Abstract
Advances in flow cytometry techniques and the availability of monoclonal antibodies that detect key functional molecules on lymphocytes have contributed greatly to a more precise diagnosis of the chronic lymphoproliferative disorders. In addition to the diagnostic value, the expression of certain markers such as p53 or CD38 provides relevant prognostic information to the clinician. Beyond their diagnostic and prognostic value, immunological markers play a major role in the detection of minimal residual disease, enabling the clinician to estimate more accurately the response to chemotherapy. Those monoclonal antibodies that are relevant to the characterisation of the chronic lymphoproliferative disorders and that could be incorporated in a routine practice are discussed.
Keywords: monoclonal antibodies, chronic lymphocytic leukaemia, cyclin D1, p53, CD79b, CD38
There have been considerable advances in flow cytometry, which have been reflected by the development of new methodologies and monoclonal antibodies that identify important functional molecules in the lymphocytes. Among these are monoclonal antibodies that recognise polypeptides of the B cell receptor and proteins involved in the regulation and control of the cell cycle and apoptosis. The application of these markers to the study of chronic lymphoproliferative disorders has made a major impact on the diagnosis and characterisation of these diseases and, to some extent, has provided important prognostic information.
It is now possible to detect not only membrane but also intracellular (nuclear and cytoplasmic) proteins by means of flow cytometry. The detection of intracellular proteins can be achieved by treating the cells with commercially available permeabilising reagents and fixative solutions, which allow the monoclonal antibody to penetrate the cell and react with the molecule that it recognises.1 In addition, the availability of monoclonal antibodies conjugated to a variety of fluorochromes has made it possible to perform triple or quadruple immunostaining routinely, allowing the expression of a particular antigen to be estimated in a discrete lymphoid subpopulation.
“It is now possible to detect not only membrane but also intracellular (nuclear and cytoplasmic) proteins by means of flow cytometry”
In addition to the panel of monoclonal antibodies routinely used in the past decade,2 and recommended in 1994 by the general haematology task force of the British Committee for Standards in Haematology,3 several others have been shown to provide relevant diagnostic and/or prognostic information, and could be incorporated into the panel of markers in a routine practice. The characteristics of these monoclonal antibodies and the molecules that they recognise, in addition to their relevance for the characterisation of the chronic lymphoproliferative disorders, are described below
MONOCLONAL ANTIBODIES TO CD79B
CD79 is a heterodimeric molecule comprising two polypeptide chains: the α-chain or mb1 (CD79a) and the β-chain or B29 (CD79b). CD79b is non-covalently bound to the immunoglobulin (Ig) in the surface of the B cell to form the B cell antigen receptor complex, and is essential for signal transduction after surface Ig crosslinking.4 Therefore, B29 is a key functional molecule in B cells. Monoclonal antibodies against the CD79b cluster (such as SN8 and CB3-1) recognise an external epitope of the B29 or β-chain of the B cell receptor complex. In normal B cell differentiation, B29 (CD79b) is first expressed in cells that have Ig μ chains and remains expressed throughout B cell differentiation up to the plasma cell stage. Cells from most chronic B cell disorders—for example, most B cell lymphomas and B cell prolymphocytic leukaemia—are CD79b positive. However, CD79b is either absent or aberrantly (weakly) expressed in neoplastic B cells from chronic lymphocytic leukaemia (CLL) and hairy cell leukaemia.5–8 Although early reports using a non-conjugated CD79b monoclonal antibody (SN8) documented that most CLL cases are CD79b negative,6,7 more recent studies with the use of a phycoerythrin conjugated monoclonal antibody against CD79b (CB3-1) have shown, by both standard and quantitative flow cytometry, that a substantial proportion of CLL cases are CD79b positive, albeit displaying very weak expression.8,9 Furthermore, it has been reported that CLL cases with detectable CD79b mRNA have point mutations or deletions in the two cDNAs encoding the B29 transmembrane and cytoplasmic domains, perhaps underlying the loss of signal transduction in CLL cells9 and accounting for altered B cell receptor expression.10 The low numbers of CD79b molecules in CLL cells correlates with the weak expression of surface Ig and the membrane CD22 characteristics of this disease.11 In addition to its functional relevance, CD79b is a useful monoclonal antibody to distinguish CLL from other B cell disorders if the intensity of staining is considered. In the scoring system for the diagnosis of CLL, the use of CD79b increases the accuracy of the diagnosis of CLL, and this molecule should be measured along with the other five markers listed in table 1.12
Table 1.
