Abstract
Aims: Transporter proteins known to mediate multidrug resistance (MDR) in tumour cells—MDR1 P-glycoprotein (P-gp) and multidrug resistance related protein 1 (MRP1)—are thought to be involved in protecting the lungs against inhaled toxic pollutants. Recently, several new transporter family members have been identified—for example, MRP2, MRP3, and breast cancer resistance protein (BCRP). To study the possible contribution of these proteins and the earlier defined MDR1 and MDR3 P-gp molecules, MRP1, and the major vault protein (MVP) to lung functioning, their expression was analysed in normal lung tissue of humans and several animal species.
Methods: Frozen sections of normal lung tissues were examined for the expression of the multidrug resistance associated proteins, using an extended panel of monoclonal antibodies that specifically detect these proteins in immunohistochemical techniques.
Results: In line with earlier reports, the expression of MDR1 P-gp and MRP1 was readily detected in the apical and basolateral membranes, respectively, of the epithelial cell layers of the lungs. In addition, prominent cytoplasmic MVP staining was detected in these layers. In contrast, the recently discovered transporters were either undetectable or they were present at very low values in lung tissue. Immunohistochemical staining in tissues from mice, rats, and guinea pigs points to a strong evolutionary conservation for these transporter proteins.
Conclusions: These results show that the “classic” MDR related molecules, MDR1 P-gp, MRP1, and MVP, should be considered the most important transporters in normal lung physiology. It will be of great interest to investigate differences in expression of both classic and newly defined transporters between normal individuals and—for example, patients with various bronchopulmonary pathological conditions.
Keywords: multidrug resistance transporter molecules, monoclonal antibodies, lung
Respiratory tissue comes into contact with a broad variety of potentially harmful and toxic substances that are present in inhaled air. Although microorganisms, dust, and toxic particles are largely removed in the upper airways via ciliary movements and mucus, protection against harmful substances is also critically important in the lower airways. Surfactant, important for lung elasticity and produced by type II pneumocytes, is bacteriocidal and the epithelial lining fluid and the alveolar macrophages play a crucial role in the removal of macromolecules. Other candidates that may play a role in the defence against toxic materials are the transporter molecules, originally characterised for their roles in multidrug resistance (MDR; reviewed by Moscow et al).1 Increased expression of these transporter molecules in tumour cells renders these cells resistant to structurally and functionally unrelated cytotoxic drugs. The proteins involved in this phenomenon are the well known MDR1 P-glycoprotein (P-gp; ABCB1),2 multidrug resistance protein 1 (MRP1; ABCC1),3 and the major vault protein (MVP),4 and the more recently discovered MRP2 (ABCC2),5 MRP3 (ABCC3),6 and the breast cancer resistance protein (BCRP; ABCG2).7 Except for the MVP molecule, all of these proteins are members of the ATP binding cassette (ABC) transporter family.8 They act as efflux pumps, resulting in decreased intracellular concentrations of natural product drugs.
“Respiratory tissue comes into contact with a broad variety of potentially harmful and toxic substances that are present in inhaled air”
The 170 kDa MDR1 P-gp is the prototypic MDR transporter protein that transports a broad range of hydrophobic, amphiphilic substrates, in addition to organic cations, including anticancer drugs.2 MDR3 P-gp (ABCB4) is 77% identical to MDR1 P-gp, but for this protein no contribution to MDR has been established. The protein is essential for the secretion of phosphatidylcholine into the bile,9 and lack of expression of MDR3 P-gp in the liver is responsible for type 3 progressive familial intrahepatic cholestasis.10 The 190 kDa MRP1 protein is the major cysteinyl leukotriene C4 (LTC4) transporter,11 and it confers a similar resistance phenotype to MDR1 P-gp,12 although these two proteins share only 14% amino acid identity. The transporter shows a propensity for transporting organic anions. In addition, MRP1 is probably involved in antioxidant defence mechanisms through transport of the anionic tripeptide glutathione and xenobiotic-glutathione conjugates. In humans at least eight homologues of MRP1 are now known to be expressed,13–16 namely: MRP2–9. MRP2 is found in the liver and is responsible for the hepatobiliary excretion of a broad range of organic anions, including glutathione and bilirubin glucuronides,17 and mutations in the MRP2 gene cause the Dubin-Johnson syndrome.5 Apparently, the substrate specificity of MRP2 is very similar to that of MRP1.17 MRP3 is the closest homologue of MRP1,6 and it is also capable of transporting several anticancer drugs.18 The involvement of the other members of the MRP family in MDR in tumour cells has not yet been established, but recently it was found that MRP4 and MRP5 can mediate transport of nucleoside analogues.19,20
BCRP is a 70 kDa transporter protein that probably acts as a (hetero/homo) dimer in transporting MDR drugs. This transporter is known to be involved in mitoxantrone and topotecan resistance,21,22 and may also be involved in the resistance to other drugs. The MVP molecule is not a family member of the ABC transporters. This approximately 100 kDa protein constitutes the major component of vault particles, large cytoplasmic ovoid shaped structures with yet unknown function(s). Importantly, several reports have shown a connection between the expression of MVP/vaults and MDR (see Scheffer and colleagues23 and references therein).
To facilitate further studies on the contribution of these molecules to MDR and their physiological functions—for example, in lung tissues—we have developed a panel of monoclonal antibodies that specifically detect the MDR1 P-gp and MDR3 P-gp molecules, MRP1, MRP2, MRP3, BCRP, and MVP.24–29 Studies on the presence of MDR1 P-gp, MRP1, and MVP in normal human tissues, including lung and liver, have been reported,30–33 and the results suggest that the normal physiological function of these molecules is to provide protection against toxic substances. Here, we used the extended monoclonal antibody panel, including at least two different monoclonal antibodies for each MDR related molecule, to investigate further the presence and possible roles of the recently discovered transporter molecules in the lungs. Their expression was examined in frozen sections reflecting different airway levels of normal human lung tissue and, as a control, in liver tissue. Because detection in archival material would be most convenient for in depth studies on clinical specimens, we also explored the applicability of the extended monoclonal antibody panel in formalin fixed, paraffin wax embedded tissues. Moreover, because animal models can provide invaluable information in mechanistic studies on lung diseases, such as asthma, we further investigated the applicability of these monoclonal antibodies to frozen sections of lung tissues from the rat, mouse, and guinea pig.
