Abstract
Extramedullary myeloid cell tumour (EMMT) localised to the mediastinum is a rare manifestation of acute myeloid leukaemia, forming less than 4% of all cases of EMMT. In contrast to other types of EMMT, cytogenetic characteristics of this rare entity are relatively unknown. This report describes a patient with EMMT who had evidence of superior vena cava syndrome and normal peripheral blood counts at diagnosis. The results from an initial biopsy specimen were consistent with a diagnosis of mediastinal large B cell lymphoma. A diagnosis of acute myeloid leukaemia was made three months after initial diagnosis by bone marrow examination. Review of the initial biopsy specimen showed strong positivity for myeloperoxidase, revealing that the patient had been initially misdiagnosed as having large B cell lymphoma. Cytogenetic studies revealed a near triploid and near tetraploid karyotype with structural abnormalities in 12 and three metaphases, respectively. Review of the literature showed that a near tetraploid or triploid karyotype is found in most of the reported cases of mediastinal EMMT. Thus, the presence of a near triploid/tetraploid karyotype and mediastinal EMMT may represent a specific subset of EMMT. The biological relevance of this observation is discussed.
Keywords: Extramedullary myeloid cell tumour, cytogenetics, myeloid leukaemia
Malignant myeloid precursor cells can find a niche to proliferate outside their normal bone marrow habitat in almost all tissues of the body,1 an unusual feature initially reported almost two centuries ago.2 When they do so, they most often invade the periosteum and bone, soft tissues, skin, and lymph nodes.2 Characteristically, they form tumorous aggregates or masses initially described as granulocytic sarcomas,3 and later referred to as extramedullary myeloid tumours (EMMTs).4 They can present before, at the same time, or after a diagnosis of the acute myeloid process.5 Awareness in recent years of the probability of misdiagnosing EMMTs when they present as an isolated process1,4,6 has been of no avail. Indeed, in a recent retrospective study, all cases presenting without a previous history of myeloproliferative disorder had an initial incorrect diagnosis, mostly of lymphoma.7
In the past decade, information gained from cytogenetic studies has contributed to identifying specific subsets of EMMT.5,8 Comprehensive information provided by a new case studied at our institution, and from a literature review, suggests that mediastinal EMMT probably constitutes a distinct clinicopathological entity among EMMTs, with unique karyotypic features.
CASE REPORT
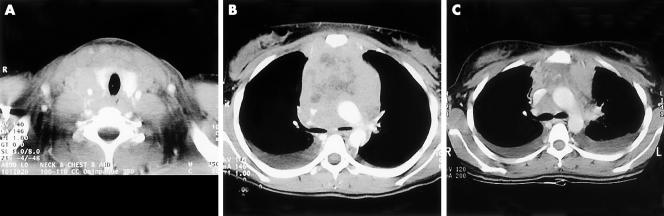
A 17 year old girl presented to her local hospital on 4 July 2000, with shortness of breath and dysphagia. Chest x ray (CXR) revealed bilateral pleural effusions with a large mediastinal mass. Physical examination revealed typical features of superior vena cava syndrome and bilateral pleural effusions. Small lymph nodes were palpable in both axillae. The white blood cell count (WBC) was 8 × 109/litre with a normal differential count, haemoglobin (Hb) was 145 g/litre, and platelets were 327 x 109/litre. Computerised tomography (CT) of the chest showed a mediastinal mass with areas of necrosis causing compression of the trachea, oesophagus, and superior vena cava (fig 1A, B). An anterior mediastinal core biopsy was interpreted as crushed material with sheets of malignant appearing lymphocytes with irregularly shaped nuclei, consistent with large B cell lymphoma. The patient refused a bone marrow aspirate and biopsy procedure. On the basis of the histology report, the patient was treated with a first cycle of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
Figure 1.