Scoring system for the diagnosis of chronic lymphoproliferative leukaemia (CLL)12
| Score points | ||
| Marker | 1 | 0 |
| SmIg | Weak | Strong |
| CD5 | Positive | Negative |
| CD23 | Positive | Negative |
| FMC7 | Negative | Positive |
| CD22 or CD79b | Weak | Strong |
Scores in CLL are >3 and in other B cell malignancies <3.
SmIg, surface immunoglobulins.
MONOCLONAL ANTIBODIES TO CYCLIN D1
Cyclin D1 is a regulatory nuclear protein of the cell cycle and a product of the PRAD/CCND1 gene locus located on chromosome 11q. Cyclin D1, together with its cyclin dependent kinase, is responsible for the transition from the G1 to the S phase of the cell cycle via phosphorylation of the retinoblastoma gene.13 Overexpression of cyclin D1 leads to the abnormal proliferation of cells with a shortened G1 phase. This may be the result of gene amplification, disturbance of regulatory mechanisms, and/or chromosome translocations. One of the most common translocations is t(11;14)(q13;q32), a cytogenetic hallmark of mantle cell lymphoma.14
Cyclin D1 overexpression can be estimated by a variety of methods, such as northern blotting to detect cyclin D1 mRNA,15–17 immunoblotting,18 reverse transcriptase polymerase chain reaction (RT-PCR),19 and, in tissue sections, by immunohistochemistry with a variety of monoclonal antibodies.20,21 It is now also possible to assess the expression of the protein by a dual parameter flow cytometry method on fixed and permeabilised cells, using a technique modified from that described for the detection of cyclin expression in tumour cell lines.22 A recent study by Elnenaei et al has shown that cyclin D1 can be detected by flow cytometry with the monoclonal antibody 5D4 in 92% of cases of mantle cell lymphoma, whereas only a very few cases of CLL and splenic lymphoma with villous lymphocytes are cyclin D1 positive.23 This study showed a good correlation between cyclin D1 expression and RT-PCR for cyclins D1, D2, and D3 and fluorescence in situ hybridisation (FISH) for the detection of the t(11;14.)(q13;q32) translocation, with 85% sensitivity and specificity. Although cyclin D1 overexpression is found in most mantle cell lymphomas and rarely in other B cell diseases, the specificity of cyclin D1 expression for mantle cell lymphoma, whether detected by flow cytometry on cell suspensions or immunohistochemistry in tissue sections, is below that for the FISH demonstration of the t(11;14) translocation.24 Thus, cells from a variety of non-haemopoietic neoplasms and multiple myeloma may overexpress cyclin D1 as a result of gene amplification, and it has also been documented in cases of hairy cell leukaemia and more rarely in cells from other B cell disorders.25,26 Nevertheless, the estimation of cyclin D1 by flow cytometry or by immunocytochemistry may be useful as a first diagnostic step in cases where mantle cell lymphoma is suspected—for example, in CD5 positive B cell disorders with a phenotype not typical of CLL (score, < 3), particularly when results are compounded with other laboratory features. As for immunohistochemistry, when results are equivocal by flow cytometry, other tests should be undertaken to exclude the diagnosis of mantle cell lymphoma.