MATERIALS AND METHODS
Tissues and sections
Frozen tissues of normal human lung and liver were obtained from our tissue bank. The lung tissue samples originate from different regions of normal parts of removed malignant lung tissue from six individuals. Rat, mouse, and guinea pig tissues were obtained from euthanised animals, snap frozen in liquid nitrogen and stored at −80°C. Cryosections (4 μm thick) were cut, airdried overnight, fixed for seven minutes in 100% acetone, and stored at −20°C until further use. Formalin fixed, paraffin wax embedded tissue blocks of normal human tissues were from our tissue bank. Sections (4 μm thick) were cut, mounted on to poly-L-lysine pretreated slides, and dried overnight.
Monoclonal antibodies
Except for the anti-P-gp monoclonal antibody, C219, all antibodies used in our study were produced in our laboratory. These monoclonal antibodies were kept as concentrated supernatants and were used in the immunohistochemical staining at a final concentration of approximately 20 μg/ml. The C219 monoclonal antibody was purchased from Alexis (San Diego, California, USA) and used at the recommended dilution. MDR1 P-gp detection was with the JSB-1 and C219 monoclonal antibodies, MDR3 P-gp detection was with P3II-1 and P3II-26. MRP1 detection was with MRPr1, MRPm6, and MRPm5. MRP2 detection was with M2I-4, M2II-12, M2III-5, and M2III-6. MRP3 detection was with M3II-9 and M3II-21. BCRP detection was with BXP-21 and BXP-34. MVP detection was with LRP-56, LMR-5, and MVP-37. All monoclonal antibodies are murine antibodies, except for MRPr1 and LMR-5, which are rat antibodies. The characteristics of the monoclonal antibodies have been described in detail previously.24–29,31,34,35
Immunohistochemistry
Cytospin preparations and cryosections (4 μm thick) were airdried overnight and fixed for seven minutes in acetone at room temperature. Sections of routinely processed formalin fixed, paraffin wax embedded tissues (4 μm thick) were dewaxed and rehydrated. Endogenous peroxidase activity was blocked using 0.3% H2O2 in methanol for 30 minutes. Antigen was detected without pretreatment, or after microwave antigen retrieval using 1mM EDTA or 0.01M citric acid (pH 6.0) in distilled water. The slides were incubated with hybridoma supernatant for one hour at room temperature or overnight at 4°C for the paraffin wax sections. Biotinylated rabbit antimouse or antirat serum (1/150 dilution; Zymed, San Francisco, California, USA) and horseradish peroxidase (HRP) labelled streptavidin (1/500 dilution; Zymed) were used as secondary reagents. Colour development was with 0.4 mg/ml aminoethylcarbazole and 0.02% H2O2 as a chromogen. Nuclei were counterstained with haematoxylin and the slides were mounted with Kaiser's glycerol gelatin (Merck, Darmstadt, Germany).
For MVP-37 staining of cryosections, a paraformaldehyde fixation in combination with guanidine hydrochloride pretreatment was applied, as described previously.29
For tissues with high endogenous biotin activity, incubation with hybridoma supernatant was followed by HRP labelled rabbit antimouse or antirat serum (1/200; Dako, Copenhagen, Denmark). Subsequently, a 10 minute incubation with fluorescein isothiocyanate (FITC) labelled tyramine in phosphate buffered saline (PBS) containing 0.01% H2O2 was performed. The slides were examined under a fluorescence microscope (Leica DMRB, Rijswijk, the Netherlands). To obtain more permanent results and to provide a better impression of morphology, the slides were further incubated with HRP labelled rabbit F(ab`)2 anti-FITC fragments (1/100 dilution; Dako) and developed with aminoethylcarbazole/H2O2.
Negative controls consisted of replacing the primary antibody with irrelevant isotype matched control antibodies.
RESULTS
Sections of different airway levels of normal human lung tissue were cut and examined for expression of the transporter proteins. This approach allowed the analysis of bronchial epithelium and mucus producing goblet cells, seromucinous glands, smooth muscle, and nerve cells in the higher part of the lungs and bronchiolar epithelial cells, endothelial cells (type I/II), pneumocytes, and alveolar macrophages in the lower regions of the lung. In normal human liver, sections were examined for the expression of transporter proteins in the hepatocytes, bile ductules and canaliculi, and blood circulatory tissue.
Frozen sections of normal human lung and liver tissues
Table 1 summarises the results of immunohistochemical staining in the lung and liver tissues. For comparison, previously reported mRNA data (MDR1 P-gp,36 MDR3 P-gp,37 MRP1/2/3,13 and BCRP38) on the expression of the transporters are also shown in table 1. Only positive staining results obtained with the panel of MDR monoclonal antibodies will be outlined in more detail. Thus, when not specifically mentioned, lung tissue zones and cell types can be considered as unreactive.
Table 1.
