Abstract
Background/Aims: The normal intestinal epithelium is increasingly being recognised as an important component of the mucosal innate protection against microorganisms. Human neutrophil defensins 1–3 (HNP 1–3) and lysozyme are components of the systemic innate immunity. The aim of this study was to investigate the expression of HNP 1–3 and lysozyme in normal and active inflammatory bowel disease (IBD) mucosa.
Methods: Mucosal tissue sections were studied by immunohistochemistry using antibodies to neutrophil defensins 1–3 and lysozyme. Extracts of purified intestinal epithelial cells were used for immunoblotting studies and antimicrobial activity against the phoP negative strain of Salmonella typhimurium.
Results: Surface epithelial cells strongly immunoreactive for neutrophil defensins and lysozyme were seen in active ulcerative colitis and Crohn's disease (but not normal or inactive IBD) mucosal samples. Many of these cells coexpressed both of the antimicrobial proteins. Immunoblotting studies confirmed the expression of neutrophil defensins in extracts of purified ulcerative colitis epithelial cells, which also demonstrated antimicrobial activity.
Conclusion: HNP 1–3 and lysozyme are expressed in surface enterocytes of mucosa with active IBD and they may play an important role in intestinal host defence against luminal microorganisms.
Keywords: innate immunity, antimicrobial peptides, defensins, lysozyme
The host defence against microorganisms can be divided into “innate” and “adaptive” immunity. The former is pre-existing or rapidly responsive and is mediated against a broad range of microorganisms. In contrast, adaptive immunity involves complex interactions between cells of the immune system and specific microorganisms and their components. It is highly specific but takes time to develop. In the systemic circulation, polymorphonuclear cells and monocytes represent an important component of innate immunity. The microbicidal activity of these cells is mediated by a variety of factors, of which antimicrobial peptides of the α defensin family have been of considerable interest.1, 2 They are small cationic peptides containing 29–35 amino acids and, in humans, four members of the family (designated HNP 1–4) are present in appreciable quantities in the azurophil granules of polymorphonuclear cells. They can kill a wide range of Gram positive and Gram negative bacteria, fungi, and protozoa.3, 4 Lysozyme is a well recognised antimicrobial protein expressed in both polymorphonuclear cells and monocytes/macrophages and is active against Gram positive bacteria.
“Adaptive immunity involves complex interactions between cells of the immune system and specific microorganisms and their components”
In active inflammatory bowel disease (IBD), there is derangement of epithelial barrier function and migration of large numbers of circulating polymorphonuclear cells and monocytes into the intestinal mucosa (and the lumen). These cells are probably important in host protection against penetration by the large resident population of microorganisms present in the lumen.
In our study, we show that the intestinal epithelial cells of active (but not inactive) IBD mucosa express neutrophil defensin peptides (HNP 1–3) and lysozyme.
MATERIALS AND METHODS
Tissue samples
Formalin fixed, paraffin wax embedded rectal biopsies from patients with active ulcerative colitis or Crohn's disease were obtained from the pathology archive at the University Hospital, Nottingham. Further tissue samples were obtained from surgical resection specimens (colon and terminal ileum) from patients with active ulcerative colitis and Crohn's disease. Histologically normal terminal ileal and colonic mucosal tissue was obtained (more than 5 cm from tumour) from intestine resected for carcinoma.
