Abstract
A 76 year old white woman presented with a four month history of dysphagia and weight loss. Clinical, radiological, and endoscopic examination revealed a pigmented mass in the lower third of the oesophagus. The preoperative diagnosis, including biopsy examination, was that of malignant melanoma. Following oesophageal resection, the mass was found to be a localised, relatively superficial tumour with light, electron microscopic, and immunohistochemical features common to both Schwann cells and melanocytes. The patient survived 46 months after surgery and died of a stroke, with no evidence of tumour recurrence. The tumour is presented as a case of melanocytic schwannoma, with unique features when compared with oesophageal melanotic schwannomas and malignant melanomas described in the literature. The differential diagnosis is discussed and an origin from a common precursor cell of neural crest origin is postulated.
Keywords: oesophageal neoplasms, schwannoma, melanoma
Malignant melanoma is a rare aggressive tumour, accounting for 0.2% of all oesophageal tumours. Less than 160 such tumours have been documented.1 Schwannomas of the oesophagus are even less common, with only 11 cases having been described previously,2–5 of which only three were melanotic.3 Melanotic schwannoma is most often associated with spinal nerve roots, but has been described at numerous other sites and as a part of Carney's syndrome, which includes psammomatous melanotic schwannomas, myxomas, spotty pigmentation, and endocrine overactivity. It must be distinguished from classic schwannoma because its behaviour is unpredictable.3,6 Complete resection of oesophageal melanotic tumours is recommended to achieve a chance of survival.1
“Melanotic schwannoma is most often associated with spinal nerve roots, but has been described at numerous other sites and as a part of Carney's syndrome”
We describe a tumour diagnosed preoperatively as a malignant melanoma. Ultrastructural examination of the resected specimen allowed revision of the diagnosis to that of a melanocytic schwannoma.
CASE REPORT
The patient, a 76 year old white woman, presented with a four month history of dysphagia and weight loss. Endoscopy showed a pigmented polypoid mass in the lower third of the oesophagus. A preoperative histological diagnosis of malignant melanoma was made. She had no cutaneous lesions and there was no radiological evidence of advanced local infiltration. Neither she, nor any family member, had Carney's syndrome or neurofibromatosis. Subtotal oesophagectomy was performed. She had long standing cardiovascular disease and the postoperative period was complicated but the patient left hospital 27 days after surgery. She died 46 months after surgery, of stroke, with no evidence of tumour recurrence.
On resection the smooth, black, polypoid lesion measured 5 × 4 × 2 cm. Two centimetres proximal to this was a separate, flat, pigmented area measuring 2.5 × 2 cm. Excision was macroscopically complete.
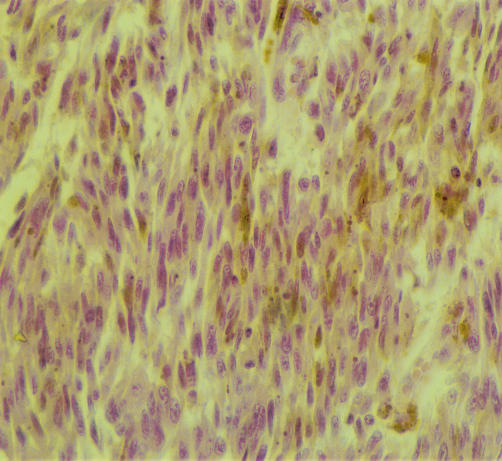
Light microscopy showed that the superficial lesion was covered by attenuated squamous epithelium. It was non-encapsulated but had a circumscribed margin. The tumour was composed of spindle cells, with scattered epithelioid cells, arranged in interlacing fascicles with focal palisading. The cells were cytologically bland, with minimal pleomorphism and no mitoses, and they contained glycogen. Abundant coarse intracytoplasmic pigment was present in most cells (fig 1). Staining with Masson-Fontana confirmed this to be melanin. Most cells were positive for S100, HMB 45, and melan A. The tumour was negative for CD34, epithelial membrane antigen, smooth muscle antigen, and desmin. Intratumoral macrophages were also melanin laden. Mucosa from the separate area showed nested junctional melanocytes without cytological atypia, and melanin in macrophages in the subepithelial stroma. Regional lymph nodes showed reactive changes only. Tumour staging was T1N0M0.
Figure 1.
The tumour consists of cytologically bland cells arranged in fascicles with local palisading. Melanin pigment is seen in most of the cells (haematoxylin and eosin).