Detection of MDR molecules with a panel of monoclonal antibodies in frozen sections of normal human lung and liver tissue
| MDR molecule | Mab | Lung epithelium | Liver canaliculi | ||
| mRNA | Staining | mRNA | Staining | ||
| MDR1 P-gp | JSB-1 | + (33) | +* | ++ (33) | ++ |
| C219 | ++* | +++ | |||
| MDR3 P-gp | P3II-1 | − (34) | − | ++ (34) | ++ |
| P3II-26 | +/− | +++ | |||
| MRP1 | MRPr1 | ++ (13) | +++*† | − (13) | − |
| MRPm6 | +*† | − | |||
| MRPm5 | +*† | − | |||
| MRP2 | M2I-4 | − (13) | − | +++ (13) | +++ |
| M2II-12 | − | ++ | |||
| M2III-5 | − | ++ | |||
| M2III-6 | ++ | ++ | |||
| MRP3 | M3II-9 | +/− (13) | − | + (13) | −§ |
| M3II-21 | − | −§ | |||
| BCRP | BXP-21 | − (35) | +/−‡ | +/− (35) | + |
| BXP-34 | +/−‡ | + | |||
| MVP | LRP-56 | ND | ++* | ND | +/− |
| LMR-5 | +* | − | |||
| MVP-37 | ++* | − |
mRNA data are from published studies, with the reference in parenthesis. *, Also variable staining in alveolar macrophages; †, also staining in seromucinous glands; ‡, also staining of endothelial cells; §, staining of bile ducts. Staining scores: −, no reactivity; +/−, very weak reactivity; +, weak reactivity; ++, strong reactivity; +++, very strong reactivity.
BCRP, breast cancer resistance protein; Mab, monoclonal antibody; MDR, multidrug resistance; MRP, multidrug resistance protein; MVP, major vault protein; ND, not determined; P-gp, P-glycoprotein.
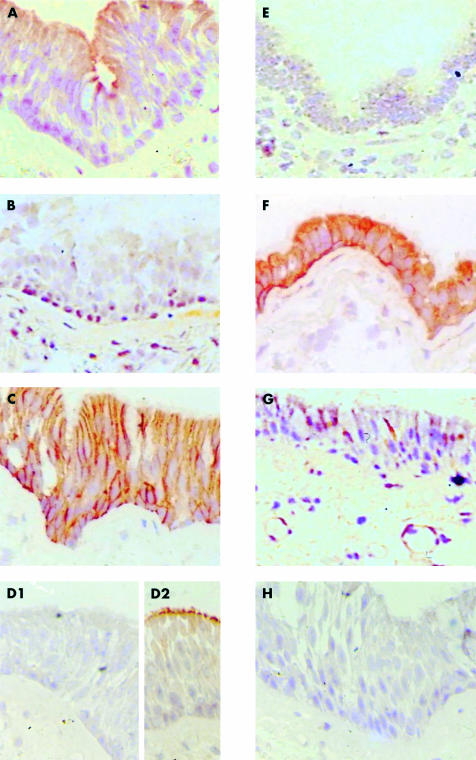
MDR1 P-gp was detected with both the JSB-1 and C219 monoclonal antibodies on the apical membranes of the bronchial and bronchiolar epithelium in the lung (fig 1A) and on the (apical) canalicular membranes in the liver (not shown; see example of canalicular staining in fig 2A). Moderate but variable staining of the alveolar macrophages was seen. The MDR3 P-gp monoclonal antibodies P3II-1 and P3II-26 showed that MDR3 P-gp, which can be readily detected at the canalicular membranes in the liver (not shown), is absent in lung tissue (fig 1B), thus confirming the mRNA data for MDR1 P-gp and MDR3 P-gp in liver and lung tissues. MRP1 was detected with the rat MRPr1 monoclonal antibody (which showed the strongest staining) and the mouse MRPm6 antibody in the basal membrane of bronchial and bronchiolar epithelium of the lung (fig 1), whereas it was not detected in liver tissue. In addition, MRP1 was present in the basal cells in the seromucinous glands of the lungs, with a higher intensity in the serous area than in the mucinous area (fig 3B). Furthermore, despite some variability between individual lung samples, consistent MRP1 expression was seen in the cytoplasm of the alveolar macrophages (fig 3A). Overall, these results agree with the mRNA data for MRP1 in lung and liver tissues. Three of the four anti-MRP2 monoclonal antibodies—M2I-4, M2II-12, and M2III-5—confirmed that MRP2 is not detectable in lung tissue (fig 1D1), as inferred from the mRNA data (table 1). An aberrant staining pattern was observed for the M2III-6 monoclonal antibody, which showed strong staining of the apical membrane of the bronchial and bronchiolar epithelial layers (fig 1D2). In contrast, without exception, all four anti-MRP2 monoclonal antibodies show the presence of MRP2 at the canalicular membranes (fig 2A). The anti-MRP3 monoclonal antibodies M3II-9 and M3II-21 showed that MRP3 is not present in lung tissue (fig 1E), whereas the protein can be detected in the liver. Staining of bile ductules was most pronounced, with some additional staining of the hepatocyte membranes (fig 2B), in line with the mRNA data for MRP3 in both lung and liver tissues. The anti-BCRP antibodies BXP-21 and BXP-34 revealed low but distinct amounts of BCRP in the epithelial layer and in the seromucinous glands of the lungs (fig 1G). Moreover, BCRP was detected in the small endothelial capillaries. In the liver, these monoclonal antibodies showed moderate staining of the canalicular membranes (not shown). These results are also in agreement with the BCRP mRNA data for these tissues (table 1). In the lung, the anti-MVP monoclonal antibodies LRP-56, LMR-5, and MVP-37 stained strongly in the cytoplasm of the bronchial and bronchiolar epithelial layers (fig 1F) and slightly less strongly in the alveolar macrophages. In the liver, MVP was very low or not detectable, as reported previously.33,39
Figure 1.
