Abstract
Modern molecular methods for precancer diagnosis have expanded the range of detectable disease to a preclinical level and provided material for histopathological correlation. The precancer scenario begins with sporadic acquisition of rare PTEN mutation bearing glands, which are morphologically unremarkable, and progresses to discrete foci of cytologically altered glands, readily visible on routinely stained sections. Clinical outcome studies of women with endometrial lesions have established threshold diagnostic features that confer increased cancer risk. This class of high risk lesions has been designated endometrial intraepithelial neoplasia (EIN). EIN is diagnosed by presence of cytological demarcation, crowded gland architecture, minimum size of 1mm, and careful exclusion of mimics. Most EIN lesions have been diagnosed as atypical endometrial hyperplasias in the World Health Organisation system. Specialised molecular and morphometric analyses have been extremely useful in redefining clinically relevant premalignant endometrial disease, but translation to improved patient care requires the informed participation of pathologists.
Keywords: endometrium, hyperplasia, diagnosis, endometrial intraepithelial neoplasia, precancer, terminology
Endometrial carcinoma is the most common malignancy of the female genital tract1 and its most frequent subtype, endometrioid carcinoma (“type I” cancers2,3), is often preceded by a histologically evident precursor lesion.1,4 Accurate and sensitive recognition of these precursors, which generally have fallen under the diagnostic umbrella of “hyperplasias”,5,6 has great clinical value as an early warning of heightened cancer risk and a potential target for preventative treatment. Public awareness of the need for early diagnosis is also on the rise, in part because of widespread media coverage of the endometrial tumorigenic effects of hormonally active medications, such as tamoxifen,7,8 and the recognition that everyday dietary or environmental exposures to compounds with oestrogenic activity9–11 are much more common than previously appreciated.
Histopathological plasticity of normal and pathological endometrial tissues alike presents formidable barriers for classification of biologically homogenous groups into reproducible morphological diagnostic categories. Premalignant lesions of the endometrium can demonstrate non-endometrioid differentiation (squamous, mucinous, and tubal changes are common)12 and, similar to normal tissues, may transiently change their architecture and cytology in response to fluctuating oestrogens and progestins. The effects of progestins on endometrial glandular cytology are a particular diagnostic problem. In a progestin rich environment, nuclei of premalignant glands tend to diminish in size and acquire a rather bland chromatin pattern, which makes them appear less “atypical”. Paradoxically, the nuclei of normal glands become enlarged and rounded—features associated with atypia.
“Histopathological plasticity of normal and pathological endometrial tissues alike presents formidable barriers for classification of biologically homogenous groups into reproducible morphological diagnostic categories”
Pathologists have applied different strategies to harness a workable diagnostic scheme from this elusive group of endometrial precancers, resulting in a variety of classification schemes,13–16 confused by overlapping terminology. A reductionist approach attempts to uncover those minimal shared features that correspond to a relevant clinical outcome. This had led to the strong association of cytological atypia with cancer risk,17–19 thereby suggesting that atypical endometrial hyperplasias are the subset of hyperplastic endometrial lesions most likely to progress to carcinoma. Interobserver reproducibility in the assessment of the presence or absence of cytological atypia is poor,15,20,21 an alarming state of affairs in light of the dominance afforded this feature in patient management. In contrast, a divisionist approach attempts to improve the homogeneity of diagnostic groups by classifying a broad spectrum of disease into a large number of discrete categories. An example is when endometrial hyperplasia cytology and architecture are separately graded on a three part scale, yielding nine permutations of endometrial hyperplasia. An unfortunate side effect is the degradation of reproducibility proportionate to the number of categories used.15 Furthermore, there is diminishing clinical benefit in having more diagnostic categories than therapeutic responses.
WHAT HAS HAPPENED LATELY?