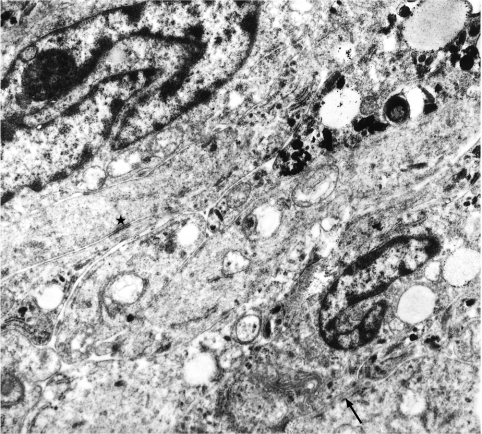
Electron microscopy showed that the tumour cells had deeply indented nuclei and long, elaborate, interlocking cytoplasmic processes, which also interdigitated laterally with neighbouring cells and formed intercellular junctions (fig 2). Melanosomes in varying stages of maturation, from the typical internal lamellar structure to dense pigmentation, occurred in most cells (fig 3A), a spectrum suggesting melanin synthesis.
Figure 2.
Tumour cells have closely apposed cytoplasmic processes with intercellular junctions (asterisk). Within the cytoplasm are melanosomes at various stages of maturation. Microfilaments are numerous within the processes. Part of an external lamina may also be seen (arrow) (original magnification, ×13 750)
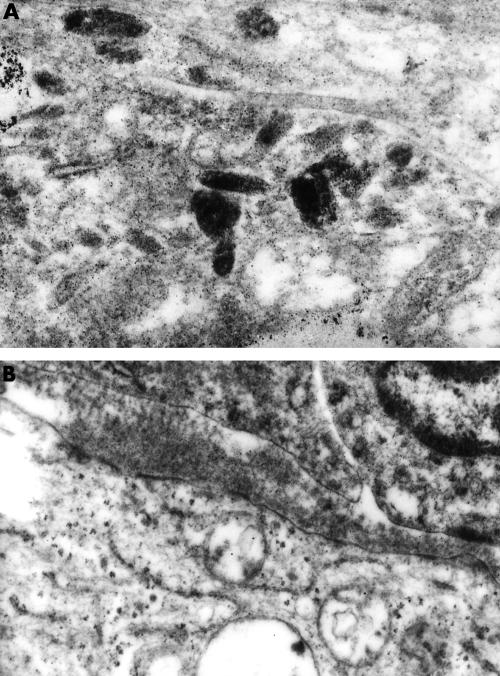
Figure 3.
(A) Cytoplasmic detail. Premelanosomes showing stages II to IV maturation (original magnification, ×27 000). (B) Long spacing collagen (Luse bodies) was seen in extracellular spaces (original magnification, ×15 500).
An incomplete external lamina surrounded groups of closely apposed cells and long spacing collagen was sometimes seen in extracellular spaces (fig 3B).
DISCUSSION
The preoperative differential diagnosis between malignant melanoma and melanotic schwannoma is difficult because the appearances in small biopsies cannot be distinguished by light microscopy. Diagnosis is often not made until after examination of the resected specimen and may, as in this case, require ultrastructural confirmation.7
Our patient fell within the age span expected for melanoma (fifth decade onwards).1 Melanotic schwannoma is a tumour of young adults, the mean age of those occurring outside Carney's syndrome being 33 years.3 The mode of presentation and macroscopic appearance of our case would fit with a diagnosis of either melanoma or schwannoma.
Primary melanomas of the oesophagus are as aggressive as melanomas at other sites, with widespread metastasis and poor prognosis (five year survival is only 5.7%).1 Although exceptions exist, the long term survival of our patient argues against a diagnosis of melanoma. The prognosis for melanotic schwannoma is impossible to predict from pathological features, but outside the spinal cord it tends to follow an indolent course.3–5
Immunohistochemistry did not permit distinction between the differential diagnoses but our case has the light microscopic features of a melanotic schwannoma: circumscription without encapsulation, high cellularity, a predominance of spindle cells, which are heavily pigmented and arranged in whorls or palisades, and a lack of cytological atypia and mitoses.2,3 However, there were no psammoma bodies or adipose tissue, as described by Assor and by Carney, the latter of whom puts great emphasis on the finding of psammomas.3 Melanoma, although renowned as a pathological mimic, usually displays obvious signs of malignancy, with cellular anaplasia and necrosis.
A confounding feature in our case is the presence of a junctional proliferation of melanocytes in nearby mucosa. Coexisting melanosis of the oesophagus is present in continuity with about 25% of cases of primary malignant melanoma and is used as a criterion in the differentiation between primary and secondary melanomas.8 However, in our case the junctional proliferation was clearly separate from the tumour and was not cytologically atypical.