Expression of MDR related proteins in frozen sections of normal human lung tissue as detected with (A) C219 (anti-MDR1 P-gp), (B) P3II-26 (anti MDR3 P-gp), (C) MRPr1 (anti-MRP1), (D1) M2II-12 (anti MRP2), (D2) M2III-6 (anti MRP2), (E) M3II-9 (anti-MRP3), (F) LRP-56 (anti-MVP), (G) BXP-34 (anti-BCRP), and (H) control monoclonal antibody. MDR1 P-gp is present in the apical membrane of the bronchial and bronchiolar epithelial layer. MRP1 is present at the basolateral membrane of the bronchial and bronchiolar layer. Abberant MRP2 staining is only seen with M2III-6. MVP is present in the cytoplasm of the bronchial and bronchiolar epithelial cells. Slides were stained with HRP labelled rabbit antimouse, FITC labelled tyramine, HRP labelled rabbit anti-FITC, and aminoethylcarbazole. BCRP, breast cancer resistance protein; FITC, fluorescein isothiocyanate; HRP, horseradish peroxidase; MDR, multidrug resistance; MRP, multidrug resistance protein; MVP, major vault protein; P-gp, P-glycoprotein.
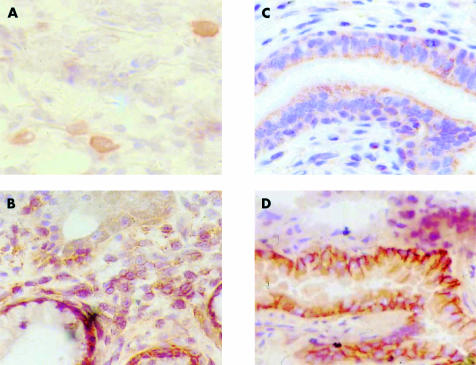
Figure 2.
Expression of MDR related proteins in normal human liver tissue. (A) MDR1 P-gp, MDR3 P-gp, MRP2, and BCRP show similar staining at the canalicular membranes of the hepatocytes (example of staining with M2I-4). (B) MRP3 is present in the bile ducts and the basolateral membranes of the hepatocytes (staining with M3II-9). (C) negative control. Slides were stained with HRP labelled rabbit antimouse, FITC labelled tyramine, HRP labelled rabbit anti-FITC, and aminoethylcarbazole. BCRP, breast cancer resistance protein; FITC, fluorescein isothiocyanate; HRP, horseradish peroxidase; MDR, multidrug resistance.
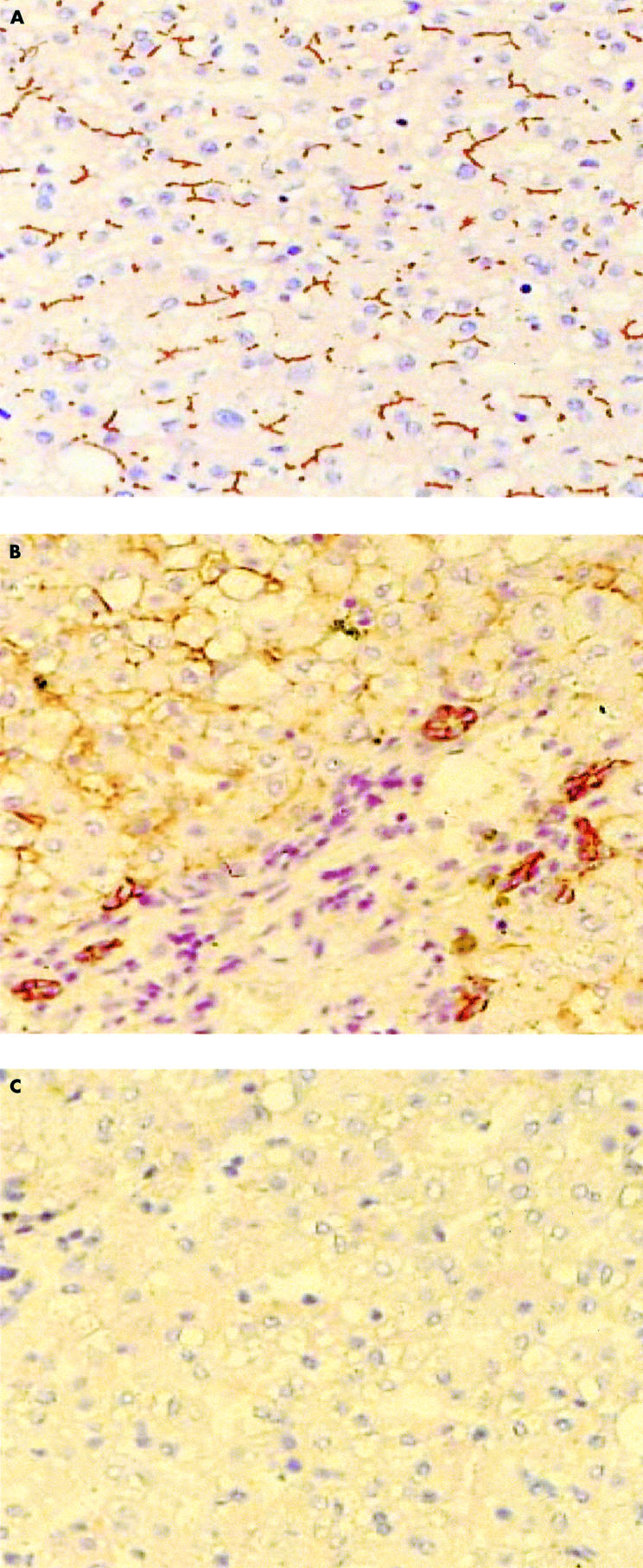
Figure 3.
Expression of MRP1 as detected with the rat MRPr1 monoclonal antibody in different types of lung tissue. (A) MRP1 staining in alveolar macrophages in frozen sections of normal human lung. (B) MRP1 staining in a seromucinous gland in a frozen section of the upper part of the lung. (C) MRP1 staining in formalin fixed, paraffin wax embedded normal human lung tissue. (D) mrp1 staining in frozen sections of mouse lung tissue. Slides were stained with HRP labelled rabbit antimouse, FITC labelled tyramine, HRP labelled rabbit anti-FITC, and aminoethylcarbazole. FITC, fluorescein isothiocyanate; HRP, horseradish peroxidase; MRP, multidrug resistance protein.