Molecular diagnostic methods compatible with paraffin wax embedded archival human tissues have permitted histopathological correlation with genetically defined premalignant endometrial disease.22 This was first done using markers capable of distinguishing monoclonal from polyclonal tissues, in search of predicted monoclonal premalignant lesions. Non-random X chromosome inactivation23–25 and clonal propagation of altered microsatellites12 confirmed that many atypical endometrial hyperplasias are indeed monoclonal, and these clones have an altered genotype that is conserved in the subsequent carcinomas they produce.21,26 Establishing lineage continuity between monoclonal putative precancers and cancer is crucial in the molecular discrimination of precancers from other “dead end” benign processes, such as the monoclonal stroma of endometrial polyps.27,28
The histopathology of monoclonal putative endometrial precancers is identical to that seen by computerised morphometric analysis to increase the risk of endometrial adenocarcinoma.21,29 Morphometric analysis of an index series of endometrial lesions of known clonal composition showed that most monoclonal putative precancers had a high risk D score, a weighted index of computer measured cytological and architectural features that are highly predictive of concurrent30 or future31,32 endometrial adenocarcinoma. Of the three variables used to calculate the D score, the architectural feature of volume percentage stroma (VPS) has by far the greatest predictive value.21,31 As glands become more crowded in monoclonal precancers, the VPS drops below a threshold of 55%.21 This establishes formal architectural features as useful cancer predictive criteria in addition to cytology. One caution is that some completely benign conditions such as normal basalis, endometrial polyps, and secretory endometrium can have VPS < 55% and must be excluded.
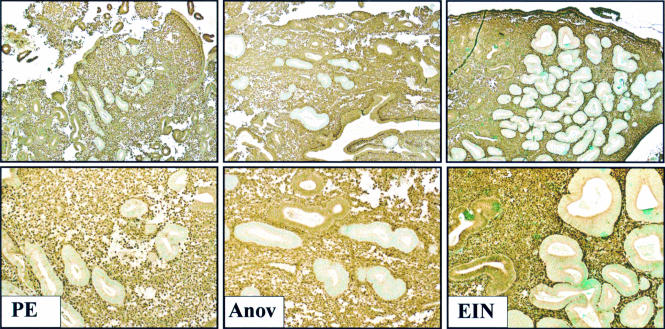
Immunohistochemical biomarkers have further pushed the detection limit of premalignant endometrial disease to a truly preclinical stage, disclosing a much higher prevalence of early stage disease than previously suspected. DNA based clonal analysis typically requires DNA isolated from a 1–2 mm diameter area of a tissue section, limiting the physical resolution of small lesions. The PTEN tumour suppressor gene33,34 is inactivated in up to 83% of endometrial adenocarcinomas.35–37 Because loss of the PTEN protein occurs at or near the time of the initiation of carcinogenesis,37–40 premalignant and malignant endometrial glands can be distinguished by the loss of this marker. Thus, PTEN immunohistochemistry shows lesion specific loss of the PTEN protein in 63% of endometrial intraepithelial neoplasia (EIN) lesions,41 confirming that these tightly aggregated glands are clonal outgrowths of genetically abnormal cells. However, immunohistochemistry has the potential to uncover individual or rare PTEN null glands that may represent a stage of disease not yet evident on routine haematoxylin and eosin histology (fig 1). Tiny clusters of architecturally and cytologically unremarkable PTEN null glands are seen in 43% and 56%, respectively, of normal proliferative and anovulatory endometria.41 These PTEN null glands of normal cycling proliferative endometria contain somatically acquired deletions and/or mutations of the PTEN gene itself, and persist between menstrual cycles.41 Persistence between menstrual cycles probably results from the inclusion of mutant cells in the regenerative population that repopulates the functionalis after each menses.
Figure 1.
Broad spectrum of premalignant endometrial disease demarcated by PTEN immunohistochemistry. PTEN immunohistochemistry with monoclonal antibody 6H2.137,41,42 delimits the distribution of PTEN null pale glands (pale counterstain) in proliferative (left, “PE”), anovulatory (middle, “Anov”), and endometrial intraepithelial neoplasia (right, “EIN”) endometria. Loss of PTEN protein expression is abnormal, caused by mutations and/or deletion of the PTEN gene itself.41 A comparison of PTEN null and expressing glands shows no distinguishing features in proliferative and anovulatory endometria: these mutant clones unrecognised by haematoxylin and eosin staining represent preclinical disease. In contrast, PTEN null glands in EIN lesions have an altered cytology and are more tightly packed (low VPS; volume percentage stroma), which permits the identification of these localising lesions by routine histology. Although PTEN immunohistochemistry is informative for educational purposes, its low sensitivity (about half of EIN lesions express the PTEN protein) and specificity (most PTEN null glands are seen in histologically normal endometrium) in the detection of clinically relevant EIN lesions makes it impractical for routine patient care. Images reprinted, with permission, fromwww.endometrium.org.
