter,31 and are excellent tools for the identification of neoplastic cells in LP-HL, in addition to their differentiation from those of CHL, which are negative in almost all instances.30
The derivation of the tumour from germinal centres is supported by:
(1) The expression of the bcl-6 gene product (fig 1I),32 CD40 (fig 1K), and CD86 by neoplastic cells.32,33
(2) The occurrence of numerous CD4+/CD57+ T cells surrounding the popcorn cells, as seen in normal germinal centres and PTGCs (fig 1F,G).34
(3) The presence of an FDC meshwork (CD21+/CD35+) within the nodules (fig 1L).35
Kraus and Haley36 have recently reported that LP-HL is characterised by Bcl-6/CD57 double stained small lymphocytes rosetting around typical CD20+/Bcl-6+ popcorn cells. These small lymphocytes correspond to a subset of CD57+ T helper cells found within the germinal centre, which coexpress Bcl-6 (B Falini et al. Presented at the Third International Symposium on Hodgkin's Lymphoma, Kolne, Germany, September 18–23, 1995). They are very useful for the differential diagnosis with PTGC, LR-CHL, and T cell rich B cell lymphoma (TCRBCL), which do not generally contain these double stained T cell rosettes.36 However, a proportion of TCRBCLs may show a pattern similar to the one observed in nodular LP-HL, supporting the view of Rüdiger et al that the borders between the two tumours are not always sharp and the diagnosis needs a combination of phenotypic features, including the CD21+ FDC pattern and the TIA-1/CD57 ratio.37 Finally, as revealed by their Ki-67 positivity, most popcorn cells are in cycle.
Genotypic findings
Further evidence indicating that the tumour is derived from germinal centre B cells has been provided by recent molecular studies, based on the single cell polymerase chain reaction (PCR).1–7 These studies have shown that popcorn cells in any given case represent monoclonal populations derived from germinal centre B cells, owing to the consistent occurrence of monoclonal Ig gene rearrangements and the high load of somatic mutations within variable region genes. Ongoing mutations are detected in about half of LP-HL cases: this finding—not observed in CHL—identifies mutating germinal centre cells as the precursors of the neoplastic elements.2,5 The pattern of mutation within these gene segments suggests that tumoral cells, their precursors, or both have been selected for expression of functional antigen receptors.2,5
Finally, to date, in situ hybridisation studies with Epstein-Barr virus (EBV) early RNA 1/2 (EBER1/2) probes, in addition to conventional Southern blot, PCR, and immunohistochemistry for the latent membrane protein 1 (LMP-1), have never detected EBV in the popcorn cells of LP-HD, in contrast to the neoplastic component of CHL.38,39 Isolated small lymphocytes from the reactive background carry EBV infection in 25% of cases of CHL.19
CLASSIC HD
This variant comprises about 95% of all HL cases and shows a typical bimodal age distribution, with a peak at 10–35 years of age and a second peak in late life.20 It is characterised by a series of clinical, morphological, phenotypic, and genotypic features, which are integrated by specific findings in the four subtypes of the process (nodular sclerosis, mixed cellularity, lymphocyte depletion, and lymphocyte rich). CHL has a peripheral B cell derivation in approximately 98% of cases, with the remaining ones originating from peripheral T cells.7,8
Clinical findings
CHL usually presents in the laterocervical lymph nodes, with peripheral extranodal involvement being very rare. About 50% of patients are in stage I or II. A mediastinal mass is seen in most patients with NS-CHL, at times showing the characteristics of “bulky” disease. Systemic symptoms—fever, night sweats, and body weight loss—are detected in approximately 25% of patients. In contrast to earlier reports, the histological subtype is not regarded as a major prognostic indicator. Without treatment, CHL has a moderately aggressive clinical course. With the present treatments, 70–80% of cases show long term survival. In the early stages of the disease, extended field irradiation has been the standard for decades and results in excellent cure rates. However, because of fatal longterm effects, especially the high rates of second solid tumours, extended field radiotherapy is now being abandoned by most study groups. Instead, mild chemotherapy for the control of occult disease is combined with involved field irradiation. In intermediate stage CHL, where combined modality treatment is the treatment of choice, extended field irradiation is substituted by involved field irradiation for the same reasons. In advanced stage CHL, eight cycles of polychemotherapy (plus additional radiotherapy for large tumour masses and residual lymphomas) for decades has cured only 50% to 60% of patients. The development of a new dose intensified regimen (such as BEACOPP) for the first time has significantly improved that prognosis. In relapsed CHL, recently published phase III studies suggest an improvement in the relapse free survival of patients using high dose chemotherapy. For a comprehensive review see Diehl and Josting.40
Morphological findings
In CHL, typical Hodgkin's and Reed-Sternberg (H&RS) cells (fig 1A) can be easily detected: their number (from few to many) differs from case to case. They may be associated with peculiar cell variants and are found within an inflammatory milieu, related to the histological subtype (see below). The lymph node structure is largely effaced, although remnants of normal follicles can be detected in some cases. The type of structural alteration is indeed characteristic in NS-CHL.
Phenotypic findings
In 1982, Schwab et al described a new monoclonal antibody, termed Ki-1, whose reactivity seemed restricted to H&RS cells and a small subset of normal lymphocytes with perifollicular location. However, the extensive application of the antibody showed that it was not specific to H&RS cells, as originally thought, but reacted with a variety of lymphoid tumours, including a previously unrecognised category, called anaplastic large cell lymphoma.41–43 Other reagents with similar characteristics have also been developed,44 and these reagents were gathered together at the third workshop on leucocyte differentiation antigens (Oxford, UK, 1986) to form the 30th cluster of monoclonal antibodies (CD30). The target recognised by these antibodies is a glycoprotein of 120 kDa, expressed by lymphoid elements after activation and formed by three distinct domains (intracytoplasmic, transmembranic, and external).11 It is encoded by a gene located at 1p36 and represents a member of the TNF receptor superfamily.45 As expected, its ligand (CD30L) belongs to a group of molecules that have homology to TNF. The external domain of CD30 is steadily cleaved by a metalloproteinase so that it can be detected and measured.11 Surface CD30 values seem to correlate with the size and diffusion of the tumour at presentation, thus representing a new possible prognostic indicator, independent of age, race, symptoms, and bulky disease.46 The CD30 molecule has also been proposed as a possible target for specific antibodies conjugated with plant toxins and administered to patients with CHL for therapeutic purposes: preliminary studies have shown these immunotoxins to have remarkable cytotoxic activity.47–50
On immunohistochemical analysis, both in paraffin wax embedded and frozen sections, the antibodies against CD30 produce different types of positivity: membrane bound or dot-like in the Golgi area (corresponding to the accumulation of the 90 kDa proteic precursor) and diffuse (fig 1M). The first two patterns are exclusive to lymphoid elements (fig 1M), with the exception of embryonic carcinoma (fig 1N),11,51 whereas the diffuse pattern can occur in a variety of malignant tumours other than lymphomas, including pancreatic carcinoma, nasopharyngeal undifferentiated carcinoma, and malignant melanoma.44 Therefore, the immunophenotypic diagnosis of HL should always be based on the application of a panel of antibodies, including reagents against cytokeratins, melanoma associated antigens, carcinoembryonic antigen, and placental alkaline phosphatase.11 Expression of the CD30 molecule by H&RS cells is seen in more than 98% of CHLs, although the intensity of the immunostaining can vary from one case to another, and even within the same case. Interestingly, the antigen is masked by fixation (especially prolonged fixation in formalin or fixation in B5): thus very efficient antigen retrieval techniques are required to achieve reliable results in routine material.52
CD15 is another valuable marker for H&RS cells (fig 1O), and is detected in about 80% of patients with CHL.42,53 CD15 is characteristic, but not specific, for H&RS cells because it can be detected (although rarely) in B and T cell lymphomas and in non-lymphoid tumours. 42,53,54
H&RS cells usually lack CD45 and EMA expression,55–59 whereas B and T cell markers are seen in a proportion of cases. In particular, CD20 (fig 1P) is found in 30–40% of CHL cases (usually EBV negative)58 and CD79a is found even less often.60 Positivity (usually weak) for one or more T cell marker is detected in a minority of H&RS cells in some cases.61,62 Under these circumstances, single cell PCR studies have so far shown T cell receptor (TCR) gene rearrangement in only three instances, with clonal Ig gene rearrangements occurring in most CHL cases with T cell marker expression.8,63 In contrast to that seen in LP-HL, the elements of CHL show variable expression of the Bcl-6 molecule (B Falini et al. Third International Symposium on Hodgkin's Lymphoma, Kolne, Germany, September 18–23, 1995). In addition, they are usually positive for the PAX 5 and MUM 1 gene products (BSAP and IRF4, respectively) and negative for BOB.1 and Oct2 (fig 1Q).7,8,64
Antibodies against the nuclear associated antigens Ki-67 (fig 1R) and proliferating cell nuclear antigen (PCNA) stain most H&RS cells, suggesting that a large number of neoplastic cells enter the cell cycle.65,66 However, in spite of this, tumour cells do not rapidly overwhelm the reactive component.65,66 This phenomenon has found a satisfactory explanation in the studies of Leoncini and co-workers, who have shown that H&RS cells have a defect in cytokinesis.67–70 In fact, only a minority of the cycling elements undergo effective mitosis, and a proportion of the cells that do not enter into the cell cycle undergo apoptosis, a step partly regulated by the bcl-2 and p53 gene products.67–70
Recent studies have suggested that phenotypic findings might have some prognostic relevance. In particular, the value of the following parameters was assessed in the course of a restrospective analysis based on 1751 patients with HL: CD30 expression, CD15 positivity, CD20 staining, age, sex, histotype, stage, B cell symptoms, haemoglobin concentrations, and the erythrocyte sedimentation rate. CD15 negative patients had a higher incidence of relapses (p = 0.0022) and a lower survival rate (p = 0.0035), independent of the remaining prognostic indicators. Similar figures were seen in CD20 positive cases.71 Although interesting, these data need to be re-evaluated because it must be confirmed that the CD15− tumours were not anaplastic large cell lymphomas (ALCLs) and that the CD20+ ones were not TCRBCLs.20
On prognostic grounds, it has also been proposed that chemoresistance and the tendency to relapse are influenced by the expression of Bcl-2, p53, p21, and PCNA (fig 1S,T).72,73 In general, tumours with H&RS cells showing expression or overexpression of one or more of these molecules seem to have a poor response to the treatment and/or short survival time.