MONOCLONAL ANTIBODIES TO CD38
CD38 is a 45 kDa transmembrane molecule. The gene encoding CD38 has been assigned to chromosome 4. The monoclonal antibody that recognises this antigen was first documented in the early 1980s as a T cell differentiation antigen,27 but its function and its potential pathogenic and/or prognostic role in leukaemia did not become apparent until the past decade.28
“CD38 is a strong prognostic marker in chronic lymphocytic leukaemia as a predictor of survival and aggressive clinical course”
CD38 belongs to a family of proteins involved in the production of calcium mobilising compounds and, in leucocytes, acts as a receptor in adhesion and signalling pathways. CD38 is expressed in a variety of non-haemopoietic and haemopoietic cells, the latter comprising early bone marrow CD34 positive precursors, thymic cells, natural killer cells, activated T cells, and B cells at early (pre-germinal and germinal centre cells) and late stages of differentiation, such as plasma cells. Despite lacking diagnostic power, it has recently become evident that CD38 is a strong prognostic marker in CLL as a predictor of survival and aggressive clinical course. Recent molecular data investigating the mutation status of the Ig variable heavy (IgVH) and light chain genes has shown that CLL may arise from a naïve pre-germinal centre cell with unmutated IgVH genes or from a memory post-germinal centre cell with mutated IgVH genes. These two groups seem to have a different prognosis and survival: significantly worse in the unmutated group.29,30 In addition, the report by Damle et al documented a correlation between CD38 expression and cases with unmutated IgVH genes, and suggested that CD38 might be a good discriminatory marker between cases with good and bad prognosis.29 In this study, CLL cases with > 30% CD38 positive cells had a significantly shorter survival than those with < 30% CD38 positive cells, and most of the former had unmutated IgVH.29 However, the correlation between CD38 expression and mutational status of IgVH has been a matter of controversy.31,32 Although the report by Damle et al suggested that CD38 expression correlated with cases having an unmutated IgVH gene,29 more recent studies indicate that CD38 is an independent prognostic marker.33–36 The prognostic value of CD38 for shorter survival and for the need for treatment has been shown in advanced and early clinical stages of the disease, including Binet stage A CLL. Because of the simplicity of CD38 assessment, unlike Ig sequencing, CD38 is a marker that should be incorporated into the routine panel for the study of CLL because of its prognostic value. From the technical point of view, it is important to assess the expression of CD38 in the leukaemic CLL lymphocytes because CD38 may be expressed in normal circulating B and T cells. The best approach is a triple platform immunostaining using the following monoclonal antibodies: CD38, CD5, and CD19.29 At present, it seems reasonable to consider as positive a result in which 30% or more of the leukaemic cells stain with CD38, but further studies are needed to confirm the best cutoff point.
MONOCLONAL ANTIBODIES TO THE P53 PROTEIN
p53 is a 393 amino acid protein encoded by the tumour suppressor gene p53 located on the short arm of chromosome 17 (17p13.1). The protein acts as a multifunctional transcription factor and is involved in cell cycle arrest, differentiation, DNA repair, and genomic stability.37,38 Mutations and deletions of the p53 gene have been shown to play a major role in disease initiation and/or progression in a variety of human cancers, including lymphoid malignancies. p53 abnormalities have been reported with a variable frequency in chronic lymphoproliferative disorders including CLL, prolymphocytic leukaemia, and B cell lymphomas, and have been shown to have prognostic significance and/or to be associated with transformation or drug resistance.39–44 Aberrations of the p53 gene lead to the accumulation of an abnormal p53 protein in the nucleus of the neoplastic cells, which can be detected by immunological methods. This is in contrast to the normal wild-type p53 protein, which cannot be detected by such methods because of its short life. There are several monoclonal antibodies that can be used to estimate abnormal p53 protein expression, either by immunohistochemistry or flow cytometry, with this last technique using fixation and permeabilisation of the cells.44 The flow cytometry method is a simple technique that could be used on a routine basis, and it has been incorporated into the CLL-4 UK clinical trial. This study will probably, in the context of a randomised setting, obtain information on the prognostic value of p53 expression in cells from patients with CLL and compare the results with the deletion of the p53 gene assessed by FISH.
MONOCLONAL ANTIBODIES TO TCR α/β AND TCR γ/δ
In T cell malignancies, a few new markers can be incorporated into a routine setting. Among these are monoclonal antibodies against T cell receptor (TCR) chains, which are highly specific for T cells and allow confirmation of the T cell nature of the neoplastic cells, particularly when results with other specific antibodies, such as CD3, are equivocal. These monoclonal antibodies should be tested together with the other T cell markers2,3 when a T cell malignancy is suspected.
“Therapeutic strategies such as stem cell transplantation aimed at disease eradication are increasingly used in these conditions, and this has resulted in the need for a precise estimate of residual leukaemic cells”
Beyond the diagnostic and prognostic value of immunological markers in chronic lymphoproliferative disorders, it is becoming apparent that markers play a major role in the detection of minimal residual disease. This is important because therapeutic strategies such as stem cell transplantation aimed at disease eradication are increasingly used in these conditions, and this has resulted in the need for a precise estimate of residual leukaemic cells. For this purpose, several strategies can be used according to the immunophenotype at diagnosis and the type of lymphoid disorder. Most studies exploit the presence of aberrant phenotypes and/or quantitative antigen abnormalities unique to leukaemic cells. For instance, residual disease in CLL or in cases of mantle cell lymphoma can be estimated by a simple double immunolabelling quantitative method using the monoclonal antibodies CD5 and CD19,45 or in CLL by a quadruple immunolabelling with monoclonal antibodies against CD19, CD20, CD79b, and CD5.46 This last study has shown that it is possible to detect one leukaemic cell within 104 or 105 cells with a sensitivity comparable to that of PCR.46
OTHER MONOCLONAL ANTIBODIES
CD20 and CD52 (Campath 1H) are monoclonal antibodies that detect antigens present in B cells (CD20) and in all lymphocytes and monocytes (CD52). Both are available as humanised chimaeric antibodies and are increasingly used as therapeutic agents in patients with lymphoproliferative disorders, either in vivo to treat or eradicate disease or in vitro for purging stem cell harvests before transplantation. Although they do not have a diagnostic or prognostic value, they should be included in the panel of markers for patients who might be considered as candidates for antibody treatment because their expression might influence the response to such treatment.