“The histopathology of monoclonal putative endometrial precancers is identical to that seen by computerised morphometric analysis to increase the risk of endometrial adenocarcinoma”
The endometrium has the highest prevalence (43%) of non-familial premalignant disease yet shown experimentally in any intact normal human tissue.41 Most of this is “latent” in that additional changes are required before it becomes clinically evident, and these occur at a low efficiency. It is not necessary to invoke any special mechanisms of mutation to explain this high rate of initiation of endometrial carcinogenesis. The rate of sporadic mutagenesis in human cells has been estimated at 10−7 mutations/gene/cell division,43 consistent with the prediction of hundreds of cells for each gram of proliferative tissue (109 cells/g) that generate “first hits” of a multistep carcinogenesis44 pathway. The PTEN gene acts as a gatekeeper for endometrial carcinogenesis, being one of the initial genetic changes seen in this process.40 As comparable biomarkers for neoplastic initiation become available in other tissue sites, it is anticipated that similarly high rates of initiation will be seen in other tissues.
DEFINING PREMALIGNANT DISEASE
A comprehensive genetic and histopathological model of endometrial carcinogenesis can now be constructed. Initiating sporadic mutations, such as loss of PTEN function, occur quite frequently within the endometrial regenerative pool, at such a high rate that they can be considered part of background “normal” genetic events. These mutant clones remain indistinguishable from non-mutant glands in normal cycling and anovulatory endometrium. Involution or expansion of mutant clones in response to non-genetic factors is one possible mechanism whereby the ambient hormonal state may modify the risk of endometrial cancer. Interestingly, the PTEN gene appears to be hormonally regulated, with greatest physiological endometrial gland expression in an oestrogen rich environment.45 Thus, the effects of diminished PTEN tumour suppressor function are probably accentuated under the very circumstances known to increase cancer risk: protracted oestrogen exposure unopposed by progestins.46,47 Mutant glands subsequently acquire additional genetic damage and then become recognisable as focal lesions by their altered cytology and crowded architecture. It is these lesions that pathologists can diagnose (table 1).
Table 1.
| Feature | Criterion | Comment |
| Architecture | Volume percentage stroma <55% | Usually localised lesion, extending to diffuse |
| Cytology | Cytological demarcation | Change within localising lesion relative to background |
| Size | Exceeds 1 mm minimum diameter | Lesion extent defined by architecture and cytology |
| Exclusions | Cancer, polyps, secretory endometrium, artifact, etc | Careful review of differential diagnosis |
The term endometrial intraepithelial neoplasia (EIN) has been proposed50 as a descriptive term for monoclonal endometrial precancers whose distinctive histopathology is characterised by those morphometric features21 that have been documented to increase the risk of cancer.30–32 This category of lesions has now fulfilled most of those postulates predicted for clinically relevant precancerous disease (table 2). Their natural history dictates that EIN lesions should be ablated by hormonal (progesterone) or surgical (hysterectomy) means.
Table 2.
Features of endometrial precancers
| Prediction | Evidence |
| Precancers differ from normal tissue | Monoclonal precancers12,21,23,25 arise from a polyclonal normal field24 |
| Mutations are acquired in precancers23,38,49–53 | |
| Precancers share some, but not all, features of cancer | Precancer–cancer lineage hierarchy26 |
| May share PTEN,37,38,55 K-ras,52–54,56 MLH151,57 changes | |
| Both are monoclonal21,23,25,58,59 | |
| Precancers increase risk for carcinoma | Increased concurrent cancer rate30 |
| Increased future cancer rate31,32 | |
| Precancers can be diagnosed | Morphometric standard21,22,29,50 |
| Endometrial intraepithelial neoplasia = precancers (table 1) | |
| Hormonal and genetic risks are linked | Hormonal modulation of the PTEN gene,45 frequently inactivated in premalignant disease37,38,41,53 |
| Precancers can be induced by manipulating genetic and/or hormonal variables | 100% of PTEN mutant heterozygote mice get endometrial “hyperplasia” and 21% of these evolve to carcinoma60 |
DIAGNOSING PREMALIGNANT DISEASE
EIN is diagnosed by a pathologist using haematoxylin and eosin stained sections prepared from a representative endometrial sample. Although computerised morphometry has been a useful tool in identifying features characteristic of EIN,18,21,30–32,61 such equipment is not required for routine diagnosis. Subjective interpretation of stated criteria by a pathologist at a standard microscope is often adequate, or a simple ocular grid may be used as a counting device for area measurements (VPS). Diagnostic accuracy may be severely compromised by exogenous progestin containing hormonal treatments. For this reason, primary diagnosis or follow up surveillance of a suspected EIN lesion should be based whenever possible on a sample obtained while the patient is not on therapeutic hormones. For those patients on progestins, diagnostic tissue can be obtained after two to four weeks of discontinuing exogenous hormone treatment, after completion of a withdrawal bleed.