(A) Horizontal scan of the base of the neck showing a large non-vascular soft tissue mass measuring 6 cm in transverse diameter engulfing and encasing the neurovascular bundle and extending to the cervical oesophagus. (B) Large anterosuperior mediastinal mass measuring 7 cm in transverse diameter engulfing and constricting the superior vena cava and extending to the aortopulmonary window. The mass contains necrotic material. There are also small pleural effusions. (C) After two cycles of CHOP chemotherapy. Significant reduction in tumour mass size with less superior vena cava compression and residual pleural effusions.
When first seen at our institution on 31 July 2000 for completion of treatment, the patient was asymptomatic. Physical examination was unremarkable. CXR and CT showed a reduction in the size of the mediastinal mass and persistent pleural effusions (fig 1C). Initial biopsy material could not be adequately interpreted. Complete blood counts revealed: WBC, 7.2 × 109/litre, with 5% myelocytes and 4% metamyelocytes; Hb, 124 g/litre; and platelets, 325 × 109/litre and scattered circulating malignant cells, which prompted a bone marrow examination, pleural fluid analysis, and biopsy of the residual mediastinal mass.
MATERIALS AND METHODS
Classification of acute myeloid leukaemia was based on standard FAB cooperative group criteria.9 Immunophenotypic studies were performed by multiparameter flow cytometry (FACS Caliber flow cytometer, Becton Dickinson, San Jose, California, USA). Flow cytometry was performed on the blast cells from the peripheral blood, bone marrow, pleural fluid, and mediastinal lymph node, gated on their abnormal light scatter characteristics using monoclonal antibodies (Becton Dickinson, Immunotech, Marseille, France) to myeloid, T cell, B cell, natural killer cell, and megakaryocytic antigens, in addition to HLA-DR, terminal deoxynucleotidyl transferase, and cytoplasmic myeloperoxidase. Several panels containing two or three conjugated antibodies each were used for immunophenotyping analyses: a membrane or cytoplasmic marker was considered positive when expressed by more than 20% of the blast cells. Immunohistochemical stains were performed on the trephine biopsy for CD45, CD3, CD20, and CD34 antigens, and myeloperoxidase. Cytochemical staining for myeloperoxidase, Sudan black B, combined esterases, periodic acid Schiff, and acid phosphatase was performed on the bone marrow aspirate specimen. Cytogenetic analysis using standard methods10,11 was performed on cells obtained from the bone marrow aspirate. Sections (3 μm thick) from the mediastinal biopsy specimen were stained with haematoxylin and eosin, in addition to immunohistochemical staining for CD45, CD20, CD3, CD34, and myeloperoxidase.
RESULTS
Morphology and immunophenotyping
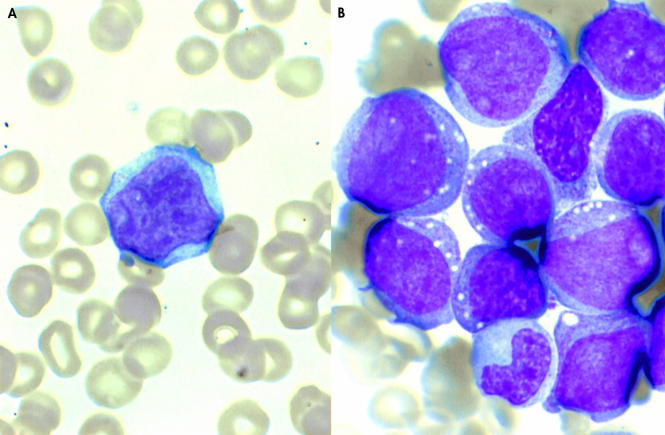
Peripheral blood, bone marrow (fig 2A,B), and pleural fluid (fig 3A) revealed the presence of large blasts with a relatively low nuclear–cytoplasmic ratio, open chromatin pattern, few prominent nucleoli, many cytoplasmic granules, and some cytoplasmic vacuolisation. Many showed cleft and convoluted nuclei. No Auer rods were seen. Flow cytometry showed that the blasts had a very high forward and side scatter, in keeping with their large size and granular cytoplasm (fig 3B). They were positive for CD45, myeloperoxidase, HLA-DR, CD4, CD15, CD11c (partial), CD64, CDw65, CD56, CD23, and CD9, and negative for CD34, CD33, and CD117. CD13 was very weakly expressed. Immunohistochemistry showed strong positivity for CD45 whereas CD34, CD20, and CD3 were negative. Cytochemistry revealed strong positivity with myeloperoxidase, Sudan black B, and chloracetate esterase, and negativity for periodic acid Schiff, acid phosphatase, and α-naphthyl acetate esterase, consistent with myeloid leukaemia.