Immunohistochemistry
Sections (5 μm thick) of formalin fixed, paraffin wax embedded intestinal tissues (terminal ileal Crohn's disease, n = 2; colonic/rectal Crohn's disease, n = 9; ulcerative colitis, n = 11; normal terminal ileum, n = 3; normal colon/rectum, n = 6) were dewaxed in xylene and rehydrated through graded alcohols. Antigen retrieval was achieved by incubation with pronase (Sigma, Poole, Dorset, UK) at 1 mg/ml. Immunoperoxidase staining was performed using a Vectastain Elite Universal ABC kit (Vector Laboratories, Burlingame, California, USA), according to the manufacturer's instructions. In brief, sections were incubated with blocking serum at room temperature for 30 minutes, and then incubated overnight at 4°C with a monoclonal antibody against HNP 1–3 at 40 ng/ml (Bachem, Feinchemikalien AG, Switzerland), a polyclonal antibody against human lysozyme (Genzyme, Cambridge, Massachusetts, USA), or a polyclonal antibody against intestinal trefoil factor (ITF; gift from Professor Podolsky, Massachusetts General Hospital, Boston, USA). Endogenous peroxidase activity was quenched by incubation in 0.3% H202 in methanol for 20 minutes. Bound antibodies were detected by incubation with a biotinylated antimouse and antirabbit IgG, followed by an avidin–biotinylated horseradish peroxidase complex. Peroxidase activity was developed with diaminobenzidine tetrahydrochloride and the sections were counterstained with haematoxylin. Control sections were incubated with phosphate buffered saline (PBS; Gibco BRL, Gaithersburg, Maryland, USA) rather than primary anti-HNP 1–3 or antilysozyme antibodies. In addition, to verify specificity of immunostaining with the anti-HNP 1–3 antibody, immunoadsorption was performed by incubating synthetic HNP 1 (Bachem), at a concentration of 20 μg/ml in anti-HNP solution, for three hours at room temperature. In subsequent studies on tissue sections, immunolabelling with immunoadsorbed anti-HNP antibody was compared with untreated antibody.
For HNP-1 immunostained sections, the number of positive cells/100 epithelial cells was determined in three fields for each section.
Isolation of epithelial cells and preparation of cell extracts
Surface epithelial cells were isolated from fresh intestinal surgical resection specimens using EDTA, as described previously.5 In brief, mucosal strips (approximately 4 g wet weight) were incubated in 1 mM EDTA on a hot plate at 37°C with continuous stirring for 30 minutes. The detached epithelial cells were washed three times in PBS by centrifugation at 400 ×g for 10 minutes. Epithelial cells isolated from IBD tissue were purified (to remove polymorphonuclear cells) using immunomagnetic beads coated with an antihuman epithelial cell antibody (BerEP4; coated beads obtained from Dynabeads; Dynal AS, Oslo, Norway) at a bead to target epithelial cell ratio of 4 : 1, according to the manufacturer's instructions. After incubation at 4°C (with stirring for 30 minutes), epithelial cells bound to the magnetic beads were separated from the suspension using a magnetic particle concentrator, and then washed three times in PBS. Cytospin preparations (with 25 000 cells/slide) of the purified epithelial cells were made, fixed in acetone, stored at −20°C, and subsequently used for haematoxylin and eosin staining. Remaining purified isolated epithelial cells were resuspended in 10% acetic acid and stirred overnight at 4°C in the presence of peptidase inhibitors (1 mM phenylmethylsulphonyl fluoride, 1 mg/ml pepstatin, and 1 mg/ml leupeptin; all Sigma). Supernatants (containing cationic peptides) were clarified by centrifugation (at 1600 ×g for 10 minutes), lyophilised, and resuspended in 0.1% acetic acid. They were subsequently frozen at −80°C for later use in immunoblotting and antimicrobial assay experiments.
Immunoblotting
Epithelial cell extracts in 0.1% acetic acid were subjected to acid urea–polyacrylamide gel electrophoresis (AU–PAGE) before immunoblotting. AU–PAGE was performed as described previously.6 In brief, acid urea (6.25M)–15% polyacrylamide minigels were prepared and pre-run with 5% acetic acid for one hour at 150 V. Epithelial cell extracts and synthetic HNP-1 (Bachem) were applied to the gel and electrophoresed with 5% acetic acid until the methyl green dye front had reached the end of the gel. Electrophoretic transfer of proteins to PVDF membranes was performed using a Trans Blot cell (Bio-Rad Laboratories, Hemel Hempstead, UK). Immunostaining was subsequently performed using anti-HNP antibody (1 μg/ml) and the Vectastain Elite ABC kit.