Formalin fixed, paraffin wax embedded lung and liver tissues
For some monoclonal antibodies in the panel reactivity (or lack of reactivity) on formalin fixed, paraffin wax embedded tissues has been reported previously25,29,39; the anti-MDR1 P-gp antibodies, the anti-MRP1 antibodies, and the anti-MRP2 antibodies perform well on this material, with a microwave antigen retrieval pretreatment in citrate buffer. However, with both MRPr1 and MRPm6 the localisation of MRP1 in the epithelial layers of the lungs differed from that seen in frozen sections. Instead of the basolateral staining seen in frozen sections, a more diffuse, cytoplasmic localisation was seen (fig 3C; compare with fig 1C). In addition, the anti-MVP monoclonal antibodies performed well on this material. However, in contrast, the newly developed anti-MDR3 P-gp monoclonal antibodies, P3II-1 and P3II-26, showed only limited reactivity, and the anti-MRP3 antibodies, M3II-9 and M3II-21, were non-reactive on tissue sections processed in this way.
To study the efficiency of the anti-BCRP monoclonal antibodies on formalin fixed, paraffin wax embedded material, several experiments were performed without pretreatment or using the two different pretreatment methods, citrate or EDTA. None of the conditions tested allowed for staining of the BCRP protein with the BXP-34 monoclonal antibody but, in contrast, the BXP-21 antibody was effective when the citrate method was used for antigen retrieval. Table 2 summarises the results and provides a semiquantitative rating for the reactivities of the monoclonal antibodies on formalin fixed, paraffin wax embedded material in comparison with the results obtained using frozen sections.
Table 2.
Detection of MDR molecules with a panel of monoclonal antibodies in formalin fixed, paraffin wax embedded normal human tissues and in frozen sections of different species
| MDR molecule | Mab | Paraffin wax embedded sections | Frozen sections | |||
| Human | Human | Rat | Mouse | Guinea pig | ||
| MDR1 P-gp | JSB-1 | + | ++ | − | − | − |
| C219 | ++ | +++ | ++ | + | ++ | |
| MDR3 P-gp | P3II-1 | +/− | ++ | +/− | − | +/− |
| P3II-26 | +/− | +++ | + | +/− | ++ | |
| MRP1 | MRPr1 | + | +++ | − | ++ | ++ |
| MRPm6 | + | + | − | − | − | |
| MRPm5 | +/− | + | − | − | − | |
| MRP2 | M2I-4 | + | +++ | − | − | − |
| M2II-12 | − | ++ | − | − | − | |
| M2III-5 | − | ++ | +++ | − | + | |
| M2III-6 | + | ++ | +++ | − | ++ | |
| MRP3 | M3II-9 | − | ++ | − | − | − |
| M3II-21 | − | ++ | − | − | − | |
| BCRP | BXP-21 | + | + | +/− | − | − |
| BXP-34 | − | + | +/− | − | − | |
| MVP | LRP-56 | + | ++ | − | − | − |
| LMR-5 | + | + | − | − | − | |
| MVP-37 | ++ | ++ | − | − | − |
Reactivity of MDR1 P-gp, MDR3 P-gp, MRP2, MRP3, and BCRP as judged on sections of liver, and reactivity of anti-MRP1 and MVP Mabs as judged on sections of lung. Staining scores: −, no reactivity; +/−, very weak reactivity; +, weak reactivity; ++, strong reactivity; +++, very strong reactivity.
BCRP, breast cancer resistance protein; Mab, monoclonal antibody; MDR, multidrug resistance; MRP, multidrug resistance protein; MVP, major vault protein; P-gp, P-glycoprotein.
Reactivity with MDR molecules in different species
For experimental studies into pulmonary diseases, it is important to know the applicability of the monoclonal antibodies in relevant animal species, notably mice, rats, and guinea pigs. Therefore, we tested the monoclonal antibody panel on frozen sections of liver and lungs from these animals. The results are also presented in table 2, extending the results published previously.25 Based on the presence or absence of the expected expression profile(s) in the examined tissues, we concluded that some of the monoclonal antibodies are highly specific for the human antigen (for example, BXP-34), whereas others (for example, P3II-26) seem to crossreact with orthologues in the other species examined. Obviously, with mouse monoclonal antibodies on mouse tissues and rat monoclonal antibodies on rat tissues, staining results are more difficult to interpret because of the high background staining. An example of mrp1 detection in mouse lung tissue with the rat monoclonal antibodies MRPr1 is shown in fig 3D.
DISCUSSION
A broad panel of monoclonal antibodies specifically detecting MDR1 P-gp, MDR3 P-gp, MRP1–3, BCRP, and MVP has become available and was used here to explore the distribution of these molecules within human lung tissues, where they might contribute to protection against exogenous toxins. Moreover, some of these transporters are assumed to play crucial roles in immune effector cell functions—for example, MDR1 P-gp and MRP1 in dendritic cells.40,41 Previously, we showed that all but one of the anti-P-gp monoclonal antibodies (C219) included in this panel are fully specific for their cognate antigens, both in western blots and cytological specimens.25,26,28 The C219 monoclonal antibody also reacts with MDR3 P-gp,42 which probably contributes to its apparently stronger immunoreactivity (compared with JSB-1) in organs coexpressing both P-gp transporters. Because all monoclonal antibodies can react with their respective antigen in frozen sections, primary analyses were performed on this type of material.