Figure 2.
(A) Peripheral blood smear (Wright-Giemsa stain; original magnification, ×600) and (B) bone marrow aspirate (Wright-Giemsa stain; original magnification, ×600), showing large blast cells with a relatively low nucleo–cytoplasmic ratio, open chromatin, few prominent nucleoli, and cytoplasmic granulation and vacuolisation.
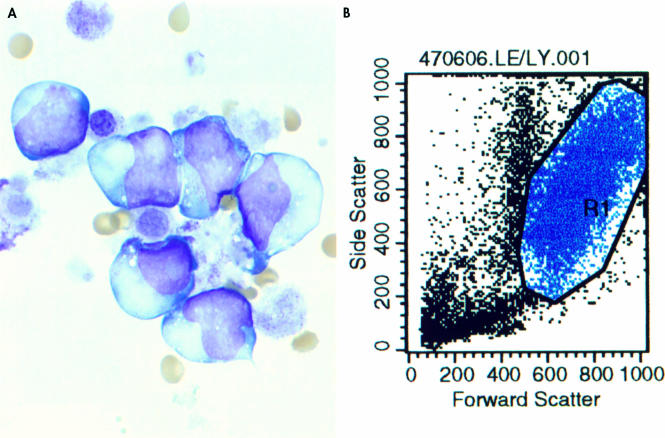
Figure 3.
(A) Pleural fluid (Wright-Giemsa stain; original magnification, ×400) showing (B) involvement by the same type of blast cells with high forward and side light scatter.
Histology
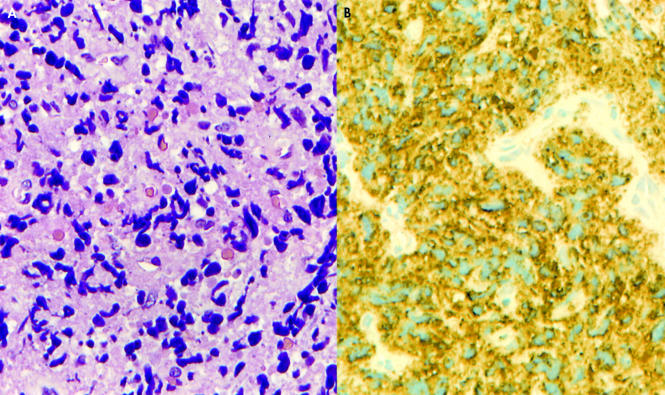
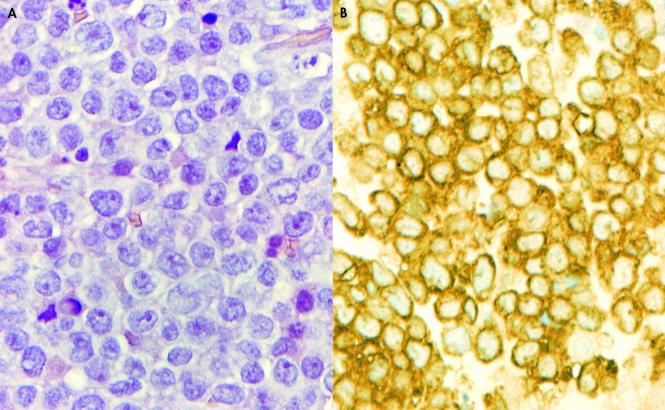
Review of the initial lymph node biopsy showed extensive crush artifact, which precluded a definitive interpretation (fig 4A). Despite poor cellular preservation, all cells were strongly positive for myeloperoxidase, excluding the B cell nature of the infiltration and confirming a diagnosis of primary EMMT (fig 4B). The subsequent biopsy also showed localised crush artifact but cell preservation was much better. The malignant infiltrate consisted of intermediate sized cells. Nuclei were relatively round and contained a single nucleolus. Cytoplasm was moderate in amount and focally granular. Auer rods were not seen (fig 5A). Immunohistochemistry showed positivity for CD45 and myeloperoxidase. All of the other antibodies, including CD34, were negative (fig 5B).
