Abstract
Aim: To determine the prevalence of Chlamydia pneumoniae DNA in infrequently examined blood vessels.
Methods: Vessels obtained from 15 men and six women at coronary artery bypass surgery were tested by a nested polymerase chain reaction (PCR) assay for C pneumoniae DNA.
Results: Chlamydia pneumoniae DNA was detected in four of six atheromatous ascending aorta specimens but in none of eight non-atheromatous aorta specimens, in six of 11 atheromatous internal mammary artery specimens but in none of seven non-atheromatous internal mammary artery specimens, in five of seven long saphenous vein specimens showing evidence of disease but in none of 12 specimens without evidence of disease, and in two of three previously grafted veins. Overall, C pneumoniae occurred significantly more often in diseased than in normal vessels (p = < 0.00001).
Conclusions: Chlamydia pneumoniae is often present in diseased areas of arteries, including the internal mammary arteries, and even in diseased areas of veins. It is not present in apparently healthy areas of either type of vessel.
Keywords: Chlamydia pneumoniae, polymerase chain reaction, arteries, veins
C hlamydia pneumoniae, an obligately intracellular micro organism, commonly causes a spectrum of upper and lower respiratory tract diseases in humans.1 It was first associated with coronary heart disease on the basis of a serological study in Finland.2 Although this and further serological data that have accrued in the past 12 years have elicited general interest and dispute,3–5 the finding of C pneumoniae in the atheromatous lesions of coronary arteries eight years ago6 intensified interest in the association. Since then, the organisms have been detected by various techniques in the atheromatous lesions of various major arteries that have been examined in more than 30 studies,7 and of the various microorganisms that could be involved in the pathogenesis of atherosclerosis, evidence for the role of C pneumoniae is relatively strong.8
“Chlamydia pneumoniae was first associated with coronary heart disease on the basis of a serological study in Finland”
We have examined normal and abnormal portions of ascending aortas, internal mammary arteries, and long saphenous veins for the following reasons. First, overall, these vessels have been examined least frequently for C pneumoniae,7 although they may be obtained readily at coronary artery bypass surgery. Second, internal mammary arteries contain atheromatous lesions less often than many other arteries, so that obtaining normal tissue from them should be easy. Third, veins that are to be used in bypass surgery are not subject to atheromatous changes and yet some have been recorded as C pneumoniae positive.9, 10
METHODS
Vessels were obtained from 21 patients (15 men and six women) at coronary artery bypass surgery. Their ages ranged from 52 to 81 years (mean, 65). Specimens of ascending aorta, internal mammary artery (right or left), and long saphenous vein from the upper leg were obtained from each of 14 patients. A sample of internal mammary artery and long saphenous vein, but not aorta, was taken from a further four patients and saphenous vein alone from one patient. In addition, three failed grafted veins were removed and examined.
Some artery and vein specimens appeared macroscopically normal, whereas others on sectioning appeared to have fatty areas or areas that seemed not as soft on pressure with a scalpel blade. In this last case, an abnormal area of vessel was sampled, and in the former, a normal area. Each piece was divided into two portions. One portion was fixed in 10% phosphate buffered formalin, embedded in paraffin wax, and sections stained with haematoxylin and eosin and elastic van Gieson stains for histopathological examination, on which evidence of atheroma was based. The adjacent portion was frozen at −70°C before being tested by a nested polymerase chain reaction (PCR) assay for C pneumoniae DNA. The PCR assay was conducted with appropriate controls and with precautions to prevent DNA cross contamination, according to a method described previously.9
RESULTS
Chlamydia pneumoniae in arteries
Of 14 aortic specimens examined, six showed atheromatous lesions that histologically comprised infiltration of the intimal and muscle layers by chronic inflammatory cells, mainly lymphocytes; of these six specimens, four were PCR positive for C pneumoniae. In contrast, of eight specimens that showed no evidence of atheroma, that is without evidence of inflammatory cell infiltration, none was PCR positive. Similarly, of 18 internal mammary artery specimens examined, 11 showed atheromatous lesions histologically; of these 11, six were PCR positive for C pneumoniae. In contrast, of seven internal mammary artery specimens for which there was no evidence of atheroma, none was PCR positive. In total, 10 of 17 atheromatous artery specimens were PCR positive in contrast to none of 15 specimens without disease (Fisher's exact test, two tailed: p = 0.00035). Advanced atheromatous lesions11 of the aorta and internal mammary artery were not seen, so that an association between the degree of atheroma and C pneumoniae positivity was not assessable.
Chlamydia pneumoniae in veins
Of 19 long saphenous vein specimens examined, seven showed sparse infiltration of the intimal and muscle layers by chronic inflammatory cells (fig 1); of these seven specimens, five were PCR positive for C pneumoniae. Intimal fibrosis was also seen in 11 of the 19 specimens and in all of the seven with inflammatory changes. Because infiltration and fibrosis were mild in all cases, it was not feasible to relate a degree of severity with C pneumoniae positivity. In contrast to inflamed tissues, of 12 specimens without evidence of infiltration histologically, none was PCR positive (Fisher's exact test, two tailed: p = 0.002). Specimens of three failed grafted veins that had been removed all showed gross intimal and subintimal thickening; two of these were PCR positive for C pneumoniae.