Antimicrobial assay
The antimicrobial activity of an extract of purified epithelial cells from active ulcerative colitis mucosa was studied using a colony forming unit (CFU) assay. A phoP mutant (CS015) of Salmonella typhimurium (gift from Dr S Miller, Massachusetts General Hospital, Boston), which is sensitive to defensins,7 was used as the test organism. An overnight culture of bacteria in 3% trypticase soy broth (TSB) was subcultured at a 1/100 dilution and grown for three hours to mid logarithmic phase at 37°C. The bacterial density was estimated photometrically, based upon the fact that an OD620 of 0.2 is equal to 5 × 107 CFU/ml, and a working dilution of 106 CFU/ml was prepared in sodium phosphate buffer–1% TSB. An aliquot of 10 μl of epithelial extract in 0.1% acetic acid, or 10 μl of 0.1% acetic acid alone as a control, was mixed with 90 μl of bacterial suspension. The initial number of CFU was determined by immediately diluting 10 μl of the assay mix 100, 10 000, and 100 000 fold with sodium phosphate buffer and keeping on ice until ready for plating. The remaining assay mix was incubated at 37°C for three hours and CFU determined again following dilution (as described above). A 50 μl aliquot of each diluted assay mix was plated in duplicate on to blood agar plates using an automated spiral plater and incubated overnight at 37°C. Bacterial colonies were counted using a plate reader, and the average number of CFU/ml calculated.
Statistics
Epithelial HNP 1–3 immunoreactivity in normal and active IBD mucosal samples was compared using Fisher's exact test. The proportions of HNP-1 positive cells were analysed by means of the Student's t test.
RESULTS
Expression of neutrophil defensins
Tissue sections
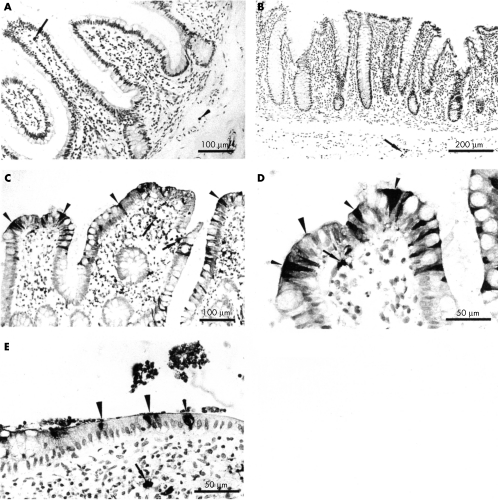
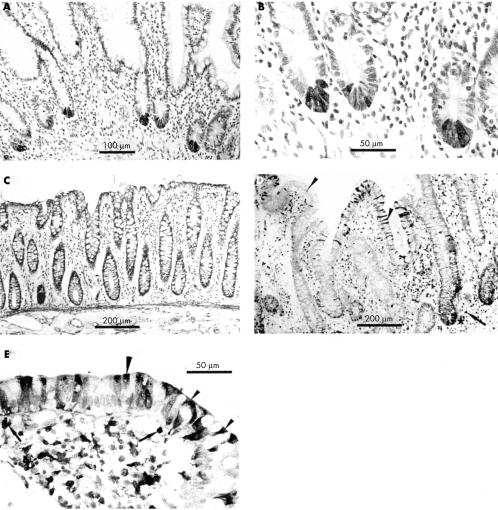
The distribution of neutrophil defensins in normal and IBD intestinal tissue sections was studied using immunohistochemistry. Immunoreactive neutrophils were seen in blood vessels of both normal and active IBD tissues, and in the lamina propria and crypt abscesses of active IBD tissues. Such neutrophils were seen only rarely in the lamina propria of some normal mucosal tissue sections. A halo of immunoreactivity was seen around many neutrophils in active IBD sections, implying the presence of secreted defensin in the vicinity of the cells (fig 1C–E). Endothelial cells of some blood vessels present within normal and inflamed intestinal mucosal sections were also immunoreactive for HNP 1–3.
Figure 1.
Expression of neutrophil defensins (HNP 1–3) in intestinal tissues. (A) Normal small intestine showing one neutrophil immunoreactive for HNP 1–3 in the villous lamina propria (arrow), and one in a blood vessel (small arrowhead). (B) Normal colon showing positively stained neutrophils in a blood vessel (arrow). No HNP immunoreactive epithelial cells are present in (A) or (B). Small intestinal sections from a patient with active Crohn's disease are shown at (C) low power and (D) higher power. (E) Colonic tissue from a patient with active ulcerative colitis. Numerous neutrophils containing defensins are present in the lamina propria, some with extracellular immunoreactive material around them (arrows, C–E). Many scattered epithelial cells are strongly immunoreactive for neutrophil defensins (some of these are shown by small arrowheads). In some cells, immunoreactivity is strongest in the apical region of the cell and at the cell surface (large arrowheads).