“The classic multidrug resistance related molecules mentioned are potentially the most important transporters in normal lung physiology—the newly discovered transporter molecules were either undetectable or present only at low values”
Indeed, distinct transporters were easily detected in lung tissue. Confirming earlier reports,30–32 MDR1 P-gp and, particularly, MRP1 were found prominently in the bronchial and bronchiolar epithelial layers. These proteins were found on opposite sites of the epithelial cell layer, with apically expressed MDR1 P-gp possibly being involved in removing environmental xenobiotics into the lumen, and basolaterally expressed MRP1 transporting toxic substances into the interstitial fluid. The aberrant staining pattern of MRP1 in sections of formalin fixed, paraffin wax embedded lung tissue, suggesting a more subapical, intracellular localisation, was seen here with two different monoclonal antibodies, and has also been reported previously with another MRP1 specific monoclonal antibody, QCRL-1.32 Therefore, this probably reflects artifactual aggregation and dislocation of the MRP1 molecule as a result of the fixation procedure, rather than discordant epitope expression. The putatively protective role of MRP1 is not limited to the epithelial layers because it is also expressed in the seromucinous glands and in alveolar macrophages. MRP1 staining in the epithelial layers varied only slightly between individual samples. However, variable numbers of stained macrophages and variable staining intensities for the macrophages were observed in individual lung samples. These observations may be related to different macrophage activational states, depending on different clinical sources. Notably, most of the “normal” lung tissue samples from the collection of our tissue bank are derived from non-affected parts of lungs obtained from patients with lung cancer, which may still show varying degrees of inflammation. Of note, MRP1 is the major transporter of LTC4, a cysteinyl leukotriene. Leukotrienes are potent proinflammatory mediators accounting for late phase asthmatic reactivity.43 Indeed, MRP1 knockout mice show strongly reduced inflammatory reactivity patterns.44 Our present results also confirm that a third MDR related protein, the major vault protein MVP, as detected by either of three different monoclonal antibodies, is highly expressed in the cytoplasm of the epithelial cells of the lung. Vaults are evolutionarily highly conserved, large ribonucleoprotein particles. The particles represent multimeric RNA–protein complexes in which MVP predominates. Although the cellular role of vaults has remained elusive, several findings support the view that vaults have a transport function by acting as a carrier, mediating bidirectional nucleocytoplasmic exchange as well as vesicular transport of compounds, including possible toxic materials (Scheffer and colleagues23 and references therein).
Our results show that the classic MDR related molecules mentioned are potentially the most important transporters in normal lung physiology—the newly discovered transporter molecules were either undetectable or present only at low values. Thus, in line with the earlier reported mRNA data, no MDR3 P-gp and MRP3 proteins were detectable in normal lung tissues. The same holds true for MRP2, which was positive with only one of the four monoclonal antibodies tested—the M2III-6 antibody. This staining of the apical membrane of the bronchial epithelium remained unconfirmed with the other three anti-MRP2 monoclonal antibodies and was not confirmed by detectable MRP2 mRNA. Moreover, M2III-6 staining was not visible in rat or guinea pig lung tissues, despite strong tissue reactivity in these species. Therefore, the M2III-6 antibody probably crossreacts with another molecule present on the human bronchial and bronchiolar epithelial surface. Its distinct localisation along the airways certainly warrants further identification of this protein(s). With regard to the other recently identified MRP homologues, such as MRP4–9, further studies are currently under way to raise appropriate monoclonal antibodies, which should help to reveal whether these closely related transporters play distinct roles in lung tissues. Interestingly, the other recently identified member of the ABC family, BCRP, showed low but distinct expression in the lung, notably in the epithelial layer and seromucinous glands, in addition to capillary endothelium. Because this molecule has been attributed an important role in the transmembrane transport of distinct classes of cytostatic drugs, and little is known about its possible contribution to physiological defence mechanisms, the importance of BCRP expression in lung tissues should be explored further. We used both acetone fixed frozen sections and formaldehyde fixed paraffin wax embedded sections in our study. Like most monoclonal antibodies raised against native molecules, fusion proteins, and/or peptides, the currently available panel shows excellent reactivities in western blotting and/or immunohistochemical methods, whereas the panel generally performs less well on paraffin wax embedded sections. However, our results indicate that antigen retrieval methods can be identified that provide acceptable conditions for the detection of each of these transporter molecules.
Take home messages.
The “classic” multidrug resistance (MDR) related molecules—MDR1 P-glycoprotein, multidrug resistance protein 1, and major vault protein—appear to be the most important transporters in normal lung physiology
Nevertheless, absence of the recently cloned members of the transporter superfamily in normal human lung does not preclude their possible role in pathological conditions
It will be of great interest to investigate differences in expression of both classic and newly defined transporters between normal individuals and—for example, patients with chronic obstructive diseases, asthma, or other pulmonary diseases
For mechanistic studies in human disease, including those affecting the lungs, experimental animal models remain indispensable, whether models involving conventional inbred or outbred mice, rats, or guinea pigs, or transgenic and knockout mice and rats. Because there is still much to be learned about the pathophysiological roles of MDR related molecules, and crucial patient tissue is often unavailable, appropriate reagents detecting animal MDR related molecules need to be defined. Our current data highlight those monoclonal antibodies that show essentially similar staining patterns in lung and liver tissues in mice, rats, and/or guinea pigs, indicating that these reagents react with true orthologues in these species. However, the panel needs to be extended; in particular, reagents detecting MRP3 and MVP/LRP need to be developed. Interestingly, all of the differently raised antihuman MVP monoclonal antibodies, including the mouse antibodies, LRP-56 and MVP-37, and the rat LMR-5, are highly specific for human epitopes. This may relate to the high degrees of homology for different species derived MVP molecules, suggesting strong evolutionary conservation, leading to low immunogenicity for larger parts of the molecule.
“For mechanistic studies in human disease, including those affecting the lungs, experimental animal models remain indispensable”
In conclusion, prominent expression of the classic MDR related transporters in normal human lung tissues supports the view that these proteins play crucial roles in the protection against xenobiotics. Nevertheless, absence of the recently cloned members of the transporter superfamily in normal human lung does not preclude their possible role in pathological conditions. Therefore, it will be interesting to explore the different expression profiles of the classic and newly defined transporters in normal individuals and—for example, patients with chronic obstructive diseases, asthma, or other pulmonary diseases.
