Abstract
Aims: The expression of bone morphogenetic proteins (BMPs) was analysed in 47 osteosarcomas to determine differences in the expression of BMP subtypes and to correlate expression with response to chemotherapy, in addition to the disease free and overall survival of patients.
Methods: The expression of BMPs was examined immunohistochemically in 47 biopsy specimens of osteosarcoma using commercially available antibodies against different subtypes (BMP-2/4 (A-20), BMP-3 (N-19), BMP-4 (N-19), BMP-5 (N-19), BMP-6 (N-19), BMP-7 (N-19), BMP-8 (N-19)). The avidin–biotin–immunoperoxidase method was used for all antibodies.
Results: The expression of BMP subtypes varied considerably: 28 of the 47 tumours expressed BMP-2/4, 24 expressed BMP-3, 41 expressed BMP-5, 31 expressed BMP-6, 43 expressed BMP-7, and 42 expressed BMP-8. High expression of BMP-6 was found in those parts of the osteosarcoma with a chondroid differentiation (p = 0.016, Mann-Whitney test). No correlation was observed between the response to chemotherapy and the expression of BMPs (p > 0.05, Mann-Whitney test). Univariate analysis showed no correlation between overall survival or progression free survival and the expression of BMPs (p > 0.05, log rank test).
Conclusions: BMP-7 and BMP-8 are highly expressed in osteosarcoma. Moreover, high expression of BMP-6 correlates with a chondroid differentiation. In contrast to conclusions derived from previous studies in which small numbers of tumours were investigated, these results indicate that the expression of BMPs does not help to predict the outcome of patients.
Keywords: bone morphogenetic protein, osteosarcoma, prognosis, immunohistochemistry
Osteosarcoma is the most common primary malignant bone tumour, accounting for approximately 20% of all primary sarcomas in bone.1 Although treatment modalities have been improved over the past decades, it is still a tumour with a high mortality rate in children and young adults; the peak incidence is registered in the second decade of life. Most osteosarcomas are highly malignant tumours arising within bone. According to their main histological component they may be divided into various subtypes, such as osteoblastic, chondroblastic fibroblastic, teleangiectatic, and small cell osteosarcoma.2, 3 The general treatment consists of preoperative chemotherapy followed by surgical resection and a postoperative chemotherapy regimen modified according to the extent of tumour necrosis. In contrast to intramedullary tumours, surface osteosarcomas such as the parosteal or periosteal subtype mainly behave like low grade malignant tumours, which only require surgical treatment with wide resection margins; high grade surface osteosarcoma is an exception.
Identifying those patients who respond poorly to treatment at the time of surgery would facilitate preoperative allocation of these patients to special chemotherapy or a more aggressive regimen with the purpose of prolonging survival. Therefore, several factors such as tumour type, age at the onset of disease, tumour location, and tumour size have been investigated. Tumour size, tumour location, and response to chemotherapy were found to predict the outcome of patients.4 Among the different proteins that are expressed by osteosarcoma cells, bone morphogenetic proteins (BMPs) were postulated to be of prognostic value.5, 6 BMPs were found to be the main source of ectopic bone formation when demineralised bone fragments are implanted either subcutaneously or intramuscularly in animals.7 Devitalised tissue of human osteosarcoma also induced bone formation in athymic nude mice.8, 9 BMP subtypes 2–9 are the best characterised of all the BMPs. However, their number is still growing. The proteins belong to the transforming growth factor β (TGF-β) superfamily because they share similar amino acid sequences. Although mesenchymal cells such as osteoblasts or chondrocytes are the main targets of these proteins, certain subtypes such as BMP-6 are also important for the differentiation of epithelial cells.10
“Identifying those patients who respond poorly to treatment at the time of surgery would facilitate preoperative allocation of these patients to special chemotherapy or a more aggressive regimen with the purpose of prolonging survival”
Based on two former studies with smaller cohorts of patients,5, 6 we examined the expression of different BMPs by immunohistochemistry in 47 osteosarcoma specimens using commercially available standardised antibodies against six different BMP subtypes.
We analysed the correlations between the expression of BMPs and response to chemotherapy, overall survival, and disease free survival.
MATERIAL AND METHODS
Patients
Forty seven patients with osteosarcoma of bone were selected from the files of the department of clinical pathology at the Vienna General Hospital. All cases were reviewed to confirm the diagnosis and to select a paraffin wax block for immunohistochemical studies. The tumours were all intramedullary high grade osteosarcomas and consisted of the following types: 31 osteoblastic osteosarcomas, 14 chondroblastic osteosarcomas, one teleangiectatic, and one small cell osteosarcoma.