A simple process for diagnosing EIN lesions can be broken down into component steps as follows. Additional images and an interactive tutorial are available online (www.endometrium.org ).
Glandular crowding: VPS < 55%
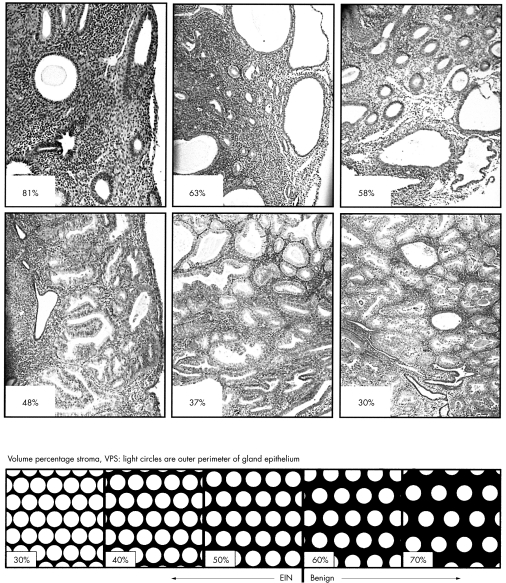
A tissue sample can be divided into stromal and glandular compartments, and their relative proportions used to assess glandular crowding (fig 2). Focusing on the stroma ensures that fragmented areas are not misinterpreted and provides a homogenous field amenable to visual assessment. EIN lesions have a stromal volume less than that of the glands.
Figure 2.
Architectural features of endometrial intraepithelial neoplasia (EIN). A visual impression of the appearance of endometria with differing volume percentage stroma (VPS) is shown diagrammatically (below) and in photomicrographs. Large, or dominant cysts, such as those shown in the middle panel, top, should be avoided in calculating VPS. VPS measurements are indicated for three benign (top) and EIN (middle) endometria which divide at a threshold VPS of 55%. The EIN lesions are further characterised by cytological changes in the areas of low VPS compared with the background (not shown). Haematoxylin and eosin photomicrographs. Images reprinted, with permission, from www.endometrium.org.
Cytological demarcation
EIN lesions have an abnormal cytology within areas where glands are more tightly packed than is seen in the background endometrium. The manner of cytological change in EIN varies considerably and can include, but is not limited to, increased variation in nuclear size and contour, a clumped or granular chromatin texture, a change in nucleoli, a change in the nuclear/cytoplasmic ratio, and altered cytoplasmic differentiation. The absolute cytological appearance is also influenced by tissue processing methods, the extent of reactive/degenerative change, and the hormonal environment. Beware diagnosing an EIN lesion if the cytology is identical between areas with crowded compared to uncrowded glands! Many of these are artifactual disruptions where the stroma is sheared and glands pushed together.
Size >1 mm
The diagnosis of EIN requires a contiguous field of glands sufficiently large to permit estimation of the VPS: lesions with a largest diameter exceeding 1–2 mm. This was the minimum size required for accurate morphometric correlation of lesion histology with clinical outcome.30–32 Lesion size is measured across the perimeter enclosing cytologically altered glands that are closely arranged (VPS < 55%).
Exclude confounding benign processes
Accurate diagnosis requires the exclusion of overlapping entities. EIN criteria are meant to be applied to endometrial functionalis. The lower uterine segment and basalis must be excluded. Furthermore, degenerative (stromal breakdown) or pure hormonal effects (anovulation) must be diagnosed as such, with the realisation that they may contain some features of altered glandular architecture or cytology that overlap with those of bona fide EIN. It is the distinction between these benign endometrial lesions and EIN that produces the greatest challenge in diagnosis. Some specific presentations that might be falsely interpreted as premalignant must be excluded, including very small atypical areas, polyps, normal secretory endometrium, and inflamed endometrium. Glands pushed together after the breakdown of the intervening stroma are commonly overinterpreted as premalignant, especially when having degenerative epithelial changes that mimic atypia. In those cases where an EIN lesion is suspected within one of these backgrounds, the diagnosis relies heavily upon contrasting the localising features of the EIN lesion to the regional context.