Genotypic findings
The origin of the RS cells of HD has long been a mystery.74 As previously discussed in the LP-HL section, micromanipulation of single RS cells from tissue sections and PCR analysis of the cells for rearranged Ig genes have shown that most of both LP-HL and CHL cases represent clonal populations of B cell lineage.1–7 In contrast to that seen in LP-HL, ongoing mutations are not detected in CHL.7 Based on the results obtained in a small series of cases, emphasis was instead given to the occurrence of mutations resulting in stop codons within originally functional variable region gene rearrangements.5 Such mutations are expected to occur in variable region genes of germinal centre B cells, but under physiological conditions “crippled” germinal centre cells (incapable of functional antibody expression) rapidly undergo apoptosis. RS cells might also have other mutations that can be crippling but may not be easy to find (for example, replacement mutations interfering with antigen binding or heavy and light chain pairing).5 However, by analysing a large number of cases, Marafioti et al have recently found that crippling mutations are absent from 75% of CHLs, indicating that crippling mutations cannot be responsible for the general absence of the Ig transcripts,7 which might be the result of downregulation of the synthesis of the transcription factors BOB.1 and Oct2 (see above).7,8 As mentioned in the previous section, the unusual occurrence of patients with CHL who have clonal TCR gene rearrangements has been reported independently by two groups.8,63 Recently, some studies have pointed to the possibility that the nuclear transcription factor NFκB is involved in the protection of H&RS cells from apoptosis, which would be expected because of their inability to produce immunoglobulins.75 The persistent activation of NFκB in H&RS cells might be caused by defects in members of the IκB family, which are the natural inhibitors of NfκB,75–78 or by the aberrant activation of IκB kinase.79 In contrast, despite the frequent expression/overexpression of p53 by neoplastic cells, no mutations of exons 4–8 of the p53 gene have been detected by H&RS cell micromanipulation, DNA amplification, or sequencing.80
The search for the ALCL associated t(2;5)(2p23;5q35) translocation and/or NPM/ALK hybrid gene products is usually negative, with a few reported exceptions in the literature,81,82 although these reports have not be confirmed in larger series, independent of the technique used (Southern blotting, reverse transcriptase–PCR, and immunohistochemical testing with anti-ALK specific antibodies).58,83–93 This negativity is relevant for the differential diagnosis between HL and ALCL in problematic cases.
No specific cytogenetic abnormalities have been reported in CHL because aberrations vary from one case to another, with frequent intraclonal variability, thus suggesting chromosomal instability.94 Some tumours show 14q alterations, as seen in B cell lymphomas, but without the occurrence of the t(14;18) translocation.94
EBV studies reveal viral integration in the genome of CHL tumour cells in a variable proportion of patients (20–80%), depending on the histotype. In particular, in Western countries, 20–40% of NS and LD cases and 50–75% of MC cases show expression of LMP-1 and/or EBER1/2, (fig 1U) but not EBV encoded nuclear antigen 2, thus showing a pattern characteristic of latency type II EBV infection.95,96 Interestingly, these figures can vary greatly according to the geographical area examined, as recently shown by Leoncini and co-workers, who found significant differences in the incidence of EBV between patients with CHL from Kenya and Italy (92% v 48%) matched for age and histotype.97 The type of EBV strain also varies between different geographical areas; in developed countries strain 1 prevails, whereas strain 2 is most prevalent in developing countries.98 HLs that are positive for EBV at diagnosis are usually also positive at relapse, with persistence of the same EBV strain.99 The exact role of EBV in the pathogenesis of human immunodeficiency (HIV) negative CHL (transforming agent as suggested by LMP-1 expression or cofactor for the maintenance of malignant growth?) is still open to question (for seropositives see below).39
NODULAR SCLEROSIS
Morphological findings
NS is the most frequent subtype of CHL in Italy and the USA, where it corresponds to 75–80% of all HL cases; however, the incidence of these subtypes varies greatly among other geographical areas.100–102 As stated by Lukes et al in 1966, the tumour is characterised by: sclerosis, lacunar cells, and nodular pattern.
Sclerosis
Fibrotic phenomena always occur in NS-CHL: they more often correspond to the formation of broad collagen bands, which originate from a regularly thickened lymph node capsule (fig 2A) and subdivide the lymphoid parenchyma into large nodules, at times visible at gross examination. Fibrotic tissue displays a typical birefractive green colour at polarised light microscopy (fig 2A, inset), a finding never seen in LD-CHL.
Figure 2.
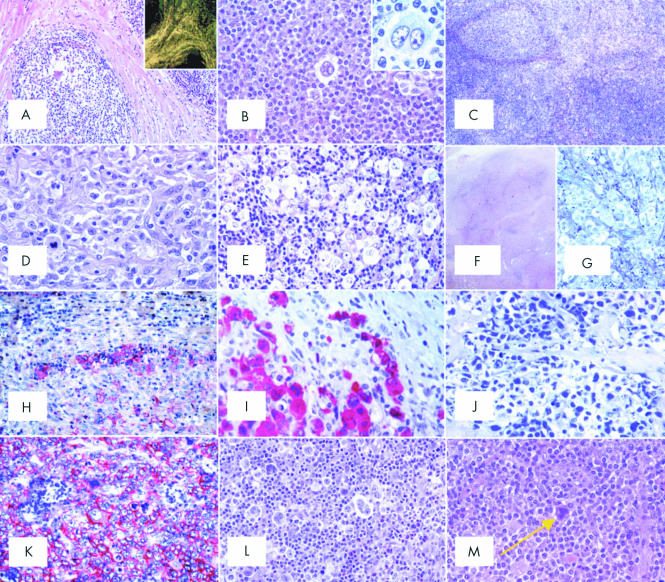
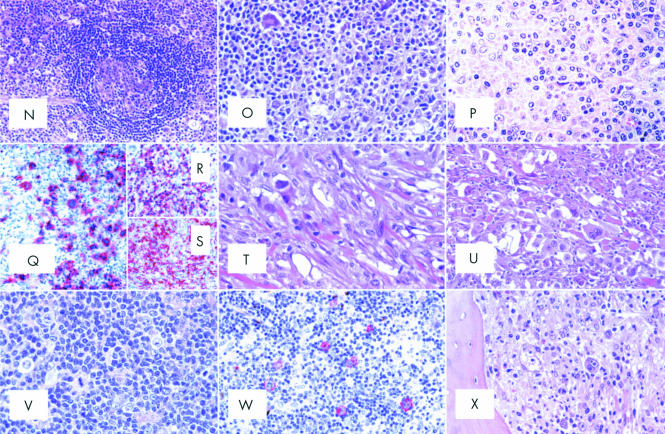
(A) Nodular sclerosing common Hodgkin's lymphoma (NS-CHL): the normal lymph node structure is largely effaced because of a nodular growth; the nodule is surrounded by thick collagen bands originating from the capsule (haematoxylin and eosin; original magnification, ×40). Inset: birefringence of collagen bands (polarised light microscopy; original magnification, ×20). (B) NS-CHL: scattered lacunar cells can easily be seen within one nodule (haematoxylin and eosin; original magnification, ×300). Inset: cytological details of a lacunar cell (Giemsa; original magnification, ×800). (C) NS-CHL: so called cellular phase; note the nodularity of the growth (haematoxylin and eosin; original magnification, ×80). (D) NS-CHL: so called syncytial variant; note the cohesive growth pattern of neoplastic cells (haematoxylin and eosin; original magnification, ×400). (E) NS-CHL: an example of grade II tumour; note the content of neoplastic cells within the nodule, which covers more than 25% of the examined area (haematoxylin and eosin; original magnification, ×300). (F) An example of anaplastic large cell lymphoma of the Hodgkin-like type (ALCL-HL): the tumour consists of nodules partly surrounded by collagen bands (haematoxylin and eosin, original magnification, ×20).(G) ALCL-HL: at higher magnification it can be seen that the collagen bands are almost exclusively formed by neoplastic cells (Giemsa; original magnification, ×300). (H) ALCL-HL: typical intrasinusoidal diffusion of neoplastic cells as shown by CD30 staining (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×200). (I) ALCL-HL: ALK protein expression by neoplastic cells (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×400). (J) Primary mediastinal large B cell lymphoma (PMLBCL): neoplastic cells sometimes have multiple nuclei, show a wide rim of clear, fragile cytoplasm, and elicit a stromal reaction with compartmentalisation (Giemsa; original magnification, ×300). (K) PMLBCL: neoplastic cells express the CD30 molecule (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×300). (L) Mixed cellularity common Hodgkin's lymphoma (MC-CHL): Hodgkin and Reed-Sternberg (H&RS) cells are easily identified; they are found with a cellular milieu consisting of small lymphocytes, some plasma cells, histiocytes, and granulocytes (haematoxylin and eosin; original magnification, ×350). (M) MC-CHL: an example of a mummified cell (arrow) (haematoxylin and eosin; original magnification, ×350). (N) MC-CHL: the tumour has a patent interfollicular location; a spared follicle with Castleman-like features can be seen (haematoxylin and eosin; original magnification, ×150). (O) MC-CHL: the tumour contains reactive epithelioid cells (haematoxylin and eosin; original magnification, ×300). (P) Peripheral T cell lymphoma not otherwise specified with a high content of epithelioid cells (so called Lennert's lymphoma): lymphoid elements show variation in size and shape (haematoxylin and eosin; original magnification, ×400). (Q) T cell rich, histiocyte rich large B cell lymphoma (TCRBCL): neoplastic cells strongly express the CD79a molecule (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×300). (R) TCRBCL: reactive T cells and histiocytes are stained by the anti-CD3 antibody (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×150). (S) TCRBCL: reactive T cells and histiocytes are stained by the anti-CD68 antibody (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×150). (T) Lymphocyte depleted common Hodgkin's lymphoma (LD-CHL), fibrotic variant: rare neoplastic cells are surrounded by thick collagen bands with a haphazard organisation, some histiocytes and scanty lymphocytes (haematoxylin and eosin; original magnification, ×400). (U) LD-CHL, sarcomatous variant: H&RS cells are quite numerous; there is a certain degree of fibrotic reaction; small lymphocytes are exceedingly rare (haematoxylin and eosin; original magnification, ×350). (V) Lymphocyte rich common Hodgkin's lymphoma (LR-CHL): mononuclear and diagnostic neoplastic elements are found within a cellular milieu mostly consisting of small lymphocytes (haematoxylin and eosin; original magnification, ×350). (W) LR-CHL: neoplastic cells express CD15 (APAAP technique, Gill's haematoxylin counterstain; original magnification, ×300). (X) CHL: typical example of bone marrow involvement; note the fibrotic reaction, and the presence of H&RS cells (haematoxylin and eosin; original magnification, ×300).
Lacunar cells
These cells are characteristic of NS-HL. Lukes et al originally described them as large elements with polylobular nuclei, small to medium sized nucleoli, and a wide rim of clear or slightly acidophilic cytoplasm, which is very sensitive to formalin fixation. This last factor causes perinuclear condensation of the cytoplasm, which remains connected to the cell membrane via some narrow filaments, forming empty “lacunar” cytoplasmic spaces (fig 2B). In fact, lacunar cells display a much higher degree of pleomorphism than was originally thought: they may be unilobular, multilobular and/or show huge nucleoli, which are indeed similar to those of typical RS cells. This morphological variability seems to depend on the characteristics of the inflammatory component present in each case.103 Although lacunar cells are easily detected, H&RS cells are rare and their identification may involve a long search. Finally, it should be stressed that some neoplastic elements appear to be “mummified” because of apoptotic changes.
Nodular pattern
The nodules, which should be detected in at least part of the lymph node involved, can contain foci of necrosis and can be very variable in terms of inflammatory cell component (from lymphocyte predominance to lymphocyte depletion).
NS-CHL: cellular phase
In NS-CHL, the amount of collagen fibres varies greatly from one case to another. In the so called cellular phase, there is a clear cut tendency to nodule formation without overt collagen band deposition (fig 2C). However, there are typical lacunar cells, often located at the periphery of the nodules or around residual follicles. The reactive component mainly consists of small lymphocytes bearing the phenotype of mantle B cells (CD20+, CD79a+, CD5+, IgM+, IgD+, CD3−).104,105 The secretion of cytokines by neoplastic cells is currently believed to cause the progressive attraction of T cells, histiocytes, plasma cells, and eosinophils, which give rise to nodules replacing the pre-existing follicles and produce the typical pattern of NS-CHL. Within the nodules, there are numerous FDCs, which seem to represent a favourable prognostic indicator.106,107
NS-CHL: syncytial
The term “syncytial” NS-CHD was coined by Butler in 1983 and then reproposed by Strickler et al in 1986.108 This variant is thought to form 16% of all NS-CHL cases,109 and to run a more aggressive clinical course,102,109 as suggested by the occurrence of mediastinal bulky disease and stage III/IV in 88% of the patients. At light microscopy, it is characterised by large sheets of neoplastic cells (partly with a lacunar appearance), which may undergo central necrosis (fig 2D).108 In the past, similar cases have been diagnosed as non-Hodgkin's lymphoma, metastatic melanoma, carcinoma or sarcoma, thymic carcinoma, or germ cell tumour. The differential diagnosis requires the application of an adequate panel of antibodies, which allows the identification of the characteristic phenotype of the tumoral cells: CD3−, CD15+, CD20−/+, CD30+, CD45−, CD79a−, cytokeratin negative, PLAP−, protein S-100−, HMB.45 melanoma associated antigen negative, EMA−, and ALK−.