In seven of 10 active Crohn's disease samples, epithelial cells were strongly immunoreactive for neutrophil defensins (figs 1C/D and 2A). Epithelial cells were negative for neutrophil defensins in one of the mucosal samples from a fibrous stricture in which there was minimal inflammation. Of 11 active ulcerative colitis mucosal samples, epithelial cells were immunoreactive for neutrophil defensins in nine (fig 1E). There was no significant difference between sections of ulcerative colitis and Crohn's disease mucosal samples in the proportion of epithelial cells positive for HNP 1–3 (mean (SEM): 10.7 (2.0) v 10.4 (2.3), respectively).
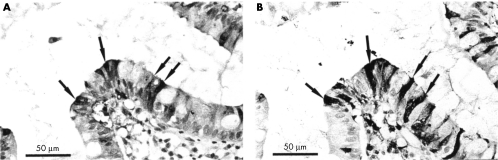
Figure 2.
Coexpression of neutrophil defensins and lysozyme. Serial sections of small intestine from a patient with active Crohn's disease. (A) Expression of neutrophil defensins in enterocytes and (B) expression of lysozyme by the same cells (arrows).
Neutrophil defensin expression in epithelial cells of the inflamed mucosal samples was focal, with positive cells predominantly near the villous tip and the surface epithelium of the small intestinal and colonic samples, respectively. Strongly immunoreactive epithelial cells were often seen adjacent to enterocytes that were not stained. The extent of neutrophil defensin immunoreactivity varied between epithelial cells. Thus, in some cells, the entire cytoplasm appeared to be positive, whereas in others neutrophil defensins were localised to the apical region of the cell (fig 1C–E). In some areas, both the apical cell surface and the overlying mucus layer were positive. In studies where primary anti-HNP antibody was immunoadsorbed with excess HNP antigen, specific immunostaining was completely abolished.
In contrast to active ulcerative colitis and Crohn's disease (epithelial HNP 1–3 immunoreactivity in nine of 11 and seven of 10 samples, respectively), none of the epithelial cells (including Paneth cells) in normal terminal ileal (fig 1A) or colonic mucosal (fig 1B) samples, or non-inflamed IBD samples, were immunoreactive for neutrophil defensins (normal colonic mucosal samples v ulcerative colitis: p < 0.01; normal terminal ileal and colonic samples v Crohn's disease samples: p < 0.01).
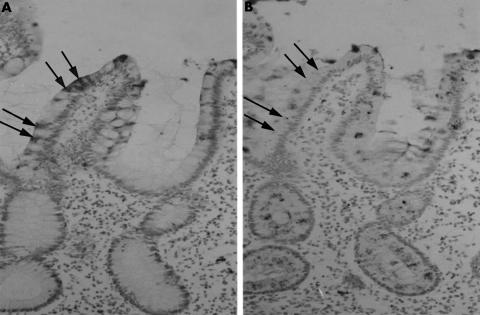
To determine whether neutrophil defensin positive epithelial cells in IBD sections were absorptive cells or goblet cells, immunohistochemical studies were performed on serial sections using anti-HNP 1–3 and anti-ITF antibodies. ITF is expressed by goblet cells,8 and cells immunoreactive for HNP 1–3 were negative for ITF (fig 3).
Figure 3.
Neutrophil defensin expressing epithelial cells are not goblet cells. Immunohistochemical studies on serial sections of small intestine from a patient with active Crohn's disease were performed using antibodies to (A) Human neutrophil defensin peptides 1–3 (HNP 1–3) and (B) intestinal trefoil factor (ITF; which is expressed by goblet cells). HNP 1–3 positive cells (arrowed) do not express ITF.