Figure 4.
(A) Initial biopsy specimen showing extensive crush artifact. Cell detail is absent and represented by streams of chromatin like material (haematoxylin and eosin stain; original magnification, ×400). (B) Anti-myeloperoxidase stain (original magnification, ×400) showing widespread positivity, confirming a diagnosis of extramedullary myeloid tumour.
Figure 5.
Second biopsy specimen (haematoxylin and eosin stain; original magnification, ×400) showing (A) sheets of intermediate sized blast like cells, with moderate cytoplasm and focal granularity. (B) Malignant cells are strongly positive for anti-myeloperoxidase.
Cytogenetics
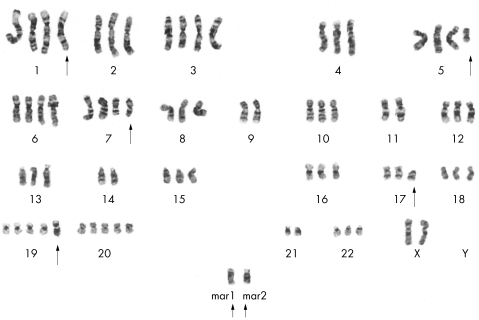
Based on the 15 metaphases analysed, two composite karyotypes were found: one with a near triploid chromosome count (72–76 chromosomes) in 12 metaphases, and the other with a near tetraploid count (81–87 chromosomes) in three metaphases. The composite karyotypes were: 72–76, {3n}, XX, −X, +1, ?inv(1)(p32q25)×2, +5, del(5)(q13q33), +6, +7, del(7)(q22)×2, −9, −11, −14, del(17)(p11.2), +19, +19, add(19)(q13.1), +20, −21, +mar1, +mar2{cp12}, and 81–87, {4n}, XX, −X, −X, ?inv(1)(p32q25)×2, −2, −4, del(5)(q13q33), del(7)(q22)×2, −9, −9, −10, −11, −11, 12, −13, −14, −14, −15, −16, −17, del(17)(p11.2), +19, +19, add(19)(q13.1)×2, +20, −21, −21, −22, +12mar{cp3}. Non-random chromosome rearrangements were found in both composite karyotypes, such as inv 1(p32;q25), del 5(q14;q33), del 7(q22), and del 17(p11.1). The tetraploid cells also had numerous marker chromosomes. A representative karyotype is shown in fig 6.
Figure 6.
Representative bone marrow karyotype: 75, {3n}, XX, −X, +1, ?inv(1)(p32q25)×2, +5, del(5)(q13q33), +6, +7, del(7)(q22)×2, −9, −11, −14, del(17)(p11.2), +19, +19, add(19)(q13.1), +20, −21, +mar1, +mar2. The arrows point to abnormal chromosomes.