New JCP online submission and review system.
We are pleased to inform authors and reviewers of the new online submission and review system at JCP. Developed by HighWire Press (CA, USA), Bench Press is a fully integrated electronic system that utilises the web to allow rapid and efficient submission of manuscripts. It also allows the peer review process to be conducted entirely online. We are one of the first journals in the BMJ Special Journals group to go online in this way. The aim, apart from saving trees, is to speed up the often frustratingly slow process (for both authors and editors) from submission to publication. Many reviewers might appreciate this too. Authors may submit their manuscript in any standard word processing software. Acceptable standard graphic formats include: jpeg, tiff, gif, and eps. The text and graphic files are automatically converted to PDF for ease of distribution and reviewing purposes. Authors are asked to approve their submission before it formally enters the reviewing process. On approval by the authors, the submission is passed to the editor and/or reviewers via the web. All transactions are secure.
To access the system click on “SUBMIT YOUR MANUSCRIPT HERE” on the JCP homepage: HYPERLINK http://www.jclinpath.com, or you can access Bench Press directly at HYPERLINK http://submit-jcp.bmjjournals.com.
We are very excited with this new development and would encourage authors and reviewers to use the online system whenever possible. As editors, we will use it all the time, the up side being lack of need to travel to the editorial office to deal with papers, the down side having no more excuses to postpone decisions on papers because we are “at a meeting”!
The system is very easy to use and should be a big improvement on the current peer review process. Full instructions can be found on Bench Press http://submit-jcp.bmjjournals.com and JCP online at http://www.jclinpath.com. Please contact Natalie Davies, Project Manager, HYPERLINK mailto: ndavies@bmjgroup.com for any further information.
H Hozel, P van Diest
Acknowledgments
This work was supported in part by the Netherlands Asthma Foundation grant AF 97.35 and Koningin Wilhelmina Fonds (KWF) grant VU 96–1256.
Abbreviations
ABC transporter, ATP binding cassette transporter
BCRP, breast cancer resistance protein
BSA, bovine serum albumin
FITC, fluorescein isothiocyanate
HRP, horseradish peroxidase
LTC4
cysteinyl leukotriene C4
MDR, multidrug resistance
MRP, multidrug resistance protein
MVP, major vault protein
PBS, phosphate buffered saline
P-gp, P-glycoprotein
REFERENCES
- 1.Moscow JA, Schneider E, Ivy SP, et al. Multidrug resistance. Cancer Chemother Biol Response Modif 1997;17:139–77. [PubMed] [Google Scholar]
- 2.Ambudkar SV, Dey S, Hrycyna CA, et al. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 1999;39:361–98. [DOI] [PubMed] [Google Scholar]
- 3.Cole SP, Deeley RG. Multidrug resistance mediated by the ATP-binding cassette transporter protein MRP. Bioessays 1998;20:931–40. [DOI] [PubMed] [Google Scholar]
- 4.Scheffer GL, Wijngaard PL, Flens MJ, et al. The drug resistance-related protein LRP is the human major vault protein. Nat Med 1995;1:578–82. [DOI] [PubMed] [Google Scholar]
- 5.Paulusma CC, Bosma PJ, Zaman GJ, et al. Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science 1996;271:1126–8. [DOI] [PubMed] [Google Scholar]
- 6.Kiuchi Y, Suzuki H, Hirohashi T, et al. cDNA cloning and inducible expression of human multidrug resistance associated protein 3 (MRP3). FEBS Lett 1998;433:149–52. [DOI] [PubMed] [Google Scholar]
- 7.Doyle LA, Yang WD, Abruzzo LV, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells [erratum in Proc Natl Acad Sci U S A1999;96:<2569]. Proc Natl Acad Sci U S A 1998;95:15665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins CF. ABC transporters—from microorganisms to man. Annu Rev Cell Biol 1992;8:67–113. [DOI] [PubMed] [Google Scholar]
- 9.Smit JJ, Schinkel AH, Oude Elferink RP, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 1993;75:451–62. [DOI] [PubMed] [Google Scholar]
- 10.de Vree JM, Jacquemin E, Sturm E, et al. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci U S A 1998;95:282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jedlitschky G, Leier I, Buchholz U, et al. ATP-dependent transport of glutathione S-conjugates by the multidrug resistance-associated protein. Cancer Res 1994;54:4833–6. [PubMed] [Google Scholar]
- 12.Grant CE, Valdimarsson G, Hipfner DR, et al. Overexpression of multidrug resistance-associated protein (MRP) increases resistance to natural product drugs. Cancer Res 1994;54:357–61. [PubMed] [Google Scholar]
- 13.Kool M, de Haas M, Scheffer GL, et al. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res 1997;57:3537–47. [PubMed] [Google Scholar]
- 14.Borst P, Evers R, Kool M, et al. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst 2000;92:1295–302. [DOI] [PubMed] [Google Scholar]
- 15.Hopper E, Belinsky MG, Zeng H, et al. Analysis of the structure and expression pattern of MRP7 (ABCC10), a new member of the MRP subfamily. Cancer Lett 2001;162:181–91. [DOI] [PubMed] [Google Scholar]
- 16.Tammur J, Prades C, Arnould I, et al. Two new genes from the human ATP-binding cassette transporter superfamily, ABCC11 and ABCC12, tandemly duplicated on chromosome 16q12. Gene 2001;273:89–96. [DOI] [PubMed] [Google Scholar]