Although there have been some studies suggesting the prognostic value of certain monoclonal antibodies, such as those recognising proteins involved in apoptosis (bcl-2 family), adhesion molecules, multidrug resistance glycoproteins, or some myelomonocytic antigens, the evidence is not solid enough to justify the inclusion of these markers in the routine study of the chronic lymphoproliferative disorders.
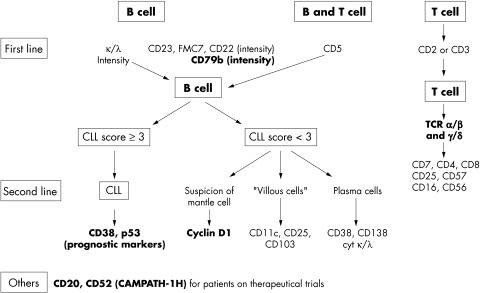
In summary, the availability and use of new markers in the study of the chronic lymphoproliferative disorders has resulted in a more precise definition of the various disease entities, improved our understanding of the pathogenesis of these disorders, and provided relevant prognostic information. Figure 1 shows the monoclonal antibodies that are useful in the characterisation of these diseases.
Figure 1.
Flow chart illustrating the monoclonal antibodies that are useful for the diagnosis of chronic lymphoproliferative disorders in routine practice (new monoclonal antibodies are shown in bold). CLL, chronic lymphocytic leukaemia; TCR, T cell receptor.
Take home messages.
The availability of monoclonal antibodies that detect key functional molecules on lymphocytes has enabled a more precise diagnosis of the chronic lymphoproliferative disorders
In addition, monoclonal antibodies that can be used to detect the expression of certain markers, such as p53 or CD38, provide relevant prognostic information to the clinician
Monoclonal antibodies that can also help in the detection of minimal residual disease, enabling the clinician to estimate more accurately the response to chemotherapy
Abbreviations
CLL, chronic lymphocytic leukaemia
FISH, fluorescence in situ hybridisation
Ig, immunoglobulin
RT-PCR, reverse transcriptase polymerase chain reaction
TCR, T cell receptor
VH, variable heavy
REFERENCES
- 1.Groeneveld K, Marvelde JG, van den Beemd MWM, et al. Flow cytometry detection of intracellular antigens for immunophenotyping of normal and malignant leukocytes. BTS technical report. Leukemia 1996;10:1383–9. [PubMed] [Google Scholar]
- 2.Matutes E. Contribution of immunophenotype in the diagnosis and classification of haemopoietic malignancies. J Clin Pathol 1995;48:194–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.General Haematology Task Force of the BCSH. Immunophenotyping in the diagnosis of chronic lymphoprolferative disorders. J Clin Pathol 1994;47:871–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benschop RJ, Cambier JC. B-cell development: signal transduction by antigen receptors and their surrogates. Curr Opin Immunol 1999;11:143–51. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki M, Luo Y, Han T, et al. Three new monoclonal antibodies that define a unique antigen associated with prolymphocytic leukemia/non-Hodgkin's lymphoma and are effectively internalised after binding to the cell surface antigen. Blood 1993;81:84–94. [PubMed] [Google Scholar]
- 6.Zomas AP, Matutes E, Morilla R, et al. Expression of the immunoglobulin-associated protein B29 in B cell disorders with monoclonal antibody SN8 (CD79b). Leukemia 1996;10:1966–70. [PubMed] [Google Scholar]
- 7.Moureau EJ, Matutes E, A'Hern RP, et al. Improvement of the chronic lymphocytic leukemia scoring system with the monoclonal antibody SN8 (CD79b). Am J Clin Pathol 1997;108:378–82. [DOI] [PubMed] [Google Scholar]
- 8.Cabezudo E, Carrara P, Morilla R, et al. Quantitative analysis of CD79b, CD5 and CD19 in mature B-cell lymphoproliferative disorders. Haematologica 1999;84:413–18. [PubMed] [Google Scholar]