Exclude carcinoma
Carcinoma should be distinguished from EIN because it must be surgically, not hormonally, ablated. It should be borne in mind that the absence of carcinoma in a tissue biopsy does not exclude the possibility that the patient has a cancer that was not sampled during the biopsy procedure. An opinion should always be rendered based upon available material, and clearly stated.
“Beware diagnosing an endometrial intraepithelial neoplasia lesion if the cytology is identical between areas with crowded compared to uncrowded glands!”
Cancers in most cases may be identified as separate from EIN by the presence of solid areas of neoplastic epithelium or a maze-like rambling of uninterrupted lumens, which indicate interruption of the normal gland–stroma relations. Poorly differentiated and papillary serous carcinomas may be identified by their distinctive appearance. In some small, distorted, or poorly oriented samples, EIN may be indistinguishable from well differentiated carcinomas. This should be mentioned in the report to avoid inappropriate progesterone treatment of an incompletely sampled endometrial adenocarcinoma. A fraction of EINs will thus be upgraded to carcinoma upon more extensive sampling—either from a follow up curettage or hysterectomy.
If the neoplastic epithelium is detached from its stroma, or present only in very small tissue fragments, it may at times be impossible to resolve the differential diagnosis between EIN and carcinoma. In these instances, a clear diagnosis of “neoplastic endometrial epithelium, cannot exclude carcinoma” will alert the clinician to the problem. Lastly, always remember that surgical treatment of endometrial cancers may be modified by the distribution of the lesion. The surgical management of a cancer completely contained within an excised polyp may be different to a well differentiated cancer that extends to involve the lower uterine segment.
Take home messages.
Recently developed molecular methods for precancer diagnosis have expanded the range of detectable disease to a preclinical level, disclosing a much higher prevalence of early disease than previously suspected
The precancer scenario begins with sporadic acquisition of rare PTEN mutation bearing glands, which are morphologically unremarkable, and additional genetic damage results in a progression to discrete foci of cytologically altered glands, readily visible on routinely stained sections
Clinical outcome studies of women with endometrial lesions have established threshold diagnostic features that confer increased cancer risk and these lesions has been designated endometrial intraepithelial neoplasia (EIN).
EIN is diagnosed by presence of cytological demarcation, glandular crowding (volume percentage stroma < 55%), minimum size of 1mm, and careful exclusion of mimics
Specialised molecular and morphometric analyses have been powerful tools in redefining clinically relevant premalignant endometrial disease, but translation to improved patient care requires the informed participation of pathologists
WHAT LIES AHEAD?
Isolated PTEN negative glands in normal endometria may well be the earliest detectable phases of endometrial tumorigenesis yet seen, but the clinical relevance of such small lesions remains to be determined.37,41 This situation is very similar to the first use of the polymerase chain reaction in the diagnosis of infectious diseases, where organisms could be detected at a burden well below that associated with clinical disease. Can one PTEN null gland in an “about to be shed” endometrium possibly increase the risk for carcinoma? Disease burden (lesion size) and the likelihood of persistence (influenced by hormonal state and location of lesion within the functionalis/basalis) are poorly understood factors that probably interact in determining the ultimate cancer risk. Sorting out the natural history of latent disease, a stage that had long been hypothesised but was undocumented, is a major challenge for the future.
The impact of molecular diagnosis and computerised morphometric analysis on the future practice of clinical pathology will be determined by pathologists. Endometrial precancer diagnosis has clearly benefited from those diagnostic insights contributed by these investigational tools. The result is revised diagnostic criteria, rather than obsolescence of standard microscopy. As long as pathologists actively participate in critical evaluation of new data, and are open minded to its constructive use, it is likely that this precedent can be followed in other organ sites.