Histological grading of NS-HL
The British national lymphoma investigation (BNLI) group has repeatedly proposed that NS-CHL should be subclassified into two grades: grade II tumours seem to represent 15–25% of all NS-CHL cases and to run a more aggressive clinical course,104,110,111 a finding not confirmed by all studies.106,112,113 In the recently developed WHO scheme, the BNLI grading system has been maintained to test its real prognostic value on larger series.6 It is based on the degree of cellularity of the nodules, the amount of sclerosis, and the number and atypia of neoplastic cells. The term grade II is applied to cases showing one of the three following patterns:
(1) More than 25% of the nodules have a cellular composition consistent with the pleomorphic or reticular subtype of NS-CHL/LDV (fig 2E).
(2) More than 80% of the nodules show a fibrotic or fibrohistiocytic composition.
(3) More than 25% of the nodules contain numerous large bizarre or anaplastic cells, in the absence of depletion of the reactive small lymphoid component.
Differential diagnosis
Anaplastic large cell lymphoma
The borders between HL and ALCL are not always sharp: this had led to the creation of the category of ALCL-Hodgkin's like (HL), which presents in about 85% of cases with a mediastinal mass and stage II disease.17,55,87,92,114–118 On practical grounds, this distinction is relevant because ALCL can be cured in 80% of cases by the administration of third generation chemotherapy regimens, whereas HL usually requires different therapeutical approaches.115 The problems in the differential diagnosis result from the fact that ALCL-HL shares architectural and cytological features with NS-CHL, including the fibrotic reaction and nodule formation (fig 2F,G). In our experience, the diagnosis of ALCL-HL should be considered only in those cases that have nodules consisting almost exclusively of basophilic blasts, with a minimal reactive cell component, which also display the phenomenon of intrasinusoidal spreading (fig 2H).116–118 When these morphological criteria are applied, most of the cases in the past diagnosed as ALCL-HL would be now reclassified as NS-CHL (grade II or syncytial) and might tentatively be termed “ALCL-like Hodgkin's disease”.92 Immunohistochemistry and molecular biology largely contribute to the differential diagnosis between HL and ALCL (table 3). In problematical cases, the expression of CD15, possibly in conjunction with positivity for B cell markers, and the lack of TCR gene rearrangements and ALK protein favour HL, whereas negativity for CD15, the expression of T cell markers and/or ALK protein, and the presence of TCR gene clonal rearrangements or the NPM/ALK hybrid gene support the diagnosis of ALCL (fig 2I). Cases that cannot be resolved by the combination of cell morphology, phenotype, and molecular data should be regarded as “unclassifiable” and submitted to a second biopsy or a treatment equally effective for ALCL and HL.6
Table 3.
Differential diagnosis between anaplastic large cell lymphoma Hodglin's-like (ALCL-HL) and classic Hodgkin's lymphoma (HL)
| ALCL-HL | HL | |
| Morphological characteristics | ||
| Neoplastic component | Usually cohesive | Usually dispersed |
| Reactive component | Often minor | Usually prevalent |
| Reed-Sternberg cells | May be present | Always present |
| Intrasinusoidal diffusion | Typical | Exceptional |
| Molecular characteristics | ||
| CD30 | + | +* |
| CD45 | +/− | − |
| CD15 | −/+ | +/− |
| EMA | +/− | rare |
| CBF.78 | + (80–90%) | −/+ (rare cells) |
| BHN.9 | + (60%)† | − (5%) |
| CD3 | −/+‡ | 10% |
| T cell rosettes | − | +/− |
| p53 | −/+ | + |
| EBV | −/+ | + or +/− |
| Clonal TCR gene rearrangement | + | ¶ |
| Clonal Ig gene rearrangement | − | + |
| PAX-5 gene product/BSAP | − | + |
| t(2;5)/ALK protein | +§ | − |
*The intensity of immunostaining may vary within the same case; †results can vary depending on the fixative used; ‡membrane bound and/or dot-like positivity; §some cases with morphology and phenotypic profile consistent with ALCL-HL (including BSAP negativity) can lack t(2;5)/ALK protein expression: these cases need further studies to assess their definitive inclusion among anaplastic large cell lymphomas; ¶in 1–2% of the cases T cell receptor gene rearrangement occurs.
EBV, Epstein-Barr virus; EMA, epithelial membrane antigen; TCR, T cell receptor.
Primary mediastinal (thymic) large B cell lymphoma
Primary mediastinal large B cell lymphoma (PMLBCL) is a distinct clinicopathological entity, which makes up about 2.4% of malignant lymphomas,119 and more often affects young women.6,17,120–122 The presence of a certain number of T cells, eosinophils, and RS-like elements, together with sclerosis, possible nodularity, and frequent CD30 expression by neoplastic cells,123,124 may lead to a misdiagnosis of HL (fig 2J,K); the differentiation between the two processes becomes even more problematical in small biopsies obtained during mediastinoscopy or mini-thoracotomy. PMLBCL cells regularly express B cell markers (CD19, CD20, CD22, and CD79a), and in about 75% of instances the recently discovered MAL protein,125 although they are negative for CD15; these findings are useful for the differential diagnosis with HL.
Undifferentiated nasopharyngeal carcinoma
This variant occurs in young patients, producing early metastatic involvement of laterocervical nodes, which are the usual site of biopsy. On morphological grounds, neoplastic cells can resemble H&RS cells and give rise to nodular growth with sclerosis, plasmacytosis, and eosinophilia. Because the primary tumour may remain occult, morphological features can contribute to a misdiagnosis of NS-CHL.126,127 However, immunohistochemistry allows the straightforward differentiation of undifferentiated nasopharyngeal carcinoma from HL by showing positivity of the neoplastic cells for cytokeratins, EMA, and LMP-1. Positivity for LMP-1 results from the regular integration of EBV into the genome of the tumour cells, as confirmed by in situ hybridisation with EBER1/2 probes and PCR techniques.
Mixed cellularity
This histotype was originally described by Lukes et al as intermediate between LP and LD-CHD. Later, Lukes included in this category all the cases that according to his criteria remained unclassified, transforming it into a “basket”.
About 15–25% of CHL cases belong to this category. The histological picture is characterised by diffuse growth with a frequent paracortical location. The capsule is not often involved and necrosis seldom occurs. The term MC-CHL reflects the cellular composition of the reactive milieu, which consists of plasma cells, epithelioid histiocytes, eosinophils, and T cells (CD3+, CD57−), which form rosettes around neoplastic elements (fig 2L). The tumour cells correspond to H&RS cells, and are numerous and easy to find, without lacunar or popcorn variants. Some neoplastic elements, as in the NS subtype, appear to be “mummified” because of apoptotic changes (fig 2M).
Morphological variants of MC
Interfollicular variant
This variant is rarely seen and probably represents partial lymph node involvement by HL. It is characterised by the occurrence of numerous H&RS cells around reactive follicles, which display germinal centres either in the second phase of development128 or in regressive transformation (fig 2N). These germinal centres usually resemble those seen in hyaline-vascular Castleman's disease and are probably related to the release of cytokines, such as IL-6,129 by H&RS cells.130 This unusual variant of MC-HL should be taken into consideration to avoid possible confusion with follicular hyperplasia or Castleman's disease.128,131
Epithelioid cell rich variant
This variant is relatively common and shows a prominent epithelioid cell reaction with granulomata formation and occasional Langhans cells (fig 2O). In this context, typical H&RS cells are always detected, at times after a laborious search. It should be differentiated from the so called Lennert's lymphoma (LL) (fig 2P) because of the dramatic differences in terms of treatment between the two entities.104,132
Differential diagnosis
Lennert's lymphoma
LL is a peripheral T cell lymphoma with a high content of epithelioid elements and some blasts resembling RS cells.17 Some of the past cases diagnosed as atypical HD correspond to peripheral T cell lymphomas with a high content of epithelioid elements.103 The following elements are of paramount importance for the recognition of LL: (1) pronounced irregularity of the nuclear profiles of the lymphoid component, as opposed to the regular nuclear outline of reactive lymphocytes in HL.133 (2) The phenotypic profile of the atypical population, which is CD3+, CD45+, occasionally CD30+, and CD15−, although some cases can partially lack T cell markers.54,134,135 (3) Higher mitotic index.136
T cell/histiocyte rich large B cell lymphoma
TCRBCL, first described in 1984,137 is an aggressive tumour, usually presenting in stage III–IV with splenomegaly, bone marrow involvement, and mesenteric lymphadenopathy—findings that are rare at the onset of HL.138 Table 2 summarises the main differences between HL and TCRBCL (fig 2Q).27,37,139–144
Lymphocyte depletion
This variant is very rare, accounting for about 1% of HL cases, and shows the worst clinical behaviour and prognosis. In most instances, it presents in stage III–IV, with B cell symptoms and bone marrow involvement being detected in 50% of cases.145,146 At microscopic examination, it is characterised by paucity of the lymphoid component, absolute or relative abundance of RS cells, and variable fibrotic reaction. According to Lukes and Butler, two subtypes of LD-HL can be distinguished: fibrotic and reticular/sarcomatous.
Fibrotic variant
This results in complete effacement of the nodal structure with possible capsule preservation. At microscopic examination (fig 2T), it shows the following distinctive features: (1) low cellular density with scarce, although variable, amounts of small lymphocytes; (2) prominent diffuse reticulin fibre formation without organised birefringent collagen bands,131 which tends to include single neoplastic elements and is associated with the deposition of amorphous material (precollagen) around sinusoids; (3) a high variability in the number of H&RS cells, the detection of which sometimes requires a long and labourious search.
At low power, the histopathological picture can resemble the depletion phase of HIV lymphadenopathy133; therefore, careful node examination is needed to make a firm diagnosis.147
Reticular or sarcomatous variant
This is characterised by extremely large numbers of H&RS cells, some of which appear to be “mummified” (fig 2U). The growth results in diffuse effacement of the normal lymph node structure; small lymphocytes, plasma cells, histiocytes, and granulocytes are scanty; foci of necrosis are usually encountered, although their extent varies from one case to another.
Differential diagnosis
As a result of the extensive application of immunohistochemistry and molecular biology techniques, it is now evident that most of the cases diagnosed in the 1970s and early 1980s as sarcomatous LD-CHL were in fact examples of ALCL,146–148 peripheral T cell lymphoma,149 TCRBCL, PMLBCL,150 or the syncytial variant of NS-CHL. In our experience, the differential diagnosis should also include some non-lymphoid tumours, such as inflammatory fibrosarcoma,151,152 atypical Langerhans cell histiocytosis, inflammatory and giant cell malignant fibrous histiocytoma,153 lymphocyte rich well differentiated lyposarcoma,154 and undifferentiated nasopharyngeal carcinoma. Under these circumstances, immunophenotyping is essential for a correct diagnosis.