- 9.Thompson AA, Talley JA, Do HN. Aberrations of the B-cell receptor B29 (CD79b) gene in chronic lymphocytic leukemia. Blood 1997;90:1387–94. [PubMed] [Google Scholar]
- 10.Alfarano A, Indraccolo S, Circosta P, et al. An alternatively spliced form of CD79b gene may account for altered B-cell receptor expression in B-chronic lymphocytic leukemia. Blood 1999;93:2327–35. [PubMed] [Google Scholar]
- 11.Matutes E, Owusu-Ankomah K, Morilla R, et al. The immunological profile of B-cell disorders and proposal of a scoring system for the diagnosis of CLL. Leukemia 1994;8:1640–5. [PubMed] [Google Scholar]
- 12.Matutes E, Polliack A. Morphological and immunophenotypic features of chronic lymphocytic leukemia. Reviews in Clinical and Experimental Hematology 2000;4:22–47. [DOI] [PubMed] [Google Scholar]
- 13.Donellan R, Chetty R. Cyclin D1 and human neoplasia. J Clin Pathol Mol Pathol 1998;51:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raffeld M, Jaffe ES. bcl-1, t(11;14) and mantle-cell derived lymphomas. Blood 1991;78:259–63. [PubMed] [Google Scholar]
- 15.Delmer A, Ajchenbaum-Cymbalista F, Tang R, et al. Over-expression of cyclin D1 in chronic B-cell malignancies with abnormality of chromosome 11q13. Br J Haematol 1995;89:798–804. [DOI] [PubMed] [Google Scholar]
- 16.Williams ME, Swerdlow SH. Cyclin D1 overexpression in non-Hodgkin's lymphoma with chromosome 11 bcl-1 rearrangement. Ann Oncol 1994;5(suppl 1):71–3. [DOI] [PubMed] [Google Scholar]
- 17.Jadayel D, Matutes E, Dyer MJS, et al. Splenic lymphoma with villous lymphocytes: analysis of BCL-1 rearrangements and expression of the cyclin D1 gene. Blood 1994;83:3664–71. [PubMed] [Google Scholar]
- 18.Ott MM, Bartkova J, Bartek J, et al. Cyclin D1 expression in mantle-cell lymphoma is accompanied by downregulation of cyclin D3 and is not related to the proliferative activity. Blood 1997;8:3154–9. [PubMed] [Google Scholar]
- 19.Uchimaru K, Taniguchi T, Yoshikawa M, et al. Detection of cyclin D1 (bcl-1, PRAD1) overexpression by a simple competitive transcription polymerase chain reaction assay in t(11;14)(q13;q32) bearing B-cell malignancies and/or mantle-cell lymphoma. Blood 1997;89:965–74. [PubMed] [Google Scholar]
- 20.De Boer CJ, Schuuring E, Dreef E, et al. Cyclin D1 protein analysis in the diagnosis of mantle cell lymphoma. Blood 1995;86:2715–23. [PubMed] [Google Scholar]
- 21.Vasef MA, Medeiros LJ, Koo C, et al. Cyclin D1 immunohistochemical staining is useful in distinguishing mantle-cell lymphoma from other low grade neoplasms in bone marrow. Hematopathology 1997;108:302–7. [DOI] [PubMed] [Google Scholar]
- 22.Darzynkiewicz Z, Gong J, Juan G, et al. Cytometry of cyclin proteins. Cytometry 1996;25:1–13. [DOI] [PubMed] [Google Scholar]
- 23.Elnenaei MO, Jadayel DM, Matutes E, et al. Cyclin D1 by flow cytometry as a useful tool in the diagnosis of B-cell malignancies. Leuk Res 2001;25:115–23. [DOI] [PubMed] [Google Scholar]
- 24.Matutes E, Carrara P, Coignet L, et al. FISH analysis for BCL-1 rearrangements and trisomy 12 helps the diagnosis of atypical B cell leukaemias. Leukemia 1999;13:1721–6. [DOI] [PubMed] [Google Scholar]
- 25.DeBoer CJ, Kluin-Nelemans JC, Dreef E, et al. Involvement of the CCND1 gene in hairy cell leukemia. Ann Oncol 1996;7:251–6. [DOI] [PubMed] [Google Scholar]
- 26.Swerdlow SH, Yang WI, Zukerberg LR, et al. Expression of cyclin D1 protein in centrocytic/mantle-cell lymphomas with and without rearrangement of the BCL1/cyclin D1 gene. Hum Pathol 1995;26:999–1004. [DOI] [PubMed] [Google Scholar]