- 17.Jedlitschky G, Leier I, Buchholz U, et al. ATP-dependent transport of bilirubin glucuronides by the multidrug resistance protein MRP1 and its hepatocyte canalicular isoform MRP2. Biochem J 1997;327:305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kool M, van der Linden M, de Haas M, et al. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci U S A 1999;96:6914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuetz JD, Connelly MC, Sun DX, et al. MRP4: a previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med 1999;5:1048–51. [DOI] [PubMed] [Google Scholar]
- 20.Wijnholds J, Mol CA, van Deemter L, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A 2000;97:7476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maliepaard M, van Gastelen MA, de Jong LA, et al. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res 1999;59:4559–63. [PubMed] [Google Scholar]
- 22.Ross DD, Yang WD, Abruzzo LV, et al. Atypical multidrug resistance: breast cancer resistance protein messenger RNA expression in mitroxantrone-selected cell lines. J Natl Cancer Inst 1999;91:429–33. [DOI] [PubMed] [Google Scholar]
- 23.Scheffer GL, Schroeijers AB, Izquierdo MA, et al. Lung resistance-related protein/major vault protein and vaults in multidrug-resistant cancer. Curr Opin Oncol 2000;12:550–6. [DOI] [PubMed] [Google Scholar]
- 24.Scheper RJ, Bulte JW, Brakkee JG, et al. Monoclonal antibody JSB-1 detects a highly conserved epitope on the P-glycoprotein associated with multi-drug-resistance. Int J Cancer 1988;42:389–94. [DOI] [PubMed] [Google Scholar]
- 25.Scheffer GL, Kool M, Heijn M, et al. Specific detection of multidrug resistance proteins MRP1, MRP2, MRP3, MRP5 and MDR3 P-glycoprotein with a panel of monoclonal antibodies. Cancer Res 2000;60:5269–77. [PubMed] [Google Scholar]
- 26.Scheffer GL, Maliepaard M, Pijnenborg ACLM, et al. Breast cancer resistance protein is localized at the plasma membrane in mitoxantrone and topotecan resistant cell lines. Cancer Res 2000;60:2589–93. [PubMed] [Google Scholar]
- 27.Scheper RJ, Broxterman HJ, Scheffer GL, et al. Overexpression of a M(r) 110,000 vesicular protein in non-P-glycoprotein-mediated multidrug resistance. Cancer Res 1993;53:1475–9. [PubMed] [Google Scholar]
- 28.Maliepaard M, Scheffer GL, Faneyte IF, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res 2001;61:3458–64. [PubMed] [Google Scholar]
- 29.Schroeijers AB, Scheffer GL, Reurs AW, et al. Detection of the Mr 110,000 lung resistance-related protein LRP/MVP with monoclonal antibodies. J Histochem Cytochem 2001;49:1379–86. [DOI] [PubMed] [Google Scholar]
- 30.van der Valk P, van Kalken CK, Ketelaars H, et al. Distribution of multi-drug resistance-associated P-glycoprotein in normal and neoplastic human tissues. Analysis with 3 monoclonal antibodies recognizing different epitopes of the P-glycoprotein molecule. Ann Oncol 1990;1:56–64. [PubMed] [Google Scholar]
- 31.Flens MJ, Zaman GJ, van der Valk P, et al. Tissue distribution of the multidrug resistance protein. Am J Pathol 1996;148:1237–47. [PMC free article] [PubMed] [Google Scholar]
- 32.Wright SR, Boag AH, Valdimarsson G, et al. Immunohistochemical detection of multidrug resistance protein in human lung cancer and normal lung. Clin Cancer Res 1998;4:2279–89. [PubMed] [Google Scholar]
- 33.Izquierdo MA, Scheffer GL, Flens MJ, et al. Broad distribution of the multidrug resistance-related vault lung resistance protein in normal human tissues and tumors. Am J Pathol 1996;148:877–87. [PMC free article] [PubMed] [Google Scholar]
- 34.Hipfner DR, Gauldie SD, Deeley RG, et al. Detection of the M(r) 190,000 multidrug resistance protein, MRP, with monoclonal antibodies. Cancer Res 1994;54:5788–92. [PubMed] [Google Scholar]
- 35.Flens MJ, Scheffer GL, van der Valk P, et al. Identification of novel drug resistance-associated proteins by a panel of rat monoclonal antibodies. Int J Cancer 1997;73:249–57. [DOI] [PubMed] [Google Scholar]
- 36.Chin JE, Soffir R, Noonan KE, et al. Structure and expression of the human MDR (P-glycoprotein) gene family. Mol Cell Biol 1989;9:3808–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smit JJ, Schinkel AH, Mol CA, et al. Tissue distribution of the human MDR3 P-glycoprotein. Lab Invest 1994;71:638–49. [PubMed] [Google Scholar]
- 38.Allikmets R, Schriml LM, Hutchinson A, et al. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res 1998;58:5337–9. [PubMed] [Google Scholar]
- 39.Schroeijers AB, Scheffer GL, Flens MJ, et al. Immunohistochemical detection of the human major vault protein LRP with two monoclonal antibodies in formalin-fixed, paraffin-embedded tissues. Am J Pathol 1998;152:373–8. [PMC free article] [PubMed] [Google Scholar]
- 40.Robbiani DF, Finch RA, Jager D, et al. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell 2000;103:757–68. [DOI] [PubMed] [Google Scholar]
- 41.Randolph GJ, Beaulieu S, Pope M, et al. A physiologic function for p-glycoprotein (MDR-1) during the migration of dendritic cells from skin via afferent lymphatic vessels. Proc Natl Acad Sci U S A 1998;95:6924–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Georges E, Bradley G, Gariepy J, et al. Detection of P-glycoprotein isoforms by gene-specific monoclonal antibodies. Proc Natl Acad Sci U S A 1990;87:152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Busse WW. Leukotrienes and inflammation. Am J Respir Crit Care Med 1998;157:S210–13. [PubMed] [Google Scholar]
- 44.Wijnholds J, Evers R, van Leusden MR, et al. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat Med 1997;3:1275–9. [DOI] [PubMed] [Google Scholar]