Lymphocyte rich classic Hodgkin's lymphoma
Several reports have underlined the existence of HL cases with a lymphocyte predominant background, but differing from the prototypic description of LP-HL because of the presence of some eosinophils, sclerosis, typical H&RS cells, or aberrant phenotypic features, such as the expression of CD30 and CD15.21,35,100,155,156 In 1994, the ILSG included in the REAL classification a provisional entity called lymphocyte rich common Hodgkin's disease”, which was thought to have a diffuse growth pattern in most instances (table 1).17 Following two workshops held by the European Association for Haematopathology in 1994 and the European lymphoma task force in 1995, the existence of LR-CHL has been accepted and expanded by the recognition of two subtypes of the tumour—nodular and diffuse (fig 2V—which should be differentiated from LP-HL and TCRBCL (table 2).19,37,138,157,158
On morphological grounds,19 most LR-CHL cases are characterised by a nodular background, with admixed histiocytes and absent neutrophils and eosinophils closely resembling nodular LP-HL, particularly at low power. Furthermore, a varying proportion of the neoplastic cells can exhibit features of popcorn elements. However, in contrast to LP-HL, many lymphomatous cells have the cytomorphological features of classic H&RS cells, and the nodular structures frequently show small germinal centres at their periphery. Focal areas of sclerosis can sometimes be seen.
At phenotypic analysis,19 the neoplastic cells usually express CD30 and CD15. CD20 and CD79a positivity is found in 32.5% and 8.7% of cases, respectively—figures that are much lower than those observed in LP-HL. In addition, there is a complete absence of J chain in all instances and a weak expression of EMA in only a few cases. About 50% of the examples of LR-CHL harbour EBV positive H&RS cells. The reactive component consists of abundant mantle B cells, with surface IgD and IgM expression, and variable amounts of CD3+ T cells, which produce rosettes around neoplastic elements, but seldom express CD57. CD21 immunostaining reveals a loose, ill defined meshwork of FDCs, which becomes much denser and sharper around the small residual germinal centres, when present.
The clinical characteristics of this variant of CHL, which accounts for about 6% of all HL cases,26,71 has been the object of several studies, including those promoted by the international project on lymphocyte predominant Hodgkin's disease and the German Hodgkin's lymphoma study group.19,71 These studies have shown that patients with LR-CHL differ from those with NS-CHL or MC-CHL: they are usually older than 50 and display a higher incidence of stages I–II and a subdiafragmatic location. In contrast, they rarely have bulky disease, B cell symptoms, or mediastinal or extranodal involvement. Thus, the clinical profile of LR-CHL is closer to that of LP-HL, although it has a lower frequency of stages I–II and splenic infiltration is more common. When compared with other types of CHD, LR-CHL gives rise to more frequent late relapses, although these do not behave aggressively.
Owing to its peculiar clinicopathological features, LR-CHL has been quoted as an accepted entity in the recently developed WHO scheme.6
Unclassifiable HL
In cases with lymph node partial involvement, small amounts of tissue available, or extranodal location, the classification of HL can be difficult or even impossible. In the past, these problematical cases were usually included in the MC subtype. Because it is useful to keep the subtypes of HD as homogeneous as possible for prospective clinicopathological studies, both the REAL classification and the WHO scheme list cases with ambiguous features or insufficient bioptic material as HL unclassified.
EXTRANODAL INVOLVEMENT BY HL
Although the onset is typically nodal, HL can secondarily affect extranodal organs and tissues. The criteria for the diagnosis of HD at extranodal sites vary greatly depending on the clinical history and the type of tissue involved. In fact, in needle biopsies taken from the bone marrow (fig 2X) and liver during staging procedures, the diagnosis of HL can confidently be made according to “minimal criteria”; that is, by the detection of HC in the appropriate cellular milieu.133 In contrast, the diagnosis of HL at other extranodal sites needs the recognition of typical “diagnostic” cells and appropriate phenotypic markers, especially in patients with no previous history of HD.
TREATMENT RELATED HISTOLOGICAL CHANGES
Relapses of HL at previously treated sites may show morphological findings that totally differ from those seen at the time of disease presentation. Under these circumstances, the histological picture is characterised by numerous atypical Hodgkin's cells, rare RS cells, and severe lymphocyte depletion, which can make the differentiation from a diffuse large cell lymphoma difficult.103,159
In patients with bulky disease, a residual mass is often detected after chemotherapy and radiotherapy, challenging the efficacy of the administered treatment. In our experience, histological examination of the residual mass frequently shows a fibrotic reaction with sclero-jaline changes and epithelioid cell palisades around necrotic foci, but no active tumour.
Chemotherapy and radiotherapy can produce toxic damage in organs not primarily involved in the process, such as postradiation interstitial pneumonitis, thyroid fibrosis, cardiomyopathy, or bone marrow aplasia. In addition, patients treated for HL show an increased risk of developing acute leukaemias, malignant lymphomas, and more rarely non-lymphoid tumours. This concept especially applies to individuals who have undergone MOPP chemotherapy.103 In general, necropsies performed on subjects with a previous history of HL often show that the cause of death was a treatment related complication in the absence of detectable residual disease.103
RELATIONS BETWEEN HL AND AIDS
HIV positive patients are more at risk than the normal population of developing HL, especially of the LD or MC subtype.160,161 At presentation, the tumour frequently shows extensive subdiafragmatic and extranodal involvement, whereas a mediastinal mass is less common than in HIV negative individuals.162 Liver and bone marrow lesions may be detected in the absence of splenic colonisation. Analogously, skin and lung infiltrates can occur without regional or mediastinal node involvement. In general, HL behaves differently in HIV positive patients than in HIV negative subjects, showing diverse dissemination,103 a more aggressive clinical course, and worse prognosis, thus requiring specific therapeutic protocols.163 Neoplastic elements in HIV positive HL are more often CD20+ and Bcl-6−/syn-1+ (CD138/syndecan-1). This last finding differs from that usually seen in HIV negative LP-HL (which is regularly Bcl-6+/syn-1−) and CHL (which is characterised by a mixture of Bcl-6−/syn-1+ and Bcl-6+/syn-1− RS cells).164 In addition, most if not all HIV positive HL cases display positivity of neoplastic cells for EBV, as shown by LMP-1 expression and in situ hybridisation studies. This observation suggests an active role for EBV in the process of lymphomagenesis in HIV positive HL,165 especially in the light of the well known transforming ability of LMP-1.166,167 In particular, in 89% of HIV positive cases (v 32% of the seronegative ones), the LMP-1 gene shows a 30 bp deletion, which prolong the half life of LMP-1 and allows its accumulation in infected cells.165,168
Take home messages.
Thanks to the results provided by immunophenotypic and molecular studies, Hodgkin's lymphoma (HL) is now unanimously considered to be a germinal centre related B cell lymphoma in up to 99% of cases
Significant differences exist between lymphocyte predominant HL (LP-HL) and classic HL (CHL) (which includes the lymphocyte rich, nodular sclerosing, mixed cellularity, and lymphocyte depleted subtypes) in terms of natural history, the relation to Epstein-Barr virus, cell morphology, phenotype, molecular characteristics, and clinical behaviour
Although the borders between HL and anaplastic large cell lymphoma have recently become sharp, the differential diagnosis between LP-HL and T cell rich B cell lymphoma remains at times problematical
The lack of immunoglobulin (Ig) production, which is characteristic of CHL, is more often the result of defective expression of transcription factors, such as Oct-2, BOB.1, PU.1, although at times it is caused by the occurrence of crippling VH Ig gene mutations
The search for morphological, phenotypic, and/or kinetic factors that may herald a poor response to conventional treatments is felt necessary, aiming to design and to apply more effective ad hoc strategies in selected cases
ASSOCIATION OF HL WITH NON-HODGKIN'S LYMPHOMA
The occurrence of HL and a synchronous or metachronous form of non-Hodgkin's lymphoma (NHL) in the same patient is rare. The most frequent combination of the two is a DLBCL that develops after LP-HL.22,26,169–175 However, CHL has also been reported in conjunction with different types of NHL, including follicular lymphoma (FL), DLBCL, B cell chronic lymphocytic leukaemia, and even peripheral T cell lymphoma.176–192
There are three possible explanations for the occurrence of this association: (1) both neoplasms arise coincidentally from two unrelated lymphoid elements; (2) the HL progresses from a previous lymphoma; or (3) both lymphomas derive from a common precursor cell. For a long time, no reliable answer could be given to these challenging questions. However, in the past few years, the introduction of single cell PCR has allowed the molecular analysis of some cases showing simultaneous or subsequent occurrence of HL and NHL. Former reports on the subject suggested a direct progression from NHL to HL (either classic or lymphocyte predominant).172–175,185,187,188 Three more recent contributions, however, have revealed that the two tumoral components may stem from the same precursor cell.190–192 In particular, these contributions focused on cases that showed either the simultaneous occurrence of FL190 or DLBCL192 and CHL, or the development of CHL in patients with a previous history of FL191 or TCRBCL.190 In all these cases, NHL cells and H&RS cells displayed the same monoclonal Ig gene rearrangements, in addition to the presence of somatic mutations, a finding which further strengthens the concept that H&RS cells are derived from mature germinal centre B cell elements.
ADDENDUM
After the completion of the present review article, an interesting contribution appeared in the literature focusing on the differences of PU.1 expression in LP-HL and CHL. This transcription factor, which is regularly expressed in LP-HL, may be used for the differentiation of LP-HL not only from CHL, but also from TCRBCL.193
Acknowledgments
This paper was supported by grants from AIRC (Milan) and MURST (Rome).