“A confounding feature in our case is the presence of a junctional proliferation of melanocytes in nearby mucosa”
It seems reasonable to assume that this melanogenic primary oesophageal tumour has arisen from a cell of neural crest origin. The neural crest gives rise to a range of cells, including melanocytes and Schwann cells. During embryogenesis, melanocytes migrate from the neural crest to other sites, including the oesophagus.9 Cells of neural crest origin retain the capability in culture to differentiate along alternative lines,10 and it seems reasonable to assume that they may do so in vivo. Benign melanotic schwannoma and malignant melanoma may represent two ends of a spectrum of tumours arising from a common precursor cell of neural crest origin. In those cases of melanotic schwannoma that metastasise, it is interesting that the metastases have features more in common with melanoma than with the primary tumour.3,6
Light microscopy indicated that the reported tumour has bland rather than aggressive features, favouring the diagnosis of melanotic schwannoma over malignant melanoma. However, only ultrastructural examination proves that the neoplastic cells have characteristics of both Schwann cells and melanocytes, thus allowing the precise diagnosis. The tumour is a unique case of primary melanocytic schwannoma of the oesophagus, occurring without psammoma bodies and outside Carney's syndrome.
Take home messages .
In this patient the preoperative diagnosis, including biopsy examination, was that of malignant melanoma
However, after surgery the tumour was seen to have bland rather than aggressive features, and ultrastructural examination confirmed that the neoplastic cells have characteristics of both Schwann cells and melanocytes, thus allowing a firm diagnosis of melanocytic schwannoma
This tumour is a unique case of primary melanocytic schwannoma of the oesophagus, occurring without psammoma bodies and outside Carney's syndrome
An origin from a common precursor cell of neural crest origin is proposed
Acknowledgments
Access to case material and clinical details were given by Mr F J Collins, consultant thoracic surgeon, Birmingham Heartlands Hospital. We are grateful to Dr C Fisher, consultant histopathologist, The Royal Marsden Trust, London, who gave his opinion on the light microscopy of the case. The Medical Illustration Department, Birmingham Heartlands Hospital gave skilled photographic support. SJD is funded by the Oesophageal Cancer Fund (OCF), Birmingham, UK.
REFERENCES
- 1.Lam KY, Law S, Wong J. Malignant melanoma of the oesophagus: clinicopathological features, lack of p53 expression and steroid receptors and a review of the literature. Eur J Surg Oncol 1999;25:168–72. [DOI] [PubMed] [Google Scholar]
- 2.Assor D. A melanocytic tumor of the esophagus. Cancer 1975;35:1438–43. [DOI] [PubMed] [Google Scholar]
- 3.Carney JA. Psammomatous melanotic schwannoma. A distinctive, heritable tumor with special associations, including cardiac myxoma and the Cushing syndrome. Am J Surg Pathol 1990;14:206–22. [PubMed] [Google Scholar]
- 4.Hamoir MF, Minet M, Garin P, et al. Schwannoma of the cervical esophagus: case report and clinicopathologic analysis. Rev Laryngol Otol Rhinol 1998;119:51–4. [PubMed] [Google Scholar]
- 5.Prévot S, Bienvenu L, Vaillant JC, et al. Benign schwannoma of the digestive tract. Am J Surg Pathol 1999;23:431–36. [DOI] [PubMed] [Google Scholar]
- 6.Vallat-Decouvelaere A-V, Wassef M, Lot G, et al. Spinal melanotic schwannoma: a tumour with poor prognosis. Histopathology 1999;35:558–66. [DOI] [PubMed] [Google Scholar]
- 7.Scheithauer BW, Woodruff JM, Erlandson RA. Atlas of tumor pathology, Third Series, Fascicle 24. Tumors of the peripheral nervous system. Washington, DC: Armed Forces Institute of Pathology, 1999:105–76.
- 8.Raven RW, Dawson I. Malignant melanoma of the oesophagus. Br J Surg 1964;51:551–5. [DOI] [PubMed] [Google Scholar]
- 9.De la Pava S, Nigogosyan G, Pickren JW, et al. Melanosis of the esophagus. Cancer 1963;16:48–50. [DOI] [PubMed] [Google Scholar]
- 10.Nichols DH, Weston JA. Melanogenesis in cultures of peripheral nervous tissue. I. The origin and prospective fate of cells giving rise to melanocytes. Dev Biol 1977;60:217–25. [DOI] [PubMed] [Google Scholar]