The patients underwent treatment between 1992 and 1999 at the department of orthopaedics in the Vienna General Hospital. All patients were initially treated for primary tumours.
Specimens of callus tissue with reactive osteoblasts were used as controls.
Immunohistochemistry
Freshly cut serial sections were used for immunohistochemical studies with antibodies against BMP-2/4, BMP-3, BMP-5, BMP-6, BMP-7, BMP-8, respectively, and corresponding sections were stained with haematoxylin and eosin.
Immunohistochemical staining was performed on paraffin wax embedded sections using goat polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, California, USA). The antibody to BMP-2/4 is raised against a peptide mapping close to the C-terminus of the BMP-4 precursor of human origin (differs from the corresponding BMP-2 sequence by two amino acids), whereas the antibodies to BMP subtypes 3–8 are raised against a peptide mapping to the N-terminus of the corresponding BMP precursor of human origin. Sections were pretreated by autoclave heating in citrate buffer (pH 6.0) for 20 minutes at 1 Bar. Dilutions of primary antibodies varied (anti-BMP-2/4 (A-20), 1/600; anti-BMP-3 (N-19), 1/800; anti-BMP-5 (N-19), 1/600; anti-BMP-6 (N-19), 1/800; anti-BMP-7 (N-19), 1/800; and anti-BMP-8 (N-19), 1/600). After incubation at room temperature for one hour, the secondary biotinylated goat antirabbit IgG antibody (1/100 dilution) was applied for 30 minutes, followed by incubation with the avidin–biotin complex with alkaline phosphatase (ABC-AP kit; Vector Laboratories Burlingame, California, USA). The reaction was developed with a fast red chromogen system (Coulter Immunotech, Marseille, France). For all antibodies, non-specific reactivity was assessed by omitting the primary antibody.
Immunohistochemical staining for BMPs was evaluated and the specimens were scored according to the distribution of positive cells within the tumour. Immunostaining results were graded as described previously, with a slight modification11 (++, 50–95% of tumour cells positive; +, 10–49% of tumour cells positive; −, < 10% of tumour cells or no visible staining). The intensity of cytoplasmic staining was homogenous and was therefore not taken into account.
Statistical analysis
The Kruskal-Wallis test, the Mann-Whitney test, and the Friedman test with subsequent Wilcoxon tests with Bonferroni correction were used as appropriate. Overall survival was defined from the day of surgery until the death of the patient. Data concerning patients who survived until the end of the observation period were censored at their last follow up visit. Death from a cause other than osteosarcoma or survival until the end of the observation period were regarded as censoring events. Disease free survival was defined from the end of primary treatment until the first evidence of progression of disease. Univariate analysis of overall survival and disease free survival was performed as outlined by Kaplan and Meier.12 A two tailed p value ≤ 5% was considered significant. All statistical analysis was performed using the SPSS software (RE 10.0; SPSS, Chicago, Illinois, USA).
RESULTS
Clinicopathological data
The ages of patients ranged from 6 to 65 years (median, 21). The peak incidence of osteosarcomas was registered in the second decade of life. Twenty nine patients were men and 18 were women.
Three tumours were located in the pelvis, including two in the sacrum and one in the ilium. All other tumours were located in the extremities, including 23 in the femur, 14 in the tibia, two in the fibula, four in the humerus, and one in the intermediate cuneiform bone. The treatment consisted of preoperative multiagent chemotherapy according to two different protocols of the cooperative osteosarcoma study (COSS) trials13 (COSS 86c, n = 24; COSS 96, n = 23), followed by surgical resection with wide resection margins in all cases. At the time of surgery three tumours were confined to bone and 44 had invaded the adjacent soft tissue. The resected specimens were analysed histologically for response to chemotherapy according to the criteria of Salzer-Kuntschik and colleagues14; 20 patients were classified as responders and 27 as non-responders.
The duration of follow up for survivors ranged from 3.5 months to 8.5 years (median, three years) from the date of surgery. During this period, 19 patients developed metastasis exclusively in the lung and one patient had a local recurrence. Sixteen patients died of tumour progression.
Immunohistochemistry
Immunohistochemical investigation revealed cytoplasmic staining in osteoblasts of control tissue. With the exception of one osteoblastic osteosarcoma all tumours, including the single cases of small cell osteosarcoma and teleangiectatic osteosarcoma, showed variable positive labelling with all antibodies. Table 1 gives detailed results of the immunohistochemical staining. Taken together, 28 of the 47 osteosarcomas expressed BMP-2/4, 24 expressed BMP-3, 41 expressed BMP-5, 31 expressed BMP-6, 43 expressed BMP-7, and 42 expressed BMP-8.