Abbreviations
ALCL, anaplastic large cell lymphoma
ALCL-HL, ALCL Hodgkin-like type
BNLI, British national lymphoma investigation
CHL, common Hodgkin's lymphoma
DLBCL, diffuse large B cell lymphoma
EBER, Epstein-Barr virus early RNA
EBV, Epstein-Barr virus
EMA, epithelial membrane antigen
FDC, follicular dendritic cell
FL, follicular lymphoma
HC, Hodgkin's cell
HCRBCL, histiocyte rich large B cell lymphoma
HD, Hodgkin's disease
HIV, human immunodeficiency virus
HL, Hodgkin's lymphoma
H&RS, Hodgkin's and Reed-Sternberg
IL, interleukin
LD-CHL, lymphocyte depletion CHL
L/H, lymphocytic/histiocytic
LL, Lennert's lymphoma
LMP, latent membrane protein
LP-HL, lymphocyte predominant HL
LR-CHL, lymphocyte rich CHL
MC-CHL, mixed cellularity CHL
NHL, non-Hodgkin's lymphoma
NS-CHL, nodular sclerosis CHL
PCNA, proliferating cell nuclear antigen
PCR, polymerase chain reaction
PMLBCL, primary mediastinal large B cell lymphoma
PTGC, progressively transformed germinal centre
REAL, revised European–American
RS, Reed-Sternberg
TCR, T cell receptor
TCRBCL, T cell rich B cell lymphoma
TNF, tumour necrosis factor
WHO, World Health Organisation
REFERENCES
- 1.Hummel M, Marafioti T, Stein H. Immunoglobulin V genes in Reed-Sternberg cells. N Engl J Med 1996;334:405–6. [PubMed] [Google Scholar]
- 2.Marafioti T, Hummel M, Anagnostopoulos I, et al. Origin of nodular lymphocyte predominant Hodgkin's disease from a clonal expansion of highly mutated germinal center B cells. N Engl J Med 1997;337:453–8. [DOI] [PubMed] [Google Scholar]
- 3.Izban KF, Nawrocki JF, Alkan S, et al. Monoclonal IgH gene rearrangement in microdissected nodules from nodular sclerosis Hodgkin's disease. Am J Clin Pathol 1998;110:599–606. [DOI] [PubMed] [Google Scholar]
- 4.Braeuninger A, Hansmann M-L, Strickler JG, et al. Identification of common germinal-center B-cell precursors in two patients with both Hodgkin's disease and non-Hodgkin's lymphoma. N Engl J Med 1999;340:1239–47. [DOI] [PubMed] [Google Scholar]
- 5.Küppers R, Klein U, Hansmann M-L, et al. Cellular origin of human B-cell lymphomas. N Engl J Med 1999;341:1520–9. [DOI] [PubMed] [Google Scholar]
- 6.Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization classification of neoplastic diseases of the haematopoietic and lymphoid tissues: report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Histopathology 2000;36:69–87. [DOI] [PubMed] [Google Scholar]
- 7.Marafioti T, Hummel M, Foss HD, et al. Hodgkin and Reed-Sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangement but defective immunoglobulin transcription. Blood 2000;95:1443–50. [PubMed] [Google Scholar]
- 8.Seitz V, Hummel M, Marafioti T, et al. Detection of clonal T-cell receptor gamma-chain gene-rearrangements in Reed-Sternberg cells of classic Hodgkin's disease. Blood 2000;95:3020–4. [PubMed] [Google Scholar]
- 9.Jaffe ES. Introduction: Hodgkin's lymphoma—pathology, pathogenesis, and treatment. Semin Hematol 1999;36:217–19. [PubMed] [Google Scholar]
- 10.Harris NL. Hodgkin's disease: classification and differential diagnosis. Mod Pathol 1999;12:159–76. [PubMed] [Google Scholar]
- 11.Falini B, Pileri S, Pizzolo G, et al. CD30 (Ki-1) molecule: a new cytokine receptor of tumor necrosis factor superfamily as a tool for diagnosis and immunotherapy. Blood 1995;85:1–14. [PubMed] [Google Scholar]
- 12.Kadin ME, Liebowitz DN. Cytokines and cytokine receptors in Hodgkin's disease. In: Mauch P, et al, eds. Hodgkin's disease. Philadelphia: Lippincott Williams & Wilkins, 1999:139.
- 13.Poppema S, Potters M, Emens R, et al. Immune reactions in classical Hodgkin's lymphoma. Semin Hematol 1999;36:253–9. [PubMed] [Google Scholar]
- 14.Ohshima K, Sugihara M, Suzumiya J, et al. Basic fibroblast growth factor and fibrosis in Hodgkin's disease. Pathol Res Pract 1999;195:149–55. [DOI] [PubMed] [Google Scholar]
- 15.Van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltrate in Hodgkin's lymphoma. Am J Pathol 1999;154:1685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teofili L, Di Febo AL, Pierconti F, et al. Expression of c-met proto-oncogene and its ligand hepatocyte growth factor in Hodgkin's disease. Blood 2001;97:1063–9. [DOI] [PubMed] [Google Scholar]
- 17.Harris NL, Jaffe ES, Stein H, et al. A revised European–American classification of lymphoid neoplasms: a proposal from the international lymphoma study group. Blood 1994;84:1361–92. [PubMed] [Google Scholar]
- 18.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. J Clin Oncol 1999;17:3835–49. [DOI] [PubMed] [Google Scholar]
- 19.Anagnostopolous I, Hansmann M-L, Fransilla K, et al. European task force on lymphoma project on lymphocyte predominance Hodgkin disease: histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin disease with a nodular growth pattern and abundant lymphocytes. Blood 2000;96:1889–99. [PubMed] [Google Scholar]
- 20.Harris NL. Hodgkin's lymphomas: classification, diagnosis, and grading. Semin Hematol 1999;36:220–32. [PubMed] [Google Scholar]
- 21.Poppema S, Kaiserling E, Lennert K. Hodgkin's disease with lymphocytic predominance, nodular type (nodular paragranuloma) and progressively transformed germinal centers: a cytohistologic study. Histopathology 1979;3:295–308. [DOI] [PubMed] [Google Scholar]
- 22.Mason DY, Banks PM, Chan J, et al. Nodular lymphocyte predominance Hodgkin's disease: a distinct clinicopathologic entity. Am J Surg Pathol 1994;18:526–30. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg SA, Kaplan HS. Evidence of an orderly progression in the spread of Hodgkin's disease. Cancer Res 1966;26:1225–31. [PubMed] [Google Scholar]
- 24.Regula DP, Hoppe RT, Weiss LM. Nodular and diffuse types of lymphocyte predominance Hodgkin's disease. N Engl J Med 1988;318:214–19. [DOI] [PubMed] [Google Scholar]
- 25.Kim H. Composite lymphoma and related disorders. Am J Clin Pathol 1993;99:445–51. [DOI] [PubMed] [Google Scholar]
- 26.von Wasielewski R, Werner M, Fischer R, et al. Lymphocyte-predominant Hodgkin's disease: an immunohistochemical analysis of 208 reviewed Hodgkin's disease cases from the German Hodgkin study group. Am J Pathol 1997;150:793–803. [PMC free article] [PubMed] [Google Scholar]
- 27.Delabie J, Vanderberghe E, Kennes C, et al. Histiocyte-rich B-cell lymphoma. A distinct clinicopathologic entity possibly related to lymphocyte predominant Hodgkin's disease, paragranuloma subtype. Am J Surg Pathol 1992;16:37–48. [PubMed] [Google Scholar]
- 28.Lennert K. Malignant lymphomas other than Hodgkin's disease. New York: Springer-Verlag, 1978.
- 29.Nguyen PL, Ferry JA, Harris NL. Progressive transformation of germinal centers and nodular lymphocyte predominance Hodgkin's disease. A comparative immunohistochemical study. Am J Surg Pathol 1999;23:27–33. [DOI] [PubMed] [Google Scholar]
- 30.Stein H, Marafioti T, Foss H-D, et al. Downregulation of BOB.1/OBF.1 and Oct2 in classical Hodgkin's disease but not in lymphocyte predominant Hodgkin's disease correlates with immunoglobulin transcription. Blood 2001;97:496–501. [DOI] [PubMed] [Google Scholar]
- 31.Laumen H, Nielsen PJ, Wirth T. The BOB.1/OBF.1 co-activator is essential for octamer-dependent transcription in B cells. Eur J Immunol 2000;30:458–69. [DOI] [PubMed] [Google Scholar]
- 32.Munro J, Freedman A, Aster J, et al. In vivo expression of the B7 costimulatory molecule by subsets of antigen-presenting cells and the malignant cells of Hodgkin's disease. Blood 1994;83:793–8. [PubMed] [Google Scholar]
- 33.Carbone A, Gloghini A, Gattei V, et al. Expression of functional CD40 antigen on Reed-Sternberg cells and Hodgkin's disease cell lines. Blood 1995;85:780–9. [PubMed] [Google Scholar]
- 34.Timmens W, Visser L, Poppema S. Nodular lymphocyte predominance type of Hodgkin's disease is a germinal center lymphoma. Lab Invest 1986;54:457–61. [PubMed] [Google Scholar]
- 35.Hansmann M-L, Stein H, Dallenbach F, et al. Diffuse lymphocyte-predominant Hodgkin's disease (diffuse paragranuloma). A variant of the B-cell-derived nodular type. Am J Pathol 1991;138:29–36. [PMC free article] [PubMed] [Google Scholar]
- 36.Kraus MD, Haley J. Lymphocyte predominance Hodgkin's disease. The use of bcl-6 and CD57 in diagnosis and differential diagnosis. Am J Surg Pathol 2000;24:1068–78. [DOI] [PubMed] [Google Scholar]
- 37.Rudiger T, Ott G, Ott MM, et al. Differential diagnosis between classic Hodgkin's lymphoma, T-cell-rich B-cell lymphoma, and paragranuloma by paraffin immunohistochemistry. Am J Surg Pathol 1998;22:1184–91. [DOI] [PubMed] [Google Scholar]
- 38.Delsol G, Brousset P, Chittal S, et al. Correlation of the expression of Epstein-Barr virus latent membrane protein and in situ hybridization with biotinylated BamH1-W probes in Hodgkin's disease. Am J Pathol 1992;140:247–53. [PMC free article] [PubMed] [Google Scholar]
- 39.Niedobiteck G, Young LS, Herbst H. Epstein-Barr virus infection and the pathogenesis of malignant lymphomas. Cancer Surv 1997;30:143–62. [PubMed] [Google Scholar]
- 40.Diehl V, Josting A. Hodgkin's disease. Cancer J Sci Am 2000;6(suppl 2):S150–8. [PubMed] [Google Scholar]
- 41.Stein H, Mason DY, Gerdes J, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 1985;66:848–58. [PubMed] [Google Scholar]
- 42.Pileri S, Sabattini E, Tazzari PL, et al. Hodgkin's disease: update of findings. Haematology 1991;76:175–82. [PubMed] [Google Scholar]
- 43.Stein H, Herbst H, Anagnostopoulos I, et al. The nature of Hodgkin and Reed-Sternberg cells, their association with EBV, and their relationship to anaplastic large-cell lymphoma. Ann Oncol 1991;2:33–8. [DOI] [PubMed] [Google Scholar]
- 44.Schwarting R, Gerdes J, Durkop H, et al. Ber-H2: a new anti-Ki-1 (CD-30) monoclonal antibody directed at a formol resistant epitope. Immunohistochemical and immunobiochemical characterization. Blood 1989;74:1678–89. [PubMed] [Google Scholar]
- 45.Durkop H, Latza U, Hummel M, et al. Molecular cloning and expression of a new member of the nerve growth factor receptor family which is characteristic for Hodgkin's disease. Cell 1992;68:421–7. [DOI] [PubMed] [Google Scholar]
- 46.Nadali G, Vimante F, Chilosi M, et al. Soluble molecules as biological markers in Hodgkin's disease. Leuk Lymphoma 1997;26:99–105. [DOI] [PubMed] [Google Scholar]
- 47.Falini B, Bolognesi A, Flenghi L, et al. Response of refractory Hodgkin's disease to monoclonal anti-CD30 immunotoxin. Lancet 1992;i:1195–7. [DOI] [PubMed] [Google Scholar]
- 48.Tazzari PL, Bolognesi A, De Totero D, et al. Ber-H2 (anti CD30)-saporin immunotoxin: a new tool for the treatment of Hodgkin's disease and CD30+ lymphoma. In vitro evaluation. Br J Haematol 1992;81:203–11. [DOI] [PubMed] [Google Scholar]
- 49.Engert A, Gottstin C, Winkler U, et al. Experimental treatment of human Hodgkin's disease with ricin A-chain immunotoxins. Leuk Lymphoma 1994;13:441–8. [DOI] [PubMed] [Google Scholar]