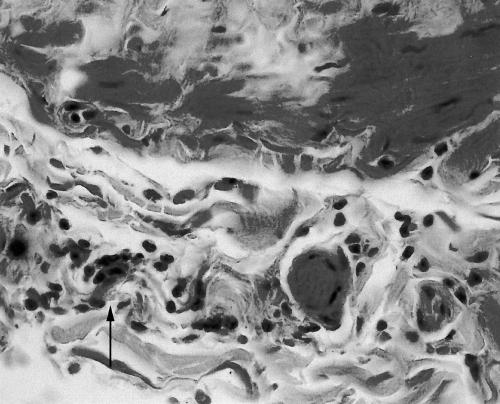
Figure 1.
Section of chlamydia positive long saphenous vein showing sparse focal lymphocytic infiltration of the muscle. Haemotoxylin and eosin; original magnification, ×400.
When considering both arteries and veins, of the 27 specimens that had signs of disease histologically, 17 were PCR positive for C pneumoniae. In contrast, of the 27 specimens for which no signs of disease were apparent histologically, none was PCR positive (χ2 test, p = < 0.00001).
DISCUSSION
We chose to use the PCR technique because of its sensitivity and specificity. We did not use immunocytochemistry because previous experience has shown that spurious staining can occur,11, 12 and neither did we use electron microscopy, which we have used previously to demonstrate C pneumoniae in tissues,11 but which is insensitive as a means of detection. In addition, although serology is arguably of value in large scale studies as an epidemiological tool,3–5 in a small study it is not rewarding to attempt to relate C pneumoniae antibody titres to the PCR positivity of vessels.5, 13
All of the three types of vessel from some subjects were C pneumoniae positive, whereas only a proportion or none from other subjects was positive. However, this is a spurious observation because examination of multiple sections of long segments of vessels would be required to make valid comparisons of positivity between vessels and between subjects. In reality, unlike the large amount of abdominal aortic tissue that may be obtained at the time of aneurysm repair,9 only thin slivers of ascending aorta were available for examination in our study. Despite this, a considerable proportion showed atheromatous changes histologically, of which two thirds were C pneumoniae positive by the PCR.
“Arteries that showed no signs of atheroma histologically were never C pneumoniae positive”
Take home messages.
Chlamydia pneumoniae is often present in diseased areas of arteries and veins, although it is not present in apparently healthy areas of either type of vessel
Vessels used for bypass surgery, whether internal mammary artery or saphenous vein or both, are often C pneumoniae positive
It is possible that those subjects who fail bypass surgery earliest are those who have been grafted with C pneumoniae positive vessels
Internal mammary artery specimens were examined by Wong et al,10 who found that two of five of these arteries that were being used for the first time in coronary artery bypass surgery were C pneumoniae positive by a PCR assay, and that four of 15 that were old grafts were positive also. Overall, 30% of the arteries were positive. Despite internal mammary arteries being favoured for coronary artery bypass surgery, about 60% of the specimens we examined showed atheromatous changes. Of all the internal mammary artery specimens, about a third were C pneumoniae positive, but of those that showed atheromatous changes just over a half were C pneumoniae positive. Arteries that showed no signs of atheroma histologically were never C pneumoniae positive. Our overall results are an endorsement of C pneumoniae being associated strongly with atheromatous lesions in arteries.
Wong et al found that 12% of saphenous vein specimens before grafting were C pneumoniae positive, whereas 38% of failed grafts were positive.10 Surprisingly, we found that, although no long saphenous vein specimen had an atheromatous appearance, more than a third of such specimens before grafting had evidence of disease in the form of focal chronic inflammatory cell infiltration. About a quarter of all the vein specimens were C pneumoniae positive but almost three quarters of the diseased specimens were C pneumoniae positive. A similar proportion of failed grafts were C pneumoniae positive also. In contrast, no specimen that was apparently infiltrate free histologically was C pneumoniae positive. Intimal fibrosis was also seen in veins, but is considered a “normal” age related change.14 The significance of the association of C pneumoniae with inflammatory lesions in veins, particularly in those before grafting, is unclear. Perhaps C pneumoniae positive lymphocytes (see below) are attracted to areas of inflammation that, for unknown reasons, already exist. On the other hand, there would certainly seem to be no a priori reason why C pneumoniae should not produce a vasculitis in these vessels, possibly as a consequence of reinfection, as suggested for other vasculitides.15 Interestingly, C pneumoniae IgG antibody titres of 256 or greater have been associated with deep vein thrombosis,16 although the association has been questioned.17 Nevertheless, the idea that C pneumoniae in veins might produce lesions, which sometimes aid thrombosis, is sufficiently plausible to deserve further consideration.