Table 1.
Immunohistochemical analysis of BMP expression in 47 osteosarcomas
| Antibody | ++ | + | − | Total BMP positive (%) |
| BMP-2/4 | 4 | 24 | 19 | 28 (59) |
| BMP-3 | 4 | 20 | 23 | 24 (51) |
| BMP-5 | 19 | 22 | 6 | 41 (87) |
| BMP-6 | 13 | 18 | 16 | 31 (68) |
| BMP-7 | 27 | 16 | 4 | 43 (91) |
| BMP-8 | 15 | 27 | 5 | 42 (90) |
Immunostaining results are graded as follows: ++, 50–95% of tumour cells positive; +, 10–49% of tumour cells positive; −, less than 10% of tumour cells positive or no visible staining.
BMP, bone morphogenetic protein.
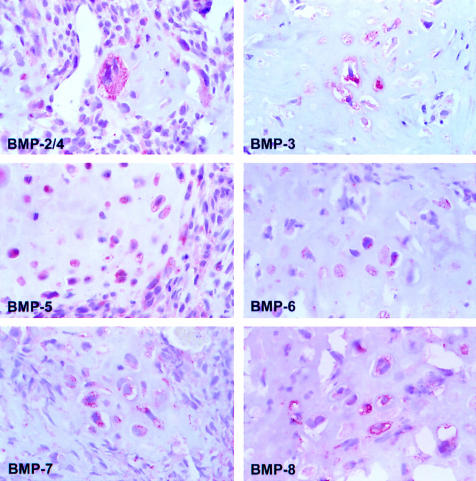
Figure 1 shows staining for the different BMPs in an osteoblastic osteosarcoma. The expression of BMP-2/4 and BMP-3 was significantly lower than that of BMP-5, BMP-7, and BMP-8. Table 2 shows details of the differences with corresponding p values.
Figure 1.
Cytoplasmic staining of bone morphogenetic proteins (BMPs) in osteoblastic osteosarcoma. Original magnification, ×400.
Table 2.
Significant differences between bone morphogenetic protein (BMP) subtypes (Wilcoxon test)
| BMPs | p Value |
| BMP-2/4 v BMP-5 | 0.015 |
| BMP-2/4 v BMP-7 | 0.015 |
| BMP-2/4 v BMP-8 | 0.015 |
| BMP-3 v BMP-5 | 0.015 |
| BMP-3 v BMP-7 | 0.015 |
| BMP-3 v BMP-8 | 0.015 |
| BMP-5 v BMP-6 | 0.015 |
| BMP-6 v BMP-7 | 0.015 |
The expression of BMP-6 correlated with chondroid differentiation (p = 0.001). Figure 2 provides detailed information about the expression of BMP-6 in osteosarcomas with chondroid differentiated parts compared with all other examined osteosarcomas. No other protein showed a correlation with an osteosarcoma subtype.
Figure 2.
The left hand figure shows the distribution of bone morphogenetic protein 6 (BMP-6) expression in all osteoblastic osteosarcomas, including the single cases of teleangiectatic and small cell osteosarcoma. The right hand figure shows the distribution in osteosarcomas with chondroid differentiated parts. High expression of BMP-6 correlated with chondroid differentiation (p = 0.001, Mann-Whitney test).
Response to chemotherapy, overall and disease free survival analysis
No correlation was found between response to chemotherapy and the expression of BMPs (p > 0.05, Mann-Whitney test). Overall and disease free survival analysis showed no significant correlation with high or low expression of the examined proteins (p > 0.05, log rank test).