Immunoblotting studies
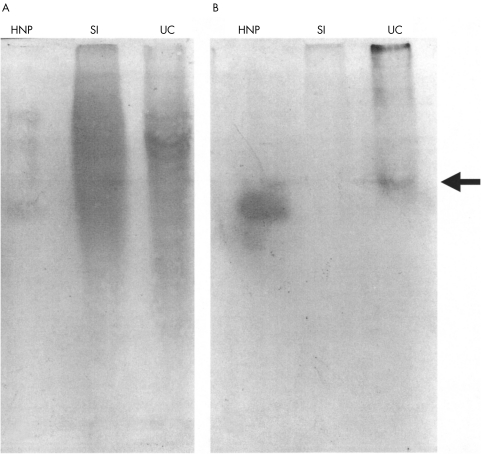
Acid extracts of epithelial cells isolated from normal and active ulcerative colitis mucosal samples were subjected to AU-PAGE followed by immunoblotting using anti-HNP 1–3 antibody. Extracts of ulcerative colitis, but not normal, mucosal epithelial cells showed a clear band of immunoreactivity, similar to that seen with synthetic HNP 1 (fig 4). Examination of cytospin preparations of the purified epithelial cells confirmed the absence of neutrophils.
Figure 4.
Detection of neutrophil defensins in epithelial cells isolated from mucosa with active ulcerative colitis. Acetic acid extracts of ulcerative colitis (UC) and normal small intestinal (SI) epithelial cells were subjected to acid urea–polyacrylamide gel electrophoresis and transferred to a PVDF membrane. Synthetic human neutrophil defensin peptide 1 (HNP-1; 2.5 μg) was also run as shown. (A) Amido black stain for protein. (B) Duplicate lanes probed with anti-HNP 1–3 antibody; a band immunoreactive for HNP is clearly seen in the lane with the UC epithelial cell extract (arrowed), but not in the lane containing the normal small intestinal epithelial cell extract.
Antimicrobial assay
Antimicrobial assays were performed using the defensin sensitive phoP mutant of S typhimurium. Acid extracts of epithelial cells (in 0.1% acetic acid) isolated and purified from ulcerative colitis mucosal samples demonstrated antimicrobial activity as illustrated by a 3 log drop in CFU after three hours of culture with the phoP mutant of S typhimurium (average CFU/ml at zero hours: 1.96 × 106; after three hours of culture with extract: 0.60 × 103). A control experiment with the same amount of 0.1% acetic acid showed an increase in CFU/ml (zero hours: 2.10 × 106; after three hours: 1.45 × 108).
Expression of lysozyme
Strong lysozyme immunoreactivity was seen in Paneth cells in the terminal ileal sections but other epithelial cells in these sections and epithelial cells of normal colonic mucosa were not labelled (fig 5A–C). In the lamina propria of both the normal colonic and ileal sections, there was weak to negative staining of mononuclear cells (macrophages).
Figure 5.
Expression of lysozyme in intestinal tissues. (A/B) Normal small intestine showing granular expression of lysozyme in Paneth cells located in the crypt base. (C) Normal colon showing absence of lysozyme expression. (D/E) Active small intestinal Crohn's disease, showing lamina propria neutrophils (arrows) and Paneth cells (asterisk) immunoreactive for lysozyme. In addition, many villous enterocytes are strongly immunoreactive for lysozyme (small arrowheads), which is often apically located (large arrowheads).
In active IBD mucosal sections, lamina propria neutrophils and macrophages were immunoreactive for lysozyme. In addition, lysozyme expressing surface epithelial cells were seen in seven of 10 Crohn's disease and seven of 11 ulcerative colitis mucosal samples. In many sections, scattered epithelial cells were strongly immunoreactive for lysozyme and were often present adjacent to negative cells. This pattern of epithelial immunoreactivity was similar to that seen in IBD sections stained with anti-HNP 1–3 antibody (fig 5D and E). Serial sections stained with anti-HNP 1–3 and antilysozyme antibodies showed coexpression of neutrophil defensins and lysozyme within several surface enterocytes (fig 2A and B). The distribution of lysozyme within enterocytes was often similar to that seen for neutrophil defensin, particularly immunoreactivity in the apical half of the cells and also in the mucus layer overlying positive cells (fig 5D and E).