We assume that the source of C pneumoniae in the lesions (whether of arteries or veins) is C pneumoniae positive peripheral blood monocytes,18 which must have easy access to the lesions. It is interesting that internal mammary arteries are used for coronary artery bypass surgery because of their anatomical location and because they are considered to be relatively atheroma free. Of course, the same may be said in terms of disease for saphenous veins. Nevertheless, our results indicate that vessels used for bypass surgery, whether internal mammary artery or saphenous vein or both, are often C pneumoniae positive. This raises the intriguing question of whether those subjects who fail bypass surgery earliest are those who have been grafted with C pneumoniae positive vessels, a question based, of course, on the so far unconfirmed notion that C pneumoniae has a role in the development of atheromatous changes or atheromatous-like changes in veins.
REFERENCES
- 1.Grayston JT. Infections caused by Chlamydia pneumoniae strain TWAR. Clin Infect Dis 1992;15:757–63. [DOI] [PubMed] [Google Scholar]
- 2.Saikku P, Leinonen M, Mattila K, et al. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet 1988;ii:983–6. [DOI] [PubMed] [Google Scholar]
- 3.Wald NJ, Law MR, Morris JK, et al. Chlamydia pneumoniae infection and mortality from ischaemic heart disease: a large prospective study. BMJ 2000;321:204–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danesh J, Whincup P, Walker M, et al. Chlamydia pneumoniae IgG titres and coronary heart disease: prospective study and meta-analysis. BMJ 2000;321:208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor-Robinson D. Chlamydia pneumoniae and ischaemic heart disease. Electronic response to: Chlamydia pneumoniae infection and mortality from ischaemic heart disease: a large prospective study. Bmj.com 2000;321 www.bmj.com/cgi/content/short/321/7255/204#responses (accessed 18 December 2001). [DOI] [PMC free article] [PubMed]
- 6.Shor A, Kuo C-C, Patton DL. Detection of Chlamydia pneumoniae in coronary arterial fatty streaks and atheromatous plaques. S Afr Med J 1992;82:158–61. [PubMed] [Google Scholar]
- 7.Taylor-Robinson D, Thomas BJ. Chlamydia pneumoniae in atherosclerotic tissue. J Infect Dis 2000;181(suppl 3):437–40. [DOI] [PubMed] [Google Scholar]
- 8.Morré SA, Stooker W, Lagrand WK, et al. Microorganisms in the aetiology of atherosclerosis. J Clin Pathol 2000;53:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong G, Thomas BJ, Mansfield AO, et al. Detection and widespread distribution of Chlamydia pneumoniae in the vascular system and its possible implications. J Clin Pathol 1996;49:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong Y, Thomas M, Tsang V, et al. The prevalence of Chlamydia pneumoniae in atherosclerotic and nonatherosclerotic blood vessels of patients attending for redo and first time coronary artery bypass graft surgery. J Am Coll Cardiol 1999;33:152–6. [DOI] [PubMed] [Google Scholar]
- 11.Shor A, Phillips JI, Ong G, et al. Chlamydia pneumoniae in atheroma: consideration of criteria for causality. J Clin Pathol 1998;51:812–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor-Robinson D, Thomas BJ. Chlamydia pneumoniae in arteries: the facts, their interpretation, and future studies. J Clin Pathol 1998;51:793–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong Y-k, Dawkins KD, Ward ME. Circulating Chlamydia pneumoniae DNA as a predictor of coronary artery disease. J Am Coll Cardiol 1999;34:1435–9. [DOI] [PubMed] [Google Scholar]
- 14.Milroy CM, Scott DJA, Beard JD, et al. Histological appearance of the long saphenous vein. J Pathol 1989;159:311–16. [DOI] [PubMed] [Google Scholar]
- 15.Ljungström L, Franzén C, Schlaug M, et al. Reinfection with Chlamydia pneumoniae may induce isolated and systemic vasculitis in small and larger vessels. Scand J Infect Dis 1997;104(suppl):37–40. [PubMed] [Google Scholar]
- 16.Lozinguez O, Arnaud E, Belec L, et al. Demonstration of an association between Chlamydia pneumoniae infection and venous thromboembolic disease. Thromb Haemostat 2000;83:887–91. [PubMed] [Google Scholar]
- 17.Koster T, Rosendaal FR, Lieuw-A-Len DD, et al. Chlamydia pneumoniae IgG seropositivity and risk of deep-vein thrombosis. Lancet 2000;355:1694–5. [DOI] [PubMed] [Google Scholar]
- 18.Boman J, Söderberg S, Forsberg J, et al. High prevalence of Chlamydia pneumoniae DNA in peripheral blood mononuclear cells in patients with cardiovascular disease and in middle-aged blood donors. J Infect Dis 1998;178:274–7. [DOI] [PubMed] [Google Scholar]