DISCUSSION
Our study documents the expression of different BMP subtypes in osteosarcoma. With the use of commercially available antibodies against six proteins of the BMP family, variable degrees of expression of these proteins were detected in osteosarcoma. Moreover, a correlation was found between BMP-6 expression and chondroid differentiation. Because BMP-6 has been shown to be one of the major mitogens of chondrogenesis in recent studies using human and mouse cell cultures of bone marrow stromal cells,15, 16 the protein may also be of importance in the formation of neoplastic cartilage. The addition of BMP-6 to micromass cultures induces the growth and maturation of these cells towards chondrocytes.15 Further investigations are needed to establish whether BMP-6 is expressed in cartilage tumours. In an earlier study,17 the expression of BMP2/4 was examined in two specimens of chondroblastic osteosarcoma, but no detectable staining was found. The divergence between these findings and ours might result from the small number of tumours examined in the earlier study and the use of different BMP subtypes
In our investigation, BMP-7 and BMP-8 were highly expressed in all tumours examined. BMP-5 also showed a high degree of expression, whereas the remaining proteins were found in about a half of the cases. BMP-2, BMP-4, and BMP-7 have been listed as some of the most important promoters of osteoblast differentiation.18 In contrast to these members of the TGF-β superfamily, BMP-8 has not yet been examined with regard to its role in osteoblast or cartilage differentiation. BMP-8 shows more than 70% sequence homology with BMP-6 or BMP-5 and has been found in mouse embryos but not in adult mice, suggesting an early developmental role.19 However, in our present study, high expression of this protein was found in most of the human osteosarcoma specimens, suggesting its importance in malignant bone tumours.
“Neither response to preoperative chemotherapy nor disease free or overall survival correlated with a high degree of expression of BMPs in osteosarcoma”
A further aim of our study was to re-evaluate BMPs as prognostic factors for patients with osteosarcoma. Based on two previous studies,5, 6 BMPs have frequently been cited as prognostic markers over the past decade. These studies measured bone morphogenetic activity in human osteosarcoma specimens. Such activity was demonstrated as ectopic formation of new bone on implanted freeze dried fractions of human osteosarcomas into athymic nude mice. Ectopic bone formation was attributed to the production of BMPs in the tumour. The cohort of examined cases in these studies was small, consisting of 20 and 30 patients, respectively.5, 6 Bone morphogenetic activity was seen in approximately one third of patients in both studies. A correlation of bone morphogenetic activity with response to chemotherapy showed relative resistance to preoperative regimens consisting of adriamycin and methotrexate in patients with bone morphogenetic activity.5 In both studies, bone morphogenetic activity correlated with a high tendency to metastasise. In one study,6 the five year survival rate of patients with bone morphogenetic activity was significantly shorter. Their explanation for this correlation is the theory of further differentiation of osteosarcomas with bone morphogenetic activity compared with those without evidence of ectopic bone formation, which could correlate with resistance to anticancer drugs. This theory was not corroborated by our study, in which we obtained direct evidence of BMP expression in osteosarcoma.
Neither response to preoperative chemotherapy nor disease free or overall survival correlated with a high degree of expression of BMPs in osteosarcoma. One reason for this striking difference may have been the diverse methods of evaluation. Immunohistochemistry does not provide evidence of bone formation induced by BMPs, but is a standardised and repeatable procedure for detecting protein expression in tumour tissue. Moreover, our study comprised a larger cohort of patients who were treated with advanced chemotherapy regimens.
A wide range of osteosarcoma markers have been reported to be of prognostic value.20 These include molecular markers such as p53, p-glycoprotein, the multidrug resistance gene, human epidermal growth factor receptor, and metallothionin expression. All these markers were investigated in tumour tissue. Serological markers would be even more helpful to modify therapeutic strategies in advance. However, reports concerning the importance of markers such as alkaline phosphatase or lactate dehydrogenase are controversial.21–23
Take home messages.
Bone morphogenetic protein 7 (BMP-7) and BMP-8 are highly expressed in osteosarcoma
High expression of BMP-6 appeared to correlate with a chondroid differentiation
Although BMPs have been frequently cited as prognostic markers over the past decade, we found that the expression of BMPs did not help to predict the outcome of patients
In summary, we detected high expression of different BMP subtypes in human osteosarcoma. BMP-7 and BMP-8 in particular are highly expressed in osteosarcomas, regardless of their differentiation. High BMP-6 expression was found in chondroid differentiated parts of osteosarcoma. In our study BMP expression did not provide predictive information about the response to preoperative chemotherapy or the outcome of patients.
Abbreviations
BMP, bone morphogenetic protein
COSS, cooperative osteosarcoma study
TGF-β, transforming growth factor β
REFERENCES
- 1.Dorfman HD, Czerniak B. Bone tumors. St Louis: Mosby,1998.
- 2.Unni KK. Osteosarcoma. In: Dahlin's bone tumors. General aspects and data on 11.087 cases, 5th ed. Philadelphia: Lippincott-Raven, 1996:143–84.
- 3.Mirra JM, Gold RH, Picci P. Osseous tumors of intramedullary origin. In: Mirra JM, ed. Bone tumors: diagnosis, treatment and prognosis, 1st ed. Philadelphia: Lea & Febiger, 1989:143–438.