- 50.Terenzi A, Bolognesi A, Pasqualucci L, et al. Anti-CD30 (Ber=H2) immunotoxins containing the type-1 ribosome-inactivating proteins momordin and PAP-S (pokeweed antiviral protein from seeds) display powerful activity against CD30+ tumour cells in vitro and in SCID mice. Br J Haematol 1996;92:872–9. [DOI] [PubMed] [Google Scholar]
- 51.Millward C, Weidner N. CD30 (Ber-H2) expression in nonhematopoietic tumors. Applied Immunohistochemistry 1998;6:164–8. [Google Scholar]
- 52.Pileri SA, Roncador G, Ceccarelli C, et al. Antigen retrieval techniques in immunohistochemistry: comparison of different methods. J Pathol 1997;183:116–23. [DOI] [PubMed] [Google Scholar]
- 53.Pileri SA, Poggi S, Sabattini E, et al. Is Hodgkin's disease a unique entity? Leuk Lymphoma 1995;15:3–6. [DOI] [PubMed] [Google Scholar]
- 54.Ascani S, Zinzani PL, Gherlinzoni F, et al. Peripheral T-cell lymphomas. Clinico-pathologic study of 168 cases diagnosed according to the REAL classification. Ann Oncol 1997;8:583–92. [DOI] [PubMed] [Google Scholar]
- 55.Falini B, Pileri S, Stein H, et al. Variable expression of leukocyte common antigen (CD45) in CD30 (Ki-1)-positive anaplastic large cell (ALC) lymphoma. Implication for the differential diagnosis between lymphoid and non-lymphoid malignancies. Hum Pathol 1990;21:624–9. [DOI] [PubMed] [Google Scholar]
- 56.Korkolopoulou P, Cordell J, Jones M, et al. The expression of the B-cell marker mb-1 (CD79a) in Hodgkin's disease. Histopathology 1994;24:511–15. [DOI] [PubMed] [Google Scholar]
- 57.Chang KL, Arber DA, Weiss LM. CD20: a review. Applied Immunohistochemistry 1996;4:1–15. [Google Scholar]
- 58.Filippa DA, Ladanyi M, Wollner N, et al. CD30 (Ki-1)-positive malignant lymphomas: clinical, immunophenotypic, histologic, and genetic characteristics and differences with Hodgkin's disease. Blood 1996;87:2905–17. [PubMed] [Google Scholar]
- 59.Chittal S, Al Saati T, Delsol G. Epithelial membrane antigen in hematolymphoid neoplasms. A review. Applied Immunohistochemistry 1997;5:203–15. [Google Scholar]
- 60.Watanabe K, Yamashita Y, Nakayama A, et al. Varied B-cell immunophenotypes of Hodgkin/Reed-Sternberg cells in classic Hodgkin's disease. Histopathology 2000;36:353–61. [DOI] [PubMed] [Google Scholar]
- 61.Falini B, Stein H, Pileri S, et al. Expression of T-cell antigens on Hodgkin's and Sternberg-Reed cells of Hodgkin's disease. A combined immunocytochemical and immunohistological study using monoclonal antibodies. Histopathology 1987;12:1229–41. [DOI] [PubMed] [Google Scholar]
- 62.Casey TT, Olson SJ, Cousar JB, et al. Immunophenotype of Reed-Sternberg cells: a study of 19 cases of Hodgkin's disease in plastic-embedded sections. Blood 1989;74:2624–8. [PubMed] [Google Scholar]
- 63.Müschen M, Rajewsky K, Bräuninger A, et al. Rare occurrence of classical Hodgkin's disease as a T-cell lymphoma. J Exp Med 2000;191:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Falini B, Fizzotti M, Pucciarini A, et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood 2000;95:2084–92. [PubMed] [Google Scholar]
- 65.Gerdes J, Van Baarlen J, Pileri S, et al. Tumor cell growth fraction in Hodgkin's disease. Am J Pathol 1987;128:390–3. [PMC free article] [PubMed] [Google Scholar]
- 66.Sabattini E, Gerdes J, Gherlinzoni F, et al. Comparison between the monoclonal antibodies Ki-67 and PC10 in 125 malignant lymphomas. J Pathol 1993;169:397–403. [DOI] [PubMed] [Google Scholar]
- 67.Leoncini L, Close P, Bothur P, et al. Apoptosis in Hodgkin's disease: an in situ endlabelling study correlated with proliferation indices and expression of cell death gene, p53 and BCL-2. Curr Diagn Pathol 1995;2:51–2. [Google Scholar]
- 68.Leoncini L, Spina D, Close P, et al. Abortive mitosis and nuclear DNA fragmentation in CD30+ large cells of Hodgkin's disease. Leuk Lymphoma 1996;22:119–24. [DOI] [PubMed] [Google Scholar]
- 69.Spina D, Leoncini L, Close P, et al. Growth vs DNA strand breaks in Hodgkin's disease: impaired proliferative ability of Hodgkin and Reed-Sternberg cells. International Journal of Cancer 1996;66:179–83. [DOI] [PubMed] [Google Scholar]
- 70.Leoncini L, Spina D, Close P, et al. Mitotic activity and nuclear DNA damage of large cells in Hodgkin's disease: comparison with the expression of p53 and bcl-2 proteins and the presence of Epstein-Barr virus. Leuk Lymphoma 1997;25:153–61. [DOI] [PubMed] [Google Scholar]
- 71.von Wasielewski R, Mengel M, Fischer R, et al. Classical Hodgkin's disease: clinical impact of the immunophenotype. Am J Pathol 1997;151:1123–30. [PMC free article] [PubMed] [Google Scholar]
- 72.Naresh KN, O'Conor GT, Soman CS, et al. A study of p53 protein, proliferating cell nuclear antigen, and p21 in Hodgkin's disease at presentation and relapse. Hum Pathol 1997;28:549–55. [DOI] [PubMed] [Google Scholar]
- 73.Smolewsky P, Robak T, Krykowski E, et al. Prognostic factors in Hodgkin's disease: multivariate analysis of 327 patients from a single institution. Clin Cancer Res 2000;6:1150–60. [PubMed] [Google Scholar]
- 74.Küppers R, Rajewsky K. The origin of Hodgkin and Reed/Sternberg cells in Hodgkin's disease. Annu Rev Immunol 1998;16:471–3. [DOI] [PubMed] [Google Scholar]
- 75.Bargou RC, Leng C, Krappmann D, et al. High level nuclear NF-κB and Oct-2 is a common feature of cultured Hodgkin/Reed-Sternberg cells. Blood 1996;87:4340–7. [PubMed] [Google Scholar]
- 76.Emmerich F, Meiser M, Hummel M, et al. Overexpression of I kappa B alpha without inhibition of NF-kappaB activity and mutations in the I kappa B alpha gene in Reed-Sternberg cells. Blood 1999;94:3129–34. [PubMed] [Google Scholar]
- 77.Cabannes E, Khan G, Aillet F, et al. Mutations in the IkBa gene in Hodgkin's disease suggest a tumour suppressor role for the IkappaBalpha. Oncogene 1999;18:3063–70. [DOI] [PubMed] [Google Scholar]
- 78.Jugnickel B, Staratschek-Jox A, Braeuninger A, et al. Clonal deleterious mutations in the IκBα gene in the malignant cells in Hodgkin's lymphoma. J Exp Med 2000;191:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krappmann D, Emmerich F, Kordes U, et al. Molecular mechanisms of constitutive NF-kappaB/Rel activation in Hodgkin/ Reed-Sternberg cells. Oncogene 1999;18:943–53. [DOI] [PubMed] [Google Scholar]
- 80.Montesinos-Rongen M, Roers A, Küppers R, et al. Mutation of the p53 gene is not a typical feature of Hodgkin and Reed-Sternberg cells in Hodgkin's disease. Blood 1999;94:1755–60. [PubMed] [Google Scholar]
- 81.Bullrich F, Morris SW, Hummel M, et al. Nucleophosmin (NPM) gene rearrangements in Ki-1 positive lymphomas. Cancer Res 1994;54:2873–7. [PubMed] [Google Scholar]
- 82.Orscheschek K, Merz H, Hell J, et al. Large-cell anaplastic lymphoma-specific translocation t(2;5)(p23;q35) in Hodgkin's disease: indication of a common pathogenesis? Lancet 1995;345:87–90. [DOI] [PubMed] [Google Scholar]
- 83.Shiota M, Nakamura S, Ichinohasama R, et al. Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: a distinct clinicopathologic entity. Blood 1995;86:1954–60. [PubMed] [Google Scholar]
- 84.Weiss LM, Lopategui JR, Sun LH, et al. Absence of the t(2;5) in Hodgkin's disease. Blood 1995;85:2845–7. [PubMed] [Google Scholar]
- 85.Xerri L, Parc P, Hassoun J, et al. Molecular analysis of the NPM-ALK rearrangement in Hodgkin's disease. J Pathol 1996;178:128–32. [DOI] [PubMed] [Google Scholar]
- 86.Nakamura S, Shiota M, Nakagawa A, et al. Anaplastic large cell lymphoma: a distinct molecular pathologic entity. A reappraisal with special reference to p80NPM/ALK expression. Am J Surg Pathol 1997;21:1420–32. [DOI] [PubMed] [Google Scholar]
- 87.Pileri SA, Mason DY, Mori S, et al. Frequent expression of the p80 NPM–ALK chimeric fusion protein in anaplastic large-cell lymphoma, lympho-histiocytic type. Am J Pathol 1997;150:1207–11. [PMC free article] [PubMed] [Google Scholar]
- 88.Hutchinson RE, Banki K, Shuster JJ, et al. Use of an anti-ALK antibody in the characterization of anaplastic large-cell lymphoma of childhood. Ann Oncol 1997;8:37–42. [PubMed] [Google Scholar]
- 89.Pulford K, Lamant L, Morris S, et al. Detection of ALK and NPM-ALK protein in normal and neoplastic cells with the monoclonal antibody ALK1. Blood 1997;89:1394–404. [PubMed] [Google Scholar]
- 90.Sarris AH, Luthra R, Papadimitracopoulou V, et al. Long-range amplification of genomic DNA detects the t(2;5)(p23;q35) in anaplastic large-cell lymphoma, but not in other non Hodgkin's lymphomas, Hodgkin's disease, or lymphomatoid papulosis. Ann Oncol 1997;8:59–63. [PubMed] [Google Scholar]
- 91.Trümper L, Daus H, Merz H, et al. NPM/ALK fusion mRNA expression in Hodgkin and Reed-Sternberg cells is rare but does occur: results from single-cell cDNA analysis. Ann Oncol 1997;8:83–7. [PubMed] [Google Scholar]
- 92.Benharroch D, Meguerian-Bedoyan Z, Lamant L, et al. ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood 1998;91:2076–84. [PubMed] [Google Scholar]
- 93.Falini B, Bigerna B, Fizzotti M, et al. ALK expression defines a distinct group of lymphomas (“ALK lymphomas”) with a wide morphologic spectrum. Am J Pathol 1998;153:875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Poppema S, Kaleta J, Hepperle B, et al. Chromosomal abnormalities in patients with Hodgkin's disease: evidence for frequent involvement of the 14q chromosomal region but infrequent bcl-2 gene rearrangement in Reed-Sternberg cells. J Natl Cancer Inst 1992;84:1789–93. [DOI] [PubMed] [Google Scholar]
- 95.Vassallo J, Brousset P, Knecht H, et al. Detection of Epstein-Barr virus in Hodgkin's disease. Applied Immunohistochemistry 1993;1:213–19. [Google Scholar]
- 96.Rowe M, Rowe DT, Gregory CD, et al. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J 1987;6:2743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leoncini L, Spina D, Nyong'o A, et al. Neoplastic cells of Hodgkin's disease show differences in EBV expression between Kenya and Italy. Int J Cancer 1996;65:781–4. [DOI] [PubMed] [Google Scholar]
- 98.Weinreb M, Day PJ, Niggli F, et al. The consistent association between Epstein-Barr virus and Hodgkin's disease in children in Kenya. Blood 1996;87:3828–36. [PubMed] [Google Scholar]
- 99.Brousset P, Schlaifer D, Meggetto F, et al. Persistence of the same viral strain in early and late relapses of Epstein-Barr virus-associated Hodgkin's disease. Blood 1994;84:2447–51. [PubMed] [Google Scholar]
- 100.Lennert K, Mohri N. Histologische klassifizierung und vorkommen des M. Hodgkin. Internist (Berl) 1974;15:57–65. [PubMed] [Google Scholar]
- 101.Diebold J, Audouin J. Maladie de Hodgkin. Une ou plusieurs maladies. Ann Pathol 1989;9:84–91. [PubMed] [Google Scholar]
- 102.MacLennan KA, Bennett MH, Tu A, et al. Relationship of histologic features to survival and relapse in nodular sclerosing Hodgkin's disease. A study of 1659 patients. Cancer 1989;64:1686–93. [DOI] [PubMed] [Google Scholar]
- 103.Grogan TM. Hodgkin's disease. In: Jaffe ES, ed. Surgical pathology of the lymph nodes and related organs. Philadelphia: Saunders, 1995:133–92.