DISCUSSION
Extramedullary myeloid tumours have been reported in almost every site in the body. However, involvement of the mediastinum is extremely rare.1,5 In 1988, Liu et al reported a case of mediastinal EMMT presenting with superior vena cava syndrome and reviewed 11 previously published cases.12 They showed that EMMT of the mediastinum could precede bone marrow and blood manifestations by many months, and that superior vena cava syndrome could represent the initial mode of presentation in several cases.12 In addition, as is typical of many other primary EMMTs, many of these were misdiagnosed as non-Hodgkin's lymphoma, leading to inappropriate treatment. Cytogenetic characteristics were not reported. Since then, Tallman et al showed that a subset of EMMTs with preferential involvement of the brain, orbit, and epidural space is strongly associated with the t(8;21) translocation.8 Of 40 reported cases, only one with t(8;21) was localised to the mediastinum.13 It was reported subsequently that most EMMTs are associated with three specific karyotypic abnormalities, namely: t(8;21), inv(16), and, t(9;11).5
Since the review of Liu et al,12 nine new cases of EMMT involving the mediastinum including the present one, have been reported with karyotypic data (summarised in table 1).14–20 Interestingly, a near triploid/tetraploid karyotype was documented in five of nine cases. Au et al reported three such cases, all in women.17 Bone marrow examination revealed the presence of large blasts with bizarre and folded nuclei, as reported in our case. Structural chromosomal abnormalities were not mentioned, as in the case published by Ajarim et al.14 Our present case shares all the characteristics reported by Au et al, such as sex, blast cell size with cleft and convoluted nuclei, superior vena cava syndrome, and karyotype. In addition, we identified several structural abnormalities, mostly involving chromosomes 1, 5, 7, 17, and 19, some of which are usually associated with myelodysplastic syndromes.21 Although four of the five patients reported with near triploidy/tetraploidy entered complete remission after induction chemotherapy, all patients died of their disease (table 1).
Table 1.
Summary of the 10 cases of extramedullary myeloid tumours involving the mediastinum that have reported karyotypic data
| Case | Age/ sex | Blast cells in PB | Bone marrow | FAB | Immunophenotype | Pleural involvement | Karyotype | Remission | Outcome | Ref |
| 1 | 21/M | Yes | Yes | M3 | N/D | No | Tetraploidy | Yes | Death, relapse | 14 |
| 2 | 45/F | Yes | Yes | M2 | CD7/13/33+, HLA-DR− | No | Del (15)(q13q15) | Yes | Death, relapse | 15 |
| 3 | 4.5/M | No | No | M4/M5 | CD33+, HLA-DR+ | Yes | t(9;11)(p22;q23) | Yes | Remission 18+ months | 16 |
| 4 | 29/F | No | No | M2 | CD34/33+, CD13− | No | Diploid/ near triploid/ near tetraploid | No | Refractory death post allotransplant | 17 |
| 5 | 46/F | Yes | Yes | M4 | CD34/7/33+, CD13− | No | Diploid/ near tetraploid | Yes | Death, relapse | 17 |
| 6 | 29/F | Yes | Yes | M1 | N/A | No | Near triploid/ near tetraploid | Yes | Death, relapse | 17 |
| 7 | 34/F | No | No | M2 | CD33+, HLA-DR+ | Yes | -10, -11 | No | Death, refractory disease | 18 |
| 8 | 42/F | No | No | M0 | CD7/34/33+, CD13+ | No | 15, +19, +20 | Yes | Death, heart failure | 19 |
| 9 | 26/M | No | Yes | RAEB | CD33+ | Yes | Normal | N/A | Death, post chemo | 20 |
| 10 | 17/F | No | N/D | M1 | CD7/65/CD56+, HLA-DR+, CD13/33− | Yes | Near triploid/ near tetraploid | No | Death | This paper |
Chemo, chemotherapy; F, female; M, male; N/A, not available; N/D, not determined; PB, peripheral blood.
Take home messages.