In the inflamed colonic mucosa, the occasional metaplastic Paneth cells were also labelled but, because of their predominant location in the crypt region and their morphology, they could easily be distinguished from the positive surface enterocytes described above.
DISCUSSION
In our study we have shown that epithelial cells in the mucosa with active IBD express neutrophil defensins HNP 1–3. Immunohistochemical studies localised the HNP expression to surface enterocytes of actively inflamed mucosa, but epithelial cells of normal and non-inflamed inflammatory bowel disease tissue samples were negative. HNP 1–3 expression by the epithelium in the inflamed mucosa was focal and largely restricted to cells on the surface and near the villous tips of the colonic and ileal samples, respectively. Specificity of HNP 1–3 immunoreactivity was confirmed by the abolition of staining after neutralisation of the antibody with synthetic HNP 1. In addition, immunoblotting studies on purified epithelial cells isolated from inflamed ulcerative colitis mucosal samples showed the presence of a single HNP immunoreactive band. The slight difference in migration between the immunoreactive bands of synthetic HNP-1 and the epithelial extract may reflect the predominance of HNP-2 and/or HNP-3 in the purified epithelial cells from inflamed ulcerative colitis mucosal samples or the binding of the defensin molecules to other cellular components. Antimicrobial activity of an acid extract of these cells was demonstrated using the defensin sensitive phoP mutant of S typhimurium. This antimicrobial activity is probably mediated by HNP 1–3 expressed by the purified epithelial cells, but it also possible that other antimicrobial molecules present in the epithelial cells contribute to this effect.6
HNP 1–3 are members of the α defensin family of antimicrobial peptides that are normally expressed in azurophil granules of neutrophils. They are small (3–4 kDa), cationic, arginine rich peptides with six invariant cysteine residues that form three stabilising disulphide bonds. They can be released extracellularly from activated neutrophils9 and have been detected in the serum of patients with septicaemia and bacterial meningitis.10 The release of defensin from neutrophils and its deposition on filarial worms and in surrounding stromal tissue in patients with onchocerciasis has been reported.11 Our immunohistochemical studies also suggest active secretion of HNP 1–3 by neutrophils in the mucosa from patients with active IBD.
Take home messages.
Human neutrophil defensins (HNP 1–3) and lysozyme are expressed in surface enterocytes of mucosa with active inflammatory bowel disease but not in inactive disease
These molecules may play an important role in intestinal host defence against luminal microorganisms.
“Immunohistochemical studies localised human neutrophil defensin expression to surface enterocytes of actively inflamed mucosa, but epithelial cells of normal and non-inflamed inflammatory bowel disease tissue samples were negative”
We also found that HNP 1–3 was expressed within vascular endothelial cells, a finding that has recently been reported elsewhere.12 However, the expression of neutrophil defensins by epithelial cells has not been reported previously. Other members of the α defensin family, HD-5 and HD-6 are expressed by Paneth cells in the terminal ileum,13, 14 where they are thought to contribute to the regulation of the crypt microflora. However, we have shown that these Paneth cells do not express neutrophil defensins.
Lysozyme is a well recognised antimicrobial protein with activity against Gram positive organisms, which is normally expressed by neutrophils, monocytes/macrophages, and Paneth cells. Although previous studies have demonstrated the expression of lysozyme by epithelial cells in the mucosa of active IBD,15, 16 our study shows that a number of epithelial cells express both HNP 1–3 and lysozyme.
Recent studies have also identified the expression of other antimicrobial activities in epithelial cells of the mucosa with active IBD. These include secretory phospholipase A2 (PLA2s) and inducible nitric oxide synthase.17, 18 Nitric oxide is known to have antimicrobial activity,19 and secretory phospholipase A2 has also been shown to be capable of mediating this function.20 Thus, in states of mucosal inflammation, intestinal epithelial cells express a variety of antimicrobial entities. Because there are a large number of bacteria normally resident in the lumen of the distal gastrointestinal tract, the expression of antimicrobial molecules in epithelial cells is likely to represent an important component of the host defence following the loss of epithelial barrier integrity. These molecules may act locally in the cell cytoplasm, at the cell surface after secretion, or intraluminally, after exfoliation of the cells. The antimicrobial molecules may also act synergistically with each other.21
Acknowledgments
These studies were supported by the Digestive Disorders Foundation (alimentary pharmacology and therapeutics fellowship to Dr Cunliffe) and by the Medical Research Council.