- 104.Banks PM. The distinction of Hodgkin's disease from T-cell lymphoma. Semin Diagn Pathol 1992;9:279–83. [PubMed] [Google Scholar]
- 105.Diebold J, Jungman P, Molina T, et al. Recent advances in Hodgkin's disease: an overview and review of the literature. Curr Diagn Pathol 1995;2:153–62. [Google Scholar]
- 106.Alavaikko MJ, Blanco G, Aine R, et al. Follicular dendritic cells have prognostic relevance in Hodgkin's disease. Am J Clin Pathol 1994;101:761–7. [DOI] [PubMed] [Google Scholar]
- 107.Baur AS, Mengi-Moraw C, Michel G, et al. Prognostic value of follicular dendritic cells in nodular sclerosing Hodgkin's disease. Histopathology 1998;32:512–20. [DOI] [PubMed] [Google Scholar]
- 108.Strickler JG, Michie SA, Warnke RA, et al. The “syncytial variant” of nodular sclerosing Hodgkin's disease. Am J Surg Pathol 1986;10:470–7. [DOI] [PubMed] [Google Scholar]
- 109.Ben-Yehuda-Salz D, Ben-Yehuda A, Polliack A, et al. Syncytial variant of nodular sclerosing Hodgkin's disease. A new clinicopathologic entity. Cancer 1990;65:1167–72. [DOI] [PubMed] [Google Scholar]
- 110.Bennett MH, MacLennan KA, Easterling MG, et al. The prognostic significance of cellular subtypes in nodular sclerosing Hodgkin's disease: an analysis of 271 non-laparotomised cases (BNLI report No 22). Clin Radiol 1983;34:497–501. [DOI] [PubMed] [Google Scholar]
- 111.Ferry JA, Linggod RM, Convery KM, et al. Hodgkin's disease, nodular sclerosing type: implications of histologic subclassification. Cancer 1993;71:457–63. [DOI] [PubMed] [Google Scholar]
- 112.D'Amore ESG, Lee CKK, Appli DM, et al. Lack of prognostic value of histopathologic parameters in Hodgkin's disease, nodular sclerosis type: a study of 123 patients with limited stage disease who had undergone laparotomy and were treated with radiation therapy. Arch Pathol Lab Med 1992;116:856–61. [PubMed] [Google Scholar]
- 113.Hess JL, Bodis S, Pinkus G, et al. Histopathologic grading of nodular sclerosis Hodgkin's disease. Lack of prognostic significance in 254 surgically staged patients. Cancer 1994;74:708–14. [DOI] [PubMed] [Google Scholar]
- 114.Pileri S, Falini B, Delsol G, et al. Lymphohistiocytic T-cell lymphoma (anaplastic large cell lymphoma CD30+/Ki-1 + with a high content of reactive histiocytes). Histopathology 1990;16:383–91. [DOI] [PubMed] [Google Scholar]
- 115.Pileri S, Bocchia M, Baroni CD, et al. Anaplastic large cell lymphoma (CD30+/Ki-1+): results of the prospective clinico-pathologic study of 69 cases. Br J Haematol 1994;86:513–23 [DOI] [PubMed] [Google Scholar]
- 116.Pileri S. Controversies on Hodgkin's disease and anaplastic large cell lymphoma. Hematopathology study group of the societa Italiana di anatomia patologica. Haematologia 1994;79:299–310. [PubMed] [Google Scholar]
- 117.Pileri SA, Piccaluga A, Poggi S, et al. Anaplastic large cell lymphoma: update of findings. Leuk Lymphoma 1995;18:17–25. [DOI] [PubMed] [Google Scholar]
- 118.Pileri SA, Poggi S, Sabattini E, et al. Anaplastic large cell lymphoma Hodgkin's like. Current Diagnostic Pathology 1995;2:57–8. [Google Scholar]
- 119.The non-Hodgkin's lymphoma classification project: a clinical evaluation of the international lymphoma study group classification of non-Hodgkin's lymphoma. Blood 1997;89:3909–18. [PubMed] [Google Scholar]
- 120.Falini B, Venturi S, Martelli M, et al. Mediastinal large B-cell lymphoma: clinical and immunohistochemical findings of 18 patients treated with two different third generation regimens. Br J Haematol 1995;89:780–9. [DOI] [PubMed] [Google Scholar]
- 121.Lazzarino M, Orlandi E, Paulli M, et al. Treatment outcome and prognostic factors for primary mediastinal (thymic) B-cell lymphoma: a multicentric study of 106 patients. J Clin Oncol 1997;15:1646–53. [DOI] [PubMed] [Google Scholar]
- 122.Aisenberg AC. Primary large cell lymphoma of the mediatinum. Semin Oncol 1999;26:251–8. [PubMed] [Google Scholar]
- 123.Higgins JP, Warnke RA. CD30 expression is common in mediastinal large B-cell lymphoma. Am J Clin Pathol 1999;112:241–7. [DOI] [PubMed] [Google Scholar]
- 124.Chadburn A, Frizzera G. Mediastinal large B-cell lymphoma vs classic Hodgkin lymphoma. Am J Clin Pathol 1999;112:155–8. [DOI] [PubMed] [Google Scholar]
- 125.Copie Bergman C, Gaulard P, Maouche CL, et al. The MAL gene is expressed in primary mediastinal large B-cell lymphoma. Blood 1999;94:3567–75. [PubMed] [Google Scholar]
- 126.Bacchi CE, Dorfman RF, Hoppe RT, et al. Metastatic carcinoma in lymph nodes simulating syncytial variant of nodular sclerosing Hodgkin's disease. Am J Clin Pathol 1991;96:589–93. [DOI] [PubMed] [Google Scholar]
- 127.Zarate-Osorno A, Jaffe ES, Medeiros LJ. Metastatic nasopharyngeal carcinoma initially presenting as cervical lymphadenopathy: a report of two cases that resembled Hodgkin's disease. Arch Pathol Lab Med 1992;116:862–5. [PubMed] [Google Scholar]
- 128.Doggett RS, Colby TV, Dorfman RF. Interfollicular Hodgkin's disease. Am J Surg Pathol 1983;7:145–9. [DOI] [PubMed] [Google Scholar]
- 129.Maheswaran PR, Ramsay AD, Norton AJ, et al. Hodgkin's disease presenting with the histological features of Castleman's disease. Histopathology 1991;18:249–53. [DOI] [PubMed] [Google Scholar]
- 130.Hsu S-M, Xie S-S, Hsu P-L, et al. Interleukin-6, but not interleukin-4, is expressed by Reed-Sternberg cells in Hodgkin's disease with or without histologic feature of Castleman's disease. Am J Pathol 1992;141:129–38. [PMC free article] [PubMed] [Google Scholar]
- 131.Lukes RJ. Criteria for involvement of lymph node, bone marrow, spleen and liver in Hodgkin's disease. Cancer Res 1971;31:1755–67 [PubMed] [Google Scholar]
- 132.Patsouris E, Noel H, Lennert K. Cytohistologic and immunohistochemical findings in Hodgkin's disease, mixed cellularity type with a high content of epithelioid cells. Am J Surg Pathol 1989;13:1014–22. [DOI] [PubMed] [Google Scholar]
- 133.Butler JJ. The histologic diagnosis of Hodgkin's disease. Semin Diagn Pathol 1992;9:252–6. [PubMed] [Google Scholar]
- 134.Hastrup N, Ralfkiaer E, Pallesen G. Aberrant phenotypes in peripheral T-cell lymphomas. J Clin Pathol 1989;42:398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pileri SA, Ascani S, Sabattini E, et al. Peripheral T-cell lymphoma: a developing concept. Ann Oncol 1998;9:797–801. [DOI] [PubMed] [Google Scholar]
- 136.Osborne BM, Uthman MO, Butler JJ, et al. Differentiation of T-cell lymphoma from Hodgkin's disease: mitotic rate and S-phase analysis. Am J Clin Pathol 1990;93:227–32. [DOI] [PubMed] [Google Scholar]
- 137.Jaffe ES, Longo DL, Cossman J, et al. Diffuse B cell lymphomas with T cell predominance in patients with follicular lymphoma or “pseudo T cell lymphoma”. Lab Invest 1984;50:2A–8A. [Google Scholar]
- 138.McBride JA, Rodriguez J, Luthra R, et al. T-cell-rich B large-cell lymphoma simulating lymphocyte-rich Hodgkin's disease. Am J Surg Pathol 1996;20:193–201. [DOI] [PubMed] [Google Scholar]
- 139.Ramsay AD, Smith WJ, Isaacson PG. T-cell-rich B-cell lymphoma. Am J Surg Pathol 1988;12:433–43. [DOI] [PubMed] [Google Scholar]
- 140.De Wolf-Peeters C, Pittaluga S. T-cell rich B-cell lymphoma: a morphological variety of non-Hodgkin's lymphomas or a clinicopathological entity? Histopathology 1995;28:381–3. [DOI] [PubMed] [Google Scholar]
- 141.Nguyen DT, Diamond LW, Hansmann M-L, et al. Differential diagnosis of L26-positive, CD15-negative Hodgkin's disease and large B-cell lymphoma with a high content of reactive T-cells: a morphologic and immunohistochemical study. Hematopathology and Molecular Hematology 1996;10:135–50. [PubMed] [Google Scholar]
- 142.De Jong D, Van Gorp J, Sie-Go D, et al. T-cell-rich B-cell non Hodgkin's lymphoma: a progressed form of follicle center cell lymphoma and lymphocyte predominance Hodgkin's disease. Histopathology 1996;28:15–24. [DOI] [PubMed] [Google Scholar]
- 143.Skinnider BF, Connors JM, Gascoyne RD. Bone marrow involvement in T-cell-rich B-cell lymphoma. Am J Clin Pathol 1997;108:570–8. [DOI] [PubMed] [Google Scholar]
- 144.Fleming MD, Shahrafei A, Dorfman D. Absence of dendritic reticulum cell staining is helpful for distinguishing T-cell-rich B-cell lymphoma from lymphocyte predominance Hodgkin's disease. Applied Immunohistochemistry 1998;6:16–22. [Google Scholar]
- 145.Kinney MC, Greer JP, Stein RS, et al. Lymphocyte depletion Hodgkin's disease: histopathologic diagnosis of marrow involvement. Am J Surg Pathol 1986;10:219–26. [PubMed] [Google Scholar]
- 146.Agnarsson BA, Kadin ME. Ki-1 positive large cell lymphoma, a morphologic and immunologic study of 19 cases. Am J Surg Pathol 1988;12:264–74. [DOI] [PubMed] [Google Scholar]
- 147.Pelstring R, Zellmer R, Sulak L, et al. Hodgkin's disease in association with human immunodeficiency virus infection. Cancer 1991;67:1865–73. [DOI] [PubMed] [Google Scholar]
- 148.Greer JP, Kinney MC, Collins RD, et al. Clinical features of 31 patients with Ki-1 anaplastic large-cell lymphoma. J Clin Oncol 1991;9:539–47. [DOI] [PubMed] [Google Scholar]
- 149.Kant JA, Hubbard SM, Longo DL, et al. The pathologic and clinical heterogeneity of lymphocyte-depleted Hodgkin's disease. J Clin Oncol 1986;4:284–94. [DOI] [PubMed] [Google Scholar]