A near tetraploid or triploid karyotype is found in most reported cases of mediastinal extramedullary myeloid cell tumour (EMMT) and may represent a specific subset of EMMT
Because of the poor prognosis conferred by this karyotype in mediastinal EMMT, early diagnosis and integration of allogeneic stem cell transplantation as part of the therapeutic regimen might improve the clinical outcome
The incidence of a triploid/tetraploid karyotype is extremely low in cases of acute myeloid leukaemia22–24 and myelodysplastic syndromes.21,25–27 The clustering of this unusual type of chromosomal abnormality in patients with mediastinal EMMT is intriguing. We suggest that the presence of a near triploid/tetraploid karyotype and mediastinal EMMT may represent a specific subset of EMMT, characterised by a preponderance of female patients, a high incidence of superior vena cava syndrome at diagnosis, and treatment refractoriness. This is in contrast to EMMT associated with the t(8;21) translocation or other cytogenetic abnormalities, which has a predilection for other anatomical sites.5,8 Because of the rarity of this entity, the biological relevance of such a finding is only speculative. The presence of a near triploid/tetraploid karyotype is probably insufficient to provide myeloblasts with either a proliferative or homing advantage to this exclusive anatomical location. Indeed, although very rare, near tetraploidy has also been reported in acute myeloid leukaemia with no extramedullary manifestations.28,29 In view of the poor prognosis conferred by this karyotype in mediastinal EMMT, early diagnosis and integration of allogeneic stem cell transplantation as part of the therapeutic regimen might improve the clinical outcome.
Abbreviations
CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone
CT, computed tomography
CXR, chest x ray
EMMT, extramedullary myeloid cell tumour
Hb, haemoglobin
WBC, white blood cell count
REFERENCES
- 1.Neiman RS, Barcos M, Berard C, et al. Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer 1981;48:1426–37. [DOI] [PubMed] [Google Scholar]
- 2.Burns A. In: Observations of surgical anatomy. Head and neck. Edinburgh: Thomas Royce, 1811:364–6.
- 3.Rappaport H. Tumours of the hematopoietic system, atlas of tumor pathology, Section III, Fascicle 8, Washington DC: Armed Forces Institute of Pathology, 1966:241–3.
- 4.Davey FR, Olson S, Kurec AS, et al. The immunophenotyping of extramedullary myeloid cell tumours in paraffin-embedded tissue sections. Am J Surg Pathol 1988;12:699–707. [DOI] [PubMed] [Google Scholar]
- 5.Byrd JC, Edenfield WJ, Shields DJ, et al. Extramedullary myeloid cell tumours in acute nonlymphocytic leukemia: a clinical review. J Clin Oncol 1995;13:1800–16. [DOI] [PubMed] [Google Scholar]
- 6.Mansi JL, Selby PJ, Carter RL, et al. Granulocytic sarcoma: a diagnosis to be considered in unusual lymphoma syndromes. Postgrad Med J 1987;63:447–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menasce LP, Banerjee SS, Beckett E, et al. Extra-medullary myeloid tumor (granulocytic sarcoma) is often misdiagnosed: a study of 26 cases. Histopathology 1999;34:391–8. [DOI] [PubMed] [Google Scholar]
- 8.Tallman MS, Hakimian D, Shaw JM, et al. Granulocytic sarcoma is associated with the 8;21 translocation in acute myeloid leukemia. J Clin Oncol 1993;11:690–7. [DOI] [PubMed] [Google Scholar]
- 9.Bennet JM, Catovsky D, Daniel MT, et al. Proposed revised criteria for the classification of acute myeloid leukemia. Ann Intern Med 1985;103:626–9. [DOI] [PubMed] [Google Scholar]
- 10.Seabright M. A rapid banding technique for human chromosomes. Lancet 1971;2:971–2. [DOI] [PubMed] [Google Scholar]
- 11.ISCN. Mitelman F, ed. An international system for human cytogenetic nomenclature. Basel: S Karger, 1995.