Abbreviations
AU
PAGE, acid urea
polyacrylamide gel electrophoresis; CFU, colony forming units; HNP, human neutrophil defensin polypeptide; IBD, inflammatory bowel disease; IFT, intestinal trefoil factor; PBS, phosphate buffered saline; TSB, trypticase soy broth
REFERENCES
- 1.Hancock REW. Peptide antibiotics. Lancet 1997;349:418–22. [DOI] [PubMed] [Google Scholar]
- 2.Mahida YR, Rose F, Chan WC. Antimicrobial peptides in the gastrointestinal tract. Gut 1997;40:161–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol 1993;11:105–28. [DOI] [PubMed] [Google Scholar]
- 4.Levy O. Antibiotic proteins of polymorphonuclear leukocytes. Eur J Haematol 1996;56:263–77. [DOI] [PubMed] [Google Scholar]
- 5.Mahida YR, Galvin A, Gray T, et al. Migration of human intestinal lamina propria lymphocytes, macrophages and eosinophils following the loss of surface epithelial cells. Clin Exp Immunol 1997;109:377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rose FRAJ, Bailey K, Keyte JW, et al. Potential role of epithelial cell-derived histone H1 proteins in innate antimicrobial defense in the human gastrointestinal tract. Infect Immun 1998;66:3255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fields PI, Groisman EA, Hefron F. A salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science 1989;243:1059–61. [DOI] [PubMed] [Google Scholar]
- 8.Podolsky DK, Lynch-Devaney K, Stow JL, et al. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem 1993;268:6694–702. [PubMed] [Google Scholar]
- 9.Ganz T. Extracellular release of antimicrobial defensins by human polymorphonuclear leukocytes. Infect Immun 1987;55:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panyutich AV, Panyutich EA, Krapivin VA, et al. Plasma defensin concentrations are elevated in patients with septicaemia or bacterial meningitis. J Lab Clin Med 1993;122:202–7. [PubMed] [Google Scholar]
- 11.Gallin MY, Jacobi AB, Büttner DW, et al. Human autoantibody to defensin: disease association with hyperreactive onchocerciasis (sowda). J Exp Med 1995;182:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnathan ES, Raghunath PN, Tomaszweski JE, et al. Immunohistochemical localization of defensin in human coronary vessels. Am J Pathol 1997;150:1009–20. [PMC free article] [PubMed] [Google Scholar]
- 13.Bevins CL, Martin-Porter E, Ganz T. Defensins and innate host defence of the gastrointestinal tract. Gut 1999;45:911–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunliffe R, Rose FRAJ, Keyte J, et al. Human defensin 5 is stored in precursor form in Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut 2001;48:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klockars M, Reitamo S, Reitamo JJ, et al. Immunohistochemical identification of lysozyme in intestinal lesions in ulcerative colitis and Crohn's disease. Gut 1977;18:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montero C, Erlandsen S. Immunocytochemical and histochemical studies on intestinal epithelial cells producing both lysozyme and mucosubstance. Anat Rec 1978;190:127–42. [DOI] [PubMed] [Google Scholar]
- 17.Haapamäki MM, Grönroos JM, Nurmi H, et al. Gene expression of group II phospholipase A2 in intestine in ulcerative colitis. Gut 1997;40:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer II, Kawka DW, Scott S, et al. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology 1996;111:871–85. [DOI] [PubMed] [Google Scholar]
- 19.Fang FC. Perspective series: host/pathogen interactions. Mechanisms of nitric oxide related antimicrobial activity. J Clin Invest 1997;99:2818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harwig SSL, Tan L, Qu X, et al. Bactericidal properties of murine intestinal phospholipase A2. J Clin Invest 1995;95:603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bals R, Wang X, Zasloff M, et al. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A 1998;95:9541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]