Abstract
Aim: To determine the bioavailability of mupirocin in human nasal secretions and to assess whether the contents of nasal secretions interact appreciably with this antibiotic.
Methods: The comparative bioavailability of mupirocin and chlorhexidine in nasal secretions was determined by bioassay after one, four, and eight hours of incubation with pooled secretions from three subjects. The interaction of mupirocin with nasal secretions was characterised by matrix assisted laser desorption time of flight mass spectrometry (MALDI-TOF).
Results: MALDI-TOF analysis showed that mupirocin was not absorbed by the main fraction of pooled nasal secretions and should remain active. In bioassay, mupirocin retained 100% of its antistaphylococcal activity in nasal secretions, whereas chlorhexidine was significantly reduced from 100 mg/litre to 1.5 mg/litre and from 1000 mg/litre to 38.5 mg/litre, irrespective of incubation time.
Conclusions: The high bioavailability of mupirocin in nasal secretions results from the lack of appreciable molecular interactions.
Keywords: mupirocin, nasal secretions, bioavailability, chlorhexidine, matrix assisted laser desorption time of flight mass spectrometer
There is renewed interest in developing intranasal antimicrobial agents to eliminate methicillin resistant and sensitive Staphylococcus aureus1 and fungi.2 High in vitro activity may not be the sole indicator of clinical efficacy because agents must be able to resist inactivation by nasal secretions. Mupirocin has been the mainstay for the elimination of nasal carriage of Staphylococcus aureus,3 including methicillin resistant strains (MRSAs), during hospital outbreaks.4–6 Chlorhexidine, which also has good antistaphylococcal activity,7 is relatively ineffective intranasally,5 and topical vancomycin has little effect.1
“High in vitro activity may not be the sole indicator of clinical efficacy because agents must be able to resist inactivation by nasal secretions”
As a simple in vitro model to identify potential intranasal efficacy, the activity of mupirocin and chlorhexidine in nasal secretions was compared, and potential complexes between nasal secretions and mupirocin examined by a revolutionary mass spectrometry method.8
METHODS
Nasal secretions were obtained from three healthy subjects (two men, one woman) after vigorous exercise for five to 10 minutes in air temperatures of ≤ 10°C: dripping and blown secretions were collected in 30 ml plastic universals.9 Mupirocin or chlorhexidine were added to 0.45 ml of pooled nasal secretions to achieve final concentrations of mupirocin or chlorhexidine of 50, 100, 250, 500, and 1000 mg/litre. After one, four, and eight hours incubation at 37°C, active values of mupirocin or chlorhexidine in 200 μl aliquots removed from each sample at each time interval were determined in a well plate assay using DST agar (CM261; Oxoid, Basingstoke, UK) buffered by 0.2 M biphasic phosphate buffer to pH 7.2 for mupirocin, or Muller-Hinton agar (CM337; Unipath) for chlorhexidine. The Oxford staphylococcus NCTC 1675 was used as the indicator organism and the inoculum size was controlled by spectrometry.9 The interaction of mupirocin with a water solubilised fraction of pooled nasal secretions, dialysed against a cellulose ester membrane with a molecular weight cutoff point of 1000 Da (Sigma, Poole, Dorset, UK) to remove salts and minor fractions released during solubilisation, was examined by a matrix assisted laser desorption time of flight mass spectrometer (MALDI-TOF MS 4; Kratos, Manchester, UK), as described previously.9