- 27.Reinherz EL, Kung PC, Goldstein G, et al. Discrete stages of intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T lineage. Proc Natl Acad Sci U S A 1980;77:1588–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deaglio S, Mehta K, Malavasi F. Human CD38: a revolutionary story of enzymes and receptors. Leuk Res 2001;25:1–12. [DOI] [PubMed] [Google Scholar]
- 29.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood 1999;94:1840–7. [PubMed] [Google Scholar]
- 30.Hamblin TJ, Davis Z, Gardiner A, et al. Unmutated Ig VH genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 1999;94:1848–54. [PubMed] [Google Scholar]
- 31.Hamblin TJ, Orchard JA, Gardiner A, et al. Immunoglobulin V genes and CD38 expression in CLL. Blood 2000;95:2455–6. [PubMed] [Google Scholar]
- 32.Damle R, Wasil T, Allen S, et al. Updated data on V gene mutation status and CD38 expression in B-CLL. Blood 2000;95:2456–7. [Google Scholar]
- 33.Ibrahim S, Keating M, Do K-A, et al. CD38 expression as an important prognostic factor in B-cell chronic lymphocytic leukemia. Blood 2001;98:181–6. [DOI] [PubMed] [Google Scholar]
- 34.Matrai Z, Lin K, Dennis M, et al. CD38 expression and Ig VH gene mutation in B-cell chronic lymphocytic leukemia. Blood 2001;97:1902–3. [DOI] [PubMed] [Google Scholar]
- 35.Del Poeta G, Maurillo L, Venditti A, et al. CD38 expression identifies two distinct prognostic subsets in B-chronic lymphocytic leukemia (B-CLL). Blood 2000;96(suppl 1):355a.10891473 [Google Scholar]
- 36.Oscier DG, Orchard JA, Ibbotson R, et al. Can CD38 expression act as a surrogate marker for Ig V gene mutational status in B-CLL? Blood 2000;96(suppl 1):835a. [Google Scholar]
- 37.Hainaut P, Hollstein M. p53 and human cancer: the first ten thousand mutations. Adv Cancer Res 2000;77:81–137. [DOI] [PubMed] [Google Scholar]
- 38.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell 1997;88:323–31. [DOI] [PubMed] [Google Scholar]
- 39.Lens D, Dyer MJS, Garcia-Marco JM, et al. p53 abnormalities in CLL are associated with excess of prolymphocytes and poor prognosis. Br J Haematol 1997;99:848–57. [DOI] [PubMed] [Google Scholar]
- 40.Hernandez L, Fest T, Cazorla M, et al. p53 mutations and protein overexpression are associated with aggressive variants of mantle-cell lymphoma. Blood 1996;87:3351–9. [PubMed] [Google Scholar]
- 41.Koduru PR, Raju K, Vadmal V, et al. Correlation between mutation in p53, p53 expression, cytogenetics, histological type and survival in patients with B-cell non-Hodgkin's lymphoma. Blood 1997;90:4078–91. [PubMed] [Google Scholar]
- 42.Lens D, de Shouwer PJ, Hamoudi RA, et al. p53 abnormalities in B-cell prolymphocytic leukemia. Blood 1997;89:2015–23. [PubMed] [Google Scholar]
- 43.Cordone I, Masi S, Mauro FR, et al. p53 expression in B-cell chronic lymphocytic leukemia: a marker of disease progression and poor prognosis. Blood 1998;91:4342–9. [PubMed] [Google Scholar]
- 44.Gruszka-Westwood AM, Hamoudi RA, Matutes E, et al. p53 abnormalities in splenic lymphoma with villous lymphocytes. Blood 2001;97:3552–8. [DOI] [PubMed] [Google Scholar]
- 45.Cabezudo E, Matutes E, Ramrattan M, et al. Analysis of residual disease in chronic lymphocytic leukemia patients and prognostic value. Leukemia 1997;11:1909–14. [DOI] [PubMed] [Google Scholar]
- 46.Rawstron AC, Kennedy B, Evans PAS, et al. Quantitation of minimal residual disease levels in chronic lymphocytic leukemia using a sensitive flow cytometric assay improves the prediction of outcome and can be used to optimize therapy. Blood 2001;98:29–35. [DOI] [PubMed] [Google Scholar]