Abbreviations
EIN, endometrial intraepithelial neoplasia
VPS, volume percentage stroma
REFERENCES
- 1.Hertig A, Sommers S. Genesis of endometrial carcinoma. I. Study of prior biopsies. Cancer 1949;2:946–56. [DOI] [PubMed] [Google Scholar]
- 2.Bokhman J. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 1983;15:10–17. [DOI] [PubMed] [Google Scholar]
- 3.Deligdisch L, Holinka C. Endometrial carcinoma: two diseases? Cancer Detect Prev 1987;10:237–46. [PubMed] [Google Scholar]
- 4.Hertig A, Sommers S, Bengloff H. Genesis of endometrial carcinoma. III. Carcinoma in situ. Cancer 1949;2:964–71. [DOI] [PubMed] [Google Scholar]
- 5.Kurman R, Norris H. Endometrial hyperplasia and metaplasia. In: Kurman R, ed. Blaustein's pathology of the female genital tract. New York: Springer-Verlag, 1987:322–37.
- 6.Silverberg S, Kurman R. Endometrial polyps and hyperplasias. In: Rosai J, Sobin LH, eds. Tumors of the uterine corpus and gestational trophoblastic disease. Washington: Armed Forces Institute of Pathology, 1991:15–45.
- 7.Bergman L, Beelen ML, Gallee MP, et al. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres' ALERT Group. Assessment of liver and endometrial cancer risk following tamoxifen. Lancet 2000;356:881–7. [DOI] [PubMed] [Google Scholar]
- 8.Deligdisch L, Kalir T, Cohen CJ, et al. Endometrial histopathology in 700 patients treated with tamoxifen for breast cancer. Gynecol Oncol 2000;78:181–6. [DOI] [PubMed] [Google Scholar]
- 9.Breithofer A, Graumann K, Scicchitano MS, et al. Regulation of human estrogen receptor by phytoestrogens in yeast and human cells. J Steroid Biochem Mol Biol 1998;67:421–9. [DOI] [PubMed] [Google Scholar]
- 10.Hopert AC, Beyer A, Frank K, et al. Characterization of estrogenicity of phytoestrogens in an endometrial-derived experimental model. Environ Health Perspect 1998;106:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodman MT, Wilkens LR, Hankin JH, et al. Association of soy and fiber consumption with the risk of endometrial cancer. Am J Epidemiol 1997;146:294–306. [DOI] [PubMed] [Google Scholar]
- 12.Jovanovic AS, Boynton KA, Mutter GL. Uteri of women with endometrial carcinoma contain a histopathologic spectrum of monoclonal putative precancers, some with microsatellite instability. Cancer Res 1996;56:1917–21. [PubMed] [Google Scholar]
- 13.Winkler B, Alvarez S, Richart R, et al. Pitfalls in the diagnosis of endometrial neoplasia. Obstet Gynecol 1984;64:185–94. [PubMed] [Google Scholar]
- 14.Sherman A, Brown S. The precursors of endometrial carcinoma. Am J Obstet Gynecol 1979;135:947–54. [DOI] [PubMed] [Google Scholar]
- 15.Bergeron C, Nogales F, Masseroli M, et al. A multicentric European study testing the reproducibility of the WHO classification of endometrial hyperplasia with a proposal of a simplified working classification for biopsy and curettage specimens. Am J Surg Pathol 1999;23:1102–8. [DOI] [PubMed] [Google Scholar]
- 16.Scully RE, Bonfiglio TA, Kurman RJ, et al. Uterine corpus. In: Histological typing of female genital tract tumors. New York: Springer-Verlag, 1994:13–31.