RESULTS
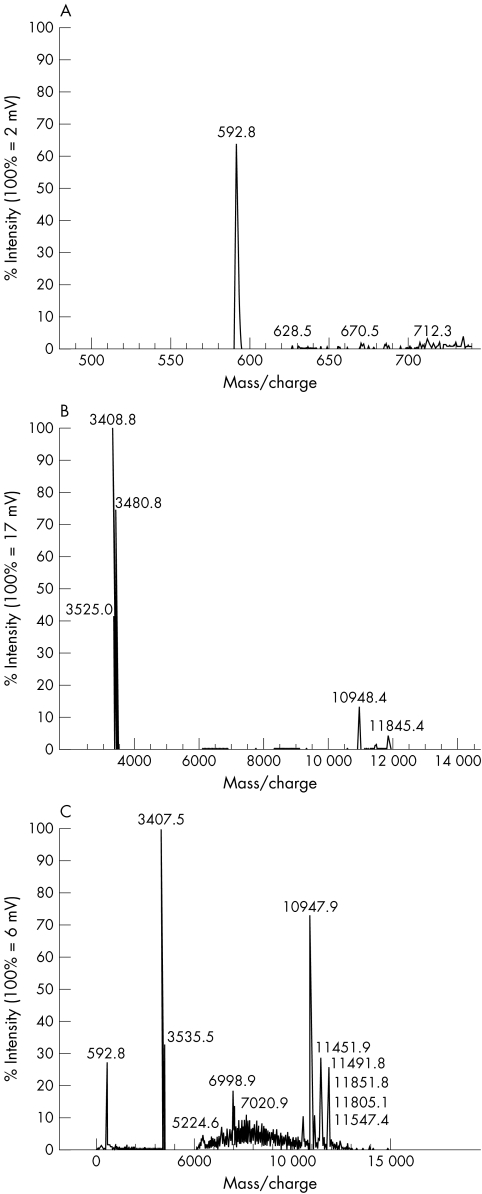
MALDI-TOF analysis showed that mupirocin, which had a mass/charge peak of 592 (fig 1A), was not absorbed by the main fraction of pooled nasal secretions (fig 1B) and should still remain active (fig 1C). This was confirmed in bioassay when, unlike chlorhexidine, the activity of mupirocin remained unaltered when incubated with nasal secretions for up to eight hours at 37°C. Table 1 shows that the activity of chlorhexidine was significantly reduced from 1.5% to 38.5% of the initial concentration of 100 and 1000 mg/litre, respectively. Recoverable activities of mupirocin and chlorhexidine were unaffected by time.
Figure 1.
Matrix assisted laser desorption time of flight mass spectrometry analysis of the interaction of mupirocin with a solubilised fraction of nasal secretions. (A) Mass spectrum of mupirocin; (B) mass spectrum of a solubilised fraction of nasal secretions; (C) interaction of mupirocin with nasal secretions.
Table 1.
Effect of nasal secretions on the activity of mupirocin or chlorhexidine at 37°C
| Average amount recovered from initial concentrations (mg/l) of | |||||
| 50 | 100 | 250 | 500 | 1000 | |
| Mupirocin | 50 | 100 | 250 | 500 | 1000 |
| Chlorhexidine | 1.2 | 1.5 | 2.0 | 12.5 | 385 |
Aliquots from the incubation mix were bioassayed after one, four, and eight hours.
DISCUSSION
In a molecular assessment, MALDI-TOF mass spectroscopy, which allows direct capture and characterisation of complex spectra,10 showed that the extract of nasal secretions did not significantly absorb mupirocin. This has not been described previously. Nasal secretions serve to eliminate substances introduced into the nose, and consist of mucopolysaccharides and mucoprotein11 which, like mupirocin, possess an overall negative charge. It is probable that negative charges on the major components of nasal secretions repel mupirocin, preventing any appreciable interactions.
“Nasal secretions serve to eliminate substances introduced into the nose, and consist of mucopolysaccharides and mucoprotein”
Take home messages .
Nasal secretions, consisting of mucopolysaccharides and mucoprotein, eliminate substances introduced into the nose
MALDI-TOF mass spectroscopy showed that the extract of nasal secretions did not significantly absorb mupirocin, explaining the clinical efficacy of this antibiotic for the treatment of nasal infections
In contrast, chlorhexidine was shown to be inactivated by nasal secretions, explaining its lack of clinical efficacy for the treatment of nasal infections
This is the opposite to chlorhexidine, which has highly positively charged quanido groups12 and is known to be inactivated by saliva which, like nasal secretions, contains negatively charged molecules.13 Indeed, bioassay confirmed that chlorhexidine is unlikely to have valuable intranasal activity because only the equivalent of 1.5 mg/litre remained from the initial 100 mg/litre and 385 from 1000 mg/litre. This is important because minimum inhibitory concentrations of chlorhexidine for several strains of MRSA have been reported to be from 2 to 8 mg/litre.7 By extrapolating our results it can be calculated that 0.26 ml of a 1% cream would be needed in each nostril to achieve a dose of 10 mg, compared with the “match head” sized amount required for mupirocin (∼ 40– 50 μl).3 Thus, the unreliability of chlorhexidine used intranasally to control MRSA carriage might result from the use of an insufficient amount.