- 4.Raida M, Sarbia M, Clement JH, et al. Expression, regulation and clinical significance of bone morphogenetic protein 6 in eosophageal squamous-cell carcinoma. Int J Cancer 1999;83:38–44. [DOI] [PubMed] [Google Scholar]
- 5.Yoshikawa H, Takaoka K, Hamada H, et al. Clinical significance of bone morphogenetic activity in osteosarcoma. A study of 20 cases. Cancer 1985;56:1682–7. [DOI] [PubMed] [Google Scholar]
- 6.Yoshikawa H, Takaoka K, Masuhara K, et al. Prognostic significance of bone morphogenetic activity in osteosarcoma tissue. Cancer 1988;61:569–73. [DOI] [PubMed] [Google Scholar]
- 7.Urist MR. Bone: formation of autionduction. Science 1965;150:893–9. [DOI] [PubMed] [Google Scholar]
- 8.Bauer FCH, Urist MR. Human osteosarcoma-derived soluble bone morphogenetic protein. Clin Orthop 1981;154:291–5. [PubMed] [Google Scholar]
- 9.Urist MR, Grant TT, Lindholm TS, et al. Induction of new bone-formation in the host bed by human bone-tumor transplants in athymic nude mice. J Bone Joint Surg 1979;61A:1207–16. [PubMed] [Google Scholar]
- 10.Bielack S, Kempf-Bielack B, Schwenzer D, et al. Neoadjuvant therapy for localized osteosarcoma of extremities. Results from the cooperative osteosarcoma study group COSS of 925 patients. Klin Padiatr 1999;211:260–70. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa H, Rettig WJ, Takaoka K, et al. Expression of bone morphogenetic proteins in human osteosarcoma. Cancer 1994;73:85–91. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Non parametric estimation from incomplete observations. Journal of the American Statistical Association 1985;53:457–81. [Google Scholar]
- 13.Winkler K, Bielack S, Delling G, et al. Effect of intraarteriel versus intravenous cisplatin in addition to systemic doxorubicin, high-dose methotrexate, and ifosfamide on histologic tumor response in osteosarcoma (COSS-86). Cancer 1990;66:1703–10. [DOI] [PubMed] [Google Scholar]
- 14.Salzer-Kuntschik M, Brand G, Delling G. Bestimmung des morphologischen Regressionsgrades nach Chemotherapie bei malignen Knochentumoren. Pathologe 1983;4:135–41. [PubMed] [Google Scholar]
- 15.Sekiya I, Colter DC, Prockop DJ. Bmp-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem Biophys Res Commun 2001;8:411–18. [DOI] [PubMed] [Google Scholar]
- 16.Akiyama H, Shukunami C, Nakamura T, et al. Differential expressions of BMP family genes during chondrogenic differentiation of mouse ATDC5 cells. Cell Struct Funct 2000;25:195–204. [DOI] [PubMed] [Google Scholar]
- 17.Mehdi R, Shimizu T, Yoshimura Y, et al. Expression of bone morphogenetic protein and its receptors in osteosarcoma and malignant fibrous histiocytoma. Jpn J Clin Oncol 2000;30:272–5. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt JM, Hwang K, Shelley RW, et al. Bone morphogenetic proteins: an update on basic biology and clinical relevance. J Orthop Res 1999;17:269–78. [DOI] [PubMed] [Google Scholar]
- 19.Ozkaynak E, Schnegelsberg PN, Jin DF, et al. Osteogenic protein-2. A new member of the transforming growth factor-beta superfamily expressed early in embryogenesis. J Biol Chem 1992;267:25220–7. [PubMed] [Google Scholar]
- 20.Trieb K, Kotz R. Proteins expressed in osteosarcoma and serum levels as prognostic factors. Int J Biochem Cell Biol 2001;33:11–17. [DOI] [PubMed] [Google Scholar]
- 21.Bacci G, Ferrari S, Picci P, et al. Methotrexate serum concentration and histologic response to multiagent primary chemotherapy for osteosarcoma of the limbs. J Chemother 1996;8:472–8. [DOI] [PubMed] [Google Scholar]
- 22.Pochgool L, Subhadharaphandou T, Dhanachai M, et al. Prognostic factors among 130 patients with osteosarcoma. Clin Orthop 1997;345:206–14. [PubMed] [Google Scholar]
- 23.Meyers PA, Heller G, Healy JH, et al. Osteogenic sarcoma with clinically detactable metastasis at initial presentation. J Clin Oncol 1993;11:449–53. [DOI] [PubMed] [Google Scholar]