- 17.Colgan TJ, Norris HJ, Foster W, et al. Predicting the outcome of endometrial hyperplasia by quantitative analysis of nuclear features using a linear discriminant function. Int J Gynecol Pathol 1983;1:347–52. [DOI] [PubMed] [Google Scholar]
- 18.Ausems EW, van der Kamp JK, Baak JP. Nuclear morphometry in the determination of the prognosis of marked atypical endometrial hyperplasia. Int J Gynecol Pathol 1985;4:180–5. [DOI] [PubMed] [Google Scholar]
- 19.Kurman R, Kaminski P, Norris H. The behavior of endometrial hyperplasia: a long term study of “untreated” hyperplasia in 170 patients. Cancer 1985;56:403–12. [DOI] [PubMed] [Google Scholar]
- 20.Kendall BS, Ronnett BM, Isacson C, et al. Reproducibility of the diagnosis of endometrial hyperplasia, atypical hyperplasia, and well-differentiated carcinoma. Am J Surg Pathol 1998;22:1012–19. [DOI] [PubMed] [Google Scholar]
- 21.Mutter GL, Baak JPA, Crum CP, et al. Endometrial precancer diagnosis by histopathology, clonal analysis, and computerized morphometry. J Pathol 2000;190:462–9. [DOI] [PubMed] [Google Scholar]
- 22.Mutter GL. Histopathology of genetically defined endometrial precancers. Int J Gynecol Pathol 2000;19:301–9. [DOI] [PubMed] [Google Scholar]
- 23.Esteller M, Garcia A, Martinez-Palones JM, et al. Detection of clonality and genetic alterations in endometrial pipelle biopsy and its surgical specimen counterpart. Lab Invest 1997;76:109–16. [PubMed] [Google Scholar]
- 24.Mutter GL, Boynton KA. X chromosome inactivation in the normal female genital tract: implications for identification of neoplasia. Cancer Res 1995;55:5080–4. [PubMed] [Google Scholar]
- 25.Mutter GL, Chaponot M, Fletcher J. A PCR assay for non-random X chromosome inactivation identifies monoclonal endometrial cancers and precancers. Am J Pathol 1995;146:501–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Mutter GL, Boynton KA, Faquin WC, et al. Allelotype mapping of unstable microsatellites establishes direct lineage continuity between endometrial precancers and cancer. Cancer Res 1996;56:4483–6. [PubMed] [Google Scholar]
- 27.Vanni R, Marras S, Andria M, et al. Endometrial polyps with predominant stromal component are characterized by a t(6;14)(p21;q24) translocation. Cancer Res 1995;55:31–3. [PubMed] [Google Scholar]
- 28.Fletcher J, Pinkus J, Lage J, et al. Clonal 6p21 rearrangement is restricted to the mesenchymal component of an endometrial polyp. Genes Chromosomes Cancer 1992;5:260–3. [DOI] [PubMed] [Google Scholar]
- 29.Mutter GL. Endometrial precancer type collection [on line]. Available: http://www.endometrium.org 2000.
- 30.Dunton C, Baak J, Palazzo J, et al. Use of computerized morphometric analyses of endometrial hyperplasias in the prediction of coexistent cancer. Am J Obstet Gynecol 1996;174:1518–21. [DOI] [PubMed] [Google Scholar]
- 31.Baak JPA, Nauta J, Wisse-Brekelmans E, et al. Architectural and nuclear morphometrical features together are more important prognosticators in endometrial hyperplasias than nuclear morphometrical features alone. J Pathol 1988;154:335–41. [DOI] [PubMed] [Google Scholar]
- 32.Orbo A, Baak JP, Kleivan I, et al. Computerised morphometrical analysis in endometrial hyperplasia for the prediction of cancer development. A long-term retrospective study from northern Norway. J Clin Pathol 2000;53:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers MP, Stolarov JP, Eng C, et al. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci U S A 1997;94:9052–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mutter GL. PTEN, a protean tumor suppressor. Am J Pathol 2001;158:1895–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Risinger JI, Hayes AK, Berchuck A, et al. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res 1997;57:4736–8. [PubMed] [Google Scholar]
- 36.Tashiro H, Blazes MS, Wu R, et al. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res 1997;57:3935–40. [PubMed] [Google Scholar]
- 37.Mutter GL, Lin MC, Fitzgerald JT, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst 2000;92:924–30. [DOI] [PubMed] [Google Scholar]
- 38.Maxwell G, Risinger J, Gumbs C, et al. Mutation of the PTEN tumor suppressor gene in endometrial hyperplasias. Cancer Res 1998;58:2500–3. [PubMed] [Google Scholar]
- 39.Yoshinaga K, Sasano H, Furukawa T, et al. The PTEN, BAX, and IGFIIR genes are mutated in endometrial atypical hyperplasia. Jpn J Cancer Res 1998;89:985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali IU. Gatekeeper for endometrium: the PTEN tumor suppressor gene. J Natl Cancer Inst 2000;92:861–3. [DOI] [PubMed] [Google Scholar]
- 41.Mutter GL, Ince TA, Baak JPA, et al. Molecular identification of latent precancers in histologically normal endometrium. Cancer Res 2001;6:4311–14. [PubMed] [Google Scholar]
- 42.Perren A, Weng L, Boag A, et al. Immunocytochemical evidence of loss of PTEN expression in primary ductal adenocarcinomas of the breast. Am J Pathol 1999;155:1253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cairns J. Mutation and cancer: the antecedents to our studies of adaptive mutation. Genetics 1998;148:1433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moolgavkar SH, Knudson AG, Jr. Mutation and cancer: a model for human carcinogenesis. J Natl Cancer Inst 1981;66:1037–52. [DOI] [PubMed] [Google Scholar]
- 45.Mutter GL, Lin MC, Fitzgerald JT, et al. Changes in endometrial PTEN expression throughout the human menstrual cycle. J Clin Endocrinol Metab 2000;85:2334–8. [DOI] [PubMed] [Google Scholar]
- 46.Curry S, Kelly S. Cancer of the female genital tract: overview. In: Osteen R, ed. Cancer manual. Boston: American Cancer Society, 1990:253–7.