Bioavailability in, and interaction with, nasal secretions could be a useful model for the development of newer analogues of mupirocin or similar agents, such as SB 205952.14 Indeed, using MALDI-TOF together with bioassay and the evaluation of killing kinetics predicted the effectiveness of 5% povidone-iodine against S aureus in a controlled trial (RLR Hill and JJ Wade. Presented at the 40th interscience congress on antimicrobial agents and chemotherapy, Toronto, 17–20 September 2000).
Acknowledgments
Thanks to A Jackson for assistance with mass spectrometry and the South London Public Health Laboratory and Joint Microbiology Research Unit for facilities at King's Denmark Hill Campus.
Abbreviations
MALDI-TOF, matrix assisted laser desorption time of flight mass spectrometry
MRSA, methicillin resistant strain
REFERENCES
- 1.Archer GL, Climo MW. Staphylococcus aureus bacteremia—consider the source. N Engl J Med 2001;344:55–6. [DOI] [PubMed] [Google Scholar]
- 2.Nagl M, Lass-Florl C, Heher A, et al. Enhanced fungicidal activity of N-chlorotaurine in nasal secretion. J Antimicrob Chemother 2001;47:871–4. [DOI] [PubMed] [Google Scholar]
- 3.Casewell MW, Hill RLR. Elimination of nasal carriage of Staphylococcus aureus with mupirocin (`pseudomonic acid')—a controlled trial. J Antimicrob Chemother 1986;17:365–72. [DOI] [PubMed] [Google Scholar]
- 4.Hill RLR, Duckworth GJ, Casewell MW. Elimination of nasal carriage of methicillin-resistant Staphylococcus aureus with mupirocin during a hospital outbreak. J Antimicrob Chemother 1988;22:377–84. [DOI] [PubMed] [Google Scholar]
- 5.Hill RLR, Casewell MW. Local treatment of MRSA carriage and colonization. In: Cafferkey MT, ed. Methicillin-resistant Staphylococcus aureus. New York: Marcel Dekker, 1992:149–70.
- 6.Working Party Report. Revised guidelines for the control of epidemic methicillin-resistant Staphylococcus aureus infection in hospitals. J Hosp Infect 1998;39:253–85. [DOI] [PubMed] [Google Scholar]
- 7.Brumfitt W, Dixson S, Hamilton-Miller JMT. Resistance to antiseptics in methicillin- and gentamicin-resistant Staphylococcus aureus. Lancet 1985:i:1442–3. [DOI] [PubMed]
- 8.Leushner J, Chiu NH. Automated mass spectrometry: a revolutionary technology for clinical diagnostics. Mol Diagn 2000;5:341–8. [DOI] [PubMed] [Google Scholar]
- 9.Hill RLR, Casewell MW. The in-vitro activity of povidone-iodine cream against Staphylococcus aureus and its bioavailability in nasal secretions. J Hosp Infect 2000;45:198–205. [DOI] [PubMed] [Google Scholar]
- 10.Palmer-Toy DE, Sarracino DA, Sgroi D, et al. Direct acquisition of matrix assisted laser desorption time of flight mass spectrometer from laser capture microdissected tissues. Clin Chem 2000;46:1513–16. [PubMed] [Google Scholar]
- 11.Schorn K, Hochstrasser K. Biochemical investigations of nasal secretions. Acta Otorhinolaryngol Belg 1979;33:603–6. [PubMed] [Google Scholar]
- 12.Hennessey TD. Antibacterial properties of Hibitane. J Clin Periodont 1977;4:36–48. [DOI] [PubMed] [Google Scholar]
- 13.Spikervet FKL, Van Saene JJM, Van Saene HFK, et al. Chlorhexidine inactivation by saliva. Oral Surg Oral Med Oral Pathol 1990;69:444–9. [DOI] [PubMed] [Google Scholar]
- 14.Wilson JM, Oliva B, Cassals R, et al. SB 205952, a novel semisynthetic monic acid analogue with at least two modes of action. Antimicrob Agents Chemother 1995;39:1925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]