- 150.Suster S, Moran CA. Pleomorphic large cell lymphomas of the mediastinum. Am J Surg Pathol 1996;20:224–32. [DOI] [PubMed] [Google Scholar]
- 151.Pileri SA, Sabattini E, Falini B. Critical commentary to “Inflammatory fibrosarcoma: another imitator of Hodgkin's disease ?” Letter to the case. Pathol Res Pract 1996;192:481–2. [DOI] [PubMed] [Google Scholar]
- 152.Mirra M, Falconieri G, Zanconati F, et al. Inflammatory fibrosarcoma: another imitator of Hodgkin's disease? Pathol Res Pract 1996;192:474–8. [DOI] [PubMed] [Google Scholar]
- 153.Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer 1978;41:2250–66. [DOI] [PubMed] [Google Scholar]
- 154.Argani P, Facchetti F, Inghirami G, et al. Lymphocyte-rich well-differentiated liposarcoma: report of nine cases. Am J Surg Pathol 1997;21:884–95. [DOI] [PubMed] [Google Scholar]
- 155.Hansmann M-L, Wacker H-H, Radzum HJ. Paragranuloma is a variant of Hodgkin's disease with predominance of B-cells. Virchows Arch A Pathol Anat Histopathol 1986;409:171–81. [DOI] [PubMed] [Google Scholar]
- 156.Hansmann M-L, Fellbaum C, Hui PK, et al. Correlation of content of B cells and Leu7-positive cells with subtype and stage in lymphocyte predominance type Hodgkin's disease. J Cancer Res Clin Oncol 1988;119:405–10. [DOI] [PubMed] [Google Scholar]
- 157.Ashton-Key M, Thorpe PA, Allen JP, et al. Follicular Hodgkin's disease. Am J Surg Pathol 1995;19:1294–9. [PubMed] [Google Scholar]
- 158.Diehl V, Stein H, Sextro M, et al. Lymphocyte predominance Hodgkin's disease: a European task force on lymphoma project [abstract]. Blood 1996;88(suppl 1):294a. [Google Scholar]
- 159.Dolginow D, Colby TV. Recurrent Hodgkin's disease in treated sites. Cancer 1981;48:1124–6. [DOI] [PubMed] [Google Scholar]
- 160.Serraino D, Pezzotti P, Dorrucci M, et al. Cancer incidence in a cohort of human immunodeficiency virus seroconverters. HIV Italian seroconversion study group. Cancer 1997;79:1004–8. [DOI] [PubMed] [Google Scholar]
- 161.Goedert JJ, Coté TR, Virgo P, et al. for the AIDS-cancer match study group: spectrum of AIDS-associated malignant disorders. Lancet 1998;351:1833–9. [DOI] [PubMed] [Google Scholar]
- 162.Serrano M, Bellas C, Campo E, et al. Hodgkin's disease in patients with antibodies to human immunodeficiency virus. Cancer 1990;65:2248–54. [DOI] [PubMed] [Google Scholar]
- 163.Tirelli U, Errante D, Dolcetti R, et al. Hodgkin's disease and human immunodeficiency virus infection: clinicopathologic and virologic features of 114 patients from the Italian cooperative group on AIDS and tumors. J Clin Oncol 1995;13:1758–67. [DOI] [PubMed] [Google Scholar]
- 164.Carbone A, Gloghini A, Larocca LM, et al. Human immunodeficiency virus-associated Hodgkin's disease derives from post-germinal center B cells. Blood 1999;93:2319–26. [PubMed] [Google Scholar]
- 165.Bellas C, Santon A, Manzanal A, et al. Pathological, immunological, and molecular features of Hodgkin's disease associated with HIV infection. Comparison with ordinary Hodgkin's disease. Am J Surg Pathol 1996;20:1520–4. [DOI] [PubMed] [Google Scholar]
- 166.Wang D, Leibowitz D, Kieff E, et al. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 1985;43:831–40. [DOI] [PubMed] [Google Scholar]
- 167.Baichwal VR, Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus. Oncogene 1988;2:461–7. [PubMed] [Google Scholar]
- 168.IARC Monographs on the evaluation of carcinogenic risks to humans, Vol. 67. Human immunodeficiency viruses and human T-cell lymphotropic viruses. Lyon, France: International Agency for Research on Cancer Health Organisation, 1996. [PMC free article] [PubMed]
- 169.Hansmann ML, Stein H, Fellbaum C, et al. Nodular paragranuloma can transform into high-grade malignant lymphoma of B type. Hum Pathol 1989;20:1169–75. [DOI] [PubMed] [Google Scholar]
- 170.Jaffe ES, Zarate-Osorno A, Kingma DW, et al. The interrelationship between Hodgkin's disease and non-Hodgkin's lymphomas. Ann Oncol 1994;5:S7–11. [DOI] [PubMed] [Google Scholar]
- 171.Kim H. Composite lymphoma and related disorders. Am J Clin Pathol 1993;99:445–51. [DOI] [PubMed] [Google Scholar]
- 172.Wickert RS, Weisenburger DD, Tierens A, et al. Clonal relationship between lymphocytic predominance Hodgkin's disease and concurrent or subsequent large B-cell lymphoma of B cell lineage. Blood 1995;86:2312–20 [PubMed] [Google Scholar]
- 173.Grainer TC, Gascoyne RD, Anderson ME, et al. Nodular lymphocyte-predominant Hodgkin's disease associated with large cell lymphoma: analysis of Ig gene rearrangements by V-J polymerase chain reaction. Blood 1996;88:657–66. [PubMed] [Google Scholar]
- 174.Pan LX, Diss TC, Peng HZ, et al. Nodular lymphocyte predominance Hodgkin's disease: a monoclonal or polyclonal B-cell disorder? Blood 1996;87:2428–34. [PubMed] [Google Scholar]
- 175.Yoshinaga H, Ohashi K, Yamamoto K, et al. Clonal identification of Burkitt's lymphoma arising from lymphocyte-predominant Hodgkin's disease. Br J Haematol 1996;95:380–2. [DOI] [PubMed] [Google Scholar]
- 176.Kadin M. Common activated helper-T-cell origin for lymphomatoid papulosis, mycosis fungoides, and some types of Hodgkin's disease. Lancet 1985;ii:864–5. [DOI] [PubMed] [Google Scholar]
- 177.Casey TT, Cousar JB, Maugum M, et al. Monomorphic lymphomas arising in patients with Hodgkin's disease. Correlation of morphologic, immunophenotypic, and molecular genetic findings in 12 cases. Am J Pathol 1990;136:81–94. [PMC free article] [PubMed] [Google Scholar]
- 178.Brecher M, Banks PM. Hodgkin's disease variant of Richter's syndrome. Report of eight cases. Am J Clin Pathol 1990;93:333–9. [DOI] [PubMed] [Google Scholar]
- 179.Gonzalez CL, Medeiros LJ, Jaffe ES. Composite lymphoma. A clinicopathologic analysis of nine patients with Hodgkin's disease and B-cell non-Hodgkin's lymphoma. Am J Clin Pathol 1991;96:81–9. [DOI] [PubMed] [Google Scholar]
- 180.Williams J, Schned A, Cotelingam JD, et al. Chronic lymphocytic leukemia with coexistent Hodgkin's disease. Implications for the origin of the Reed-Sternberg cell. Am J Surg Pathol 1991;15:33–42. [DOI] [PubMed] [Google Scholar]
- 181.Davis TH, Morton CC, Miller-Cassman R, et al. Hodgkin's disease, lymphomatoid papulosis, and cutaneous T-cell lymphoma derived from a common T-cell clone. N Engl J Med 1992;326:1115–22. [DOI] [PubMed] [Google Scholar]
- 182.Jaffe ES, Zorate-Osorno A, Medeiros LF, et al. The interrelationship of Hodgkin's disease and non-Hodgkin's lymphoma—lessons learned from composite and sequential malignancies. Semin Diagn Pathol 1992;9:297–303. [PubMed] [Google Scholar]
- 183.Harris NL. The relationship between Hodgkin's disease and non-Hodgkin's lymphoma. Semin Diagn Pathol 1992;9:304–10. [PubMed] [Google Scholar]
- 184.Zarate-Osorno A, Medeiros J, Kingma DW, et al. Hodgkin's disease following non-Hodgkin's lymphoma. A clinicopathologic and immunophenotypic study of nine cases. Am J Surg Pathol 1993;17:123–32. [PubMed] [Google Scholar]
- 185.Le Brun DP, Ngan BY, Weiss LM, et al. The bcl-2 oncogene in Hodgkin's disease arising in the setting of follicular non-Hodgkin lymphoma. Blood 1994;83:223–30. [PubMed] [Google Scholar]
- 186.Brousset P, Lamant L, Viraben R, et al. Hodgkin's disease following mycosis fungoides: phenotypic and molecular evidence for different tumour cell clones. J Clin Pathol 1996;49:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ohno T, Trenn G, Wu G, et al. The clonal relationship between nodular sclerosis Hodgkin's disease with clonal Reed-Sternberg cell population and a subsequent B-cell small noncleaved lymphoma. Mod Pathol 1998;11:485–90. [PubMed] [Google Scholar]
- 188.Ohno T, Smir BN, Weisenburger DD, et al. Origin of the Hodgkin/Reed-Sternberg cells in chronic lymphocytic leukemia with Hodgkin's transformation. Blood 1998;91:1757–61. [PubMed] [Google Scholar]
- 189.Jaffe ES, Muller-Hermelink HK. Relationship between Hodgkin's disease and non-Hodgkin's lymphomas. In: Mauch PM, Armitage JO, Diehl V, et al, eds. Hodgkin's disease. Philadelphia: Lippincott Williams & Wilkins, 1999:181–93.
- 190.Bräuninger A, Hansmann M-L, Strickler JG, et al. Identification of common germinal-center B-cell precursors in two patients with both Hodgkin's disease and non-Hodgkin's lymphoma. N Engl J Med 1999;340:1239–47. [DOI] [PubMed] [Google Scholar]
- 191.Marafioti T, Hummel M, Anagnostopoulos I, et al. Classical Hodgkin's disease and follicular lymphoma originating from the same germinal center B cell. J Clin Oncol 1999;17:3804–9. [DOI] [PubMed] [Google Scholar]
- 192.Bellan C, Lazzi S, Zassi M, et al. Immunoglobulin gene rearrangement analysis in Hodgkin's disease associated with large B-cell lymphoma in the same patient: evidence for receptor revision of immunoglobulin heavy chain variable genes in Hodgkin-Reed-Sternberg cells? Diagn Mol Pathol [In press.] [DOI] [PubMed]
- 193.Torlakovic E, Tierens A, Dang HD, et al. The transcription factor PU.1, necessary for B-cell development is expressed in lymphocyte predominance, but not classical Hodgkin's disease. Am J Pathol 2001;159:1807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]