- 47.Parazzini F, La Vecchia C, Bocciolone L, et al. The epidemiology of endometrial cancer. Gynecol Oncol 1991;41:1–16. [DOI] [PubMed] [Google Scholar]
- 48.Mutter GL. Endometrial intraepithelial neoplasia: a new standard for precancer diagnosis. Contrib Obstet Gynecol 2001;46:92–8. [Google Scholar]
- 49.Mutter GL. EIN central. Available: http://www.endometrium.org 2001.
- 50.Mutter GL, the Endometrial Collaborative Group. Endometrial intraepithelial neoplasia (EIN): will it bring order to chaos? Gynecol Oncol 2000;76:287–90. [DOI] [PubMed] [Google Scholar]
- 51.Esteller M, Catasus L, Matias-Guiu X, et al. hMLH1 promoter hypermethylation is an early event in human endometrial tumorigenesis. Am J Pathol 1999;155:1767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Enomoto T, Inoue M, Perantoni A, et al. K-ras activation in premalignant and malignant epithelial lesions of the human uterus. Cancer Res 1991;51:5304–14. [PubMed] [Google Scholar]
- 53.Mutter GL, Wada H, Faquin W, et al. K-ras mutations appear in the premalignant phase of both microsatellite stable and unstable endometrial carcinogenesis. Mol Pathol 1999;52:257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sasaki H, Nishii H, Takahashi H, et al. Mutation of the Ki-ras protooncogene in human endometrial hyperplasia and carcinoma. Cancer Res 1993;53:1906–10. [PubMed] [Google Scholar]
- 55.Levine RL, Cargile CB, Blazes MS, et al. PTEN mutations and microsatellite instability in complex atypical hyperplasia, a precursor lesion to uterine endometrioid carcinoma. Cancer Res 1998;58:3254–8. [PubMed] [Google Scholar]
- 56.Duggan BD, Felix JC, Muderspach LI, et al. Early mutational activation of the c-Ki-ras oncogene in endometrial carcinoma. Cancer Res 1994;54:1604–7. [PubMed] [Google Scholar]
- 57.Esteller M, Levine R, Baylin SB, et al. MLH1 promoter hypermethylation is associated with the microsatellite instability phenotype in sporadic endometrial carcinomas. Oncogene 1998;17:2413–17. [DOI] [PubMed] [Google Scholar]
- 58.Doherty T, Connell J, Stoerker J, et al. Analysis of clonality by polymerase chain reaction for phosphoglycerate kinase-1. Heteroduplex generator. Diagn Mol Pathol 1995;4:182–90. [DOI] [PubMed] [Google Scholar]
- 59.Shroyer K, Gudlaugsson E. Analysis of clonality in archival tissues by polymerase chain reaction amplification of PGK-1. Hum Pathol 1994;25:287–92. [DOI] [PubMed] [Google Scholar]
- 60.Stambolic V, Tsao MS, Macpherson D, et al. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res 2000;60:3605–11. [PubMed] [Google Scholar]
- 61.Baak JP, Wisse-Brekelmans EC, Fleege JC, et al. Assessment of the risk on endometrial cancer in hyperplasia, by means of morphological and morphometrical features. Pathol Res Pract 1992;188:856–9. [DOI] [PubMed] [Google Scholar]