- 12.Liu HW, Wong KL, Chan TYK, et al. Superior vena cava syndrome: a rare presenting feature of acute myeloid leukemia. Acta Haematol 1999;79:213–16. [DOI] [PubMed] [Google Scholar]
- 13.Hagihara M, Kobayashi H, Miyachi H, et al. Clinical heterogeneity in acute myelogenous leukemia with the 8;21 translocation. Keio J Med 1991;40:90–3. [DOI] [PubMed] [Google Scholar]
- 14.Ajarim DSS, Santhosh-Kumar CR, Higgy KE, et al. Granulocytic sarcoma of the thymus in acute promyelocytic leukaemia. Clin Lab Haematol 1990;12:97–9. [DOI] [PubMed] [Google Scholar]
- 15.Chubachi A, Miura I, Takahashi N, et al. Acute myelogenous leukemia associated with a mediastinal tumor. Leuk Lymphoma 1993;12:143–6. [DOI] [PubMed] [Google Scholar]
- 16.Brown NP, Rowe D, Reid MM. Granulocytic sarcoma with translocation (9;11) (p22;q23): two cases. Cancer Genet Cytogenet 1997;96:115–17. [DOI] [PubMed] [Google Scholar]
- 17.Au WY, Ma SK, Chan ACL, et al. Near tetraploidy in three cases of acute myeloid leukemia with mediastinal granulocytic sarcoma. Cancer Genet Cytogenet 1998;102:50–3. [DOI] [PubMed] [Google Scholar]
- 18.McGluggage WG, Boyd HK, Jones GC, et al. Mediastinal granulocytic sarcoma. Arch Pathol Lab Med 1998;122:545–7. [PubMed] [Google Scholar]
- 19.Asthall E, Yarranton H, Arno J, et al. Granulocytic sarcoma preceding AML M0 and the diagnostic value of CD34. J Clin Pathol 1999;52:705–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravandi-Kashani F, Cortes J, Giles FJ. Myelodysplasia presenting as granulocytic sarcoma of mediastinum causing superior vena cava syndrome. Leuk Lymphoma 2000;36:631–7. [DOI] [PubMed] [Google Scholar]
- 21.Knapp RH, Dewald GW, Pierre RV. Cytogenetic studies in 174 consecutive patients with preleukemic or myelodysplastic syndromes. Mayo Clin Proc 1985;60:507–16 [DOI] [PubMed] [Google Scholar]
- 22.Raimondi SC, Chang MN, Ravindranath Y, et al. Chromosomal abnormalities in 478 children with acute myeloid leukemia: clinical characteristics and treatment outcome in a cooperative pediatric oncology group study—POG 8821. Blood 1999;94:3707–16. [PubMed] [Google Scholar]
- 23.Mrozek K, Heinonen K, de la Chapelle A, et al. Clinical significance of cytogenetics in acute myeloid leukemia. Semin Oncol 1997;24:17–31. [PubMed] [Google Scholar]
- 24.Arthur DC, Berger R, Golomb HM, et al. The clinical significance of karyotype in acute myeloid leukemia. Cancer Genet Cytogenet 1989;40:203–16. [DOI] [PubMed] [Google Scholar]
- 25.Suciu S, Kuse R, Weh HJ, et al. Results of chromosome studies and their relation to morphology, course and prognosis in 120 patients with de novo myelodysplastic syndrome. Cancer Genet Cytogenet 1990;44:15–26 [DOI] [PubMed] [Google Scholar]
- 26.Haase D, Fonatsch C, Freund M, et al. Cytogenetic findings in 179 patients with myelodysplastic syndromes. Ann Hematol 1995;70:171–87. [DOI] [PubMed] [Google Scholar]
- 27.Heim S, Mitelman F. Chromosome abnormalities in the myelodysplastic syndromes. Clin Haematol 1986:15:1003–21. [PubMed] [Google Scholar]
- 28.Clarke MR, Edward FL, Contis LC, et al. Near-tetraploidy in adult acute myelogenous leukemia. Cancer Genet Cytogenet 1996;86:107–15. [DOI] [PubMed] [Google Scholar]
- 29.Espinet B, Sole F, Woessner S, et al. Two new cases of near-tetraploidy in adult acute myeloid leukemia. Cancer Genet Cytogenet 1998;102:131–4. [DOI] [PubMed] [Google Scholar]