Abstract
Background: After the menopause decreased concentrations of oestrogen may result in insufficient maturation of the vaginal epithelium, which can lead to a range of vaginal discomforts. This state of vaginal atrophy may be treated with oestrogen replacement treatment. Replens™, a non-hormonal alternative to oestrogen replacement treatment has been shown to be effective in relieving symptoms related to vaginal atrophy in previous studies.
Aims: To study the effect of Replens on the maturation of the vaginal epithelium and morphology of the vaginal cells and to compare the results of a recently developed cytomorphometric method with manual assessment of the degree of maturation in vaginal smears.
Methods: Vaginal smears from 38 postmenopausal women suffering from symptoms related to vaginal atrophy were analysed manually and by cytomorphometry. The maturation value (MV) and the percentages of (para)basal, intermediate, and superficial cells (maturation index; MI) were measured by both methods before and after treatment with Replens. Cytomorphometry also measured mean cellular area, mean nuclear area, and mean area ratio.
Results: A correlation was shown between the two methods in the assessment of percentages of (para) basal and intermediate cells and MV. Cytomorphometric data showed a significant increase in mean cellular area, indicating a positive effect of Replens on the maturation of the vaginal epithelium. Changes in nuclear area and ratio between nuclear and cellular areas were not significant. Treatment with Replens did not influence MI or MV, as assessed by the two methods.
Conclusions: Replens did have an effect on vaginal morphology. The automated procedure may be useful for the assessment of maturation in vaginal smears and is more sensitive to small (subvisual) changes.
Keywords: postmenopausal atrophy, vaginal maturation, maturation index, automated image analysis
A gradual decrease in the production of oestrogens from the ovaries is seen in the perimenopausal years, ultimately leading to the menopause. Symptoms of vaginal atrophy result from a combination of atrophic changes in the oestrogen dependent cells that line the vagina and a reduction in the secretion of cervical mucus, which circulates in the vaginal lumen.1 The administration of exogenous oestrogens (either systemic or topical) is a common treatment for symptoms related to vaginal atrophy.2 Under the influence of oestrogens, maturation of the vaginal epithelium is restored. However, the use of exogenous oestrogens has several adverse effects, and may therefore be contraindicated.2,3 Replens™, a polycarbophil based vaginal moisturiser, is a non-hormonal alternative to oestrogen treatment. A characteristic of this bioadhesive polymer is that it is water insoluble, but water swellable. When applied intravaginally it binds to the vaginal epithelium, releasing purified water to hydrate the underlying cells.4 The gel produces a moist film over the vaginal tissue, which remains attached to the epithelial cell surface. The hydration of the epithelium lubricates the vaginal wall and reduces the incidence of vaginal itching, irritation, and dyspareunia.3 Furthermore, Replens restores the vaginal pH to premenopausal values.2
“Replens™, a polycarbophil based vaginal moisturiser, is a non-hormonal alternative to oestrogen treatment”
Whereas the clinical effect of Replens has been shown in multiple clinical studies (VV Ragavan et al. Presented at the international congress on the menopause, Sydney, Australia, 3–7 November 1996),2–5 the effect of Replens on vaginal cytology has not yet been studied extensively. In a comparative study of Replens versus the administration of local oestrogens, nine of 15 women using Replens for 12 weeks showed reversal of atrophy in Papanicolaou stained vaginal smears.2 In a study on baboons with decreasing ovarian function, a significant improvement of histological features was observed in biopsy specimens of the vaginal epithelium after five days of Replens use.4 In a study treating 25 patients suffering from breast cancer with Replens, a significant decrease of atrophic, crowded, parabasal cells in vaginal smears was found after three months of treatment (VV Ragavan et al. Presented at the international congress on the menopause, Sydney, Australia, 3–7 November 1996). In addition, cells appeared to be larger after treatment with Replens. These data suggest increased maturation of the vaginal epithelium as a result of Replens. To our knowledge, no previous study has focused on the effect of Replens on the morphology of cells in vaginal smears.
Improvement of the maturation of subsequent vaginal smears may be studied using the maturation index (MI).6 The MI expresses the percentages of (para)basal, intermediate, and superficial cells in a predetermined number of cells in a smear. Manual assessment of the MI by an experienced cytotechnologist was found to show limited reproducibility.7 A method using cytomorphometric analysis (CMA) to assess the degree of maturation in vaginal smears has been described previously.7 This method showed better reproducibility than the classic manual method. Furthermore, when using CMA additional morphometric parameters can be obtained, which may reveal more subtle changes.
A shift in the degree of maturation, expressed in the MI, of consecutive vaginal smears would be indicative of an oestrogen-like cytological effect of Replens on the vaginal epithelium. In our study we chose to evaluate, by both manual assessment and CMA, the effect of Replens on vaginal cytology in a group of postmenopausal women with symptoms related to vaginal atrophy. Vaginal smears were taken before and after 12 weeks of treatment, and maturation of the samples was assessed by manual assessment and CMA. The improvement of vaginal maturation after 12 weeks, as compared with baseline, was studied. The aims of our study were: (1) to study the effect of Replens on the maturation of the vaginal epithelium and the morphology of vaginal cells and (2) to assess the degree of maturation of the vaginal epithelium using an automated method compared with a manual method.
MATERIALS AND METHODS
Patient groups
After giving their informed consent, women were included in our study when one or more symptoms of vaginal atrophy of moderate or severe nature were present: vaginal dryness, itching, irritation (burning), or dyspareunia. The last menstruation had to be at least one year ago and baseline vaginal smears had to show a maturation value lower than 0.6 with less than 5% superficial cells. Women had to be in good mental and physical health, as demonstrated by physical examination, including sitting blood pressure, heart rate, and respiratory rate. Cancer or any high/low grade precancerous lesions of the cervix or vagina were ruled out by cytomorphological evaluation of cervical and vaginal Papanicolaou stained smears. Fifty women were enrolled, of whom 38 completed 12 weeks of treatment with Replens. The treatment was discontinued because of insufficient therapeutic effect (n = 3), physical complaints possibly related to treatment (n = 4), the use of unacceptable concurrent medication (n = 1), or reasons not related to treatment (n = 4). No serious adverse events related to Replens treatment were recorded.
Investigational drug
Replens is a bioadhesive, polycarbophil based vaginal gel, marketed in numerous countries as a medical product for the relief of vaginal dryness in postmenopausal women. The product is packed in single use plastic applicators specifically designed for vaginal administration. Replens was administered three times a week over a period of 12 weeks.
Specimen preparation
Samples of the vaginal epithelium were obtained by rotating a special brush (Vibabrush; Rovers BV, Oss, the Netherlands) in the middle portion of the vaginal wall. Smears were applied to a glass slide, fixed with a spray fixative (Pro-Fixx; Lerner Laboratories, Pittsburgh, USA) and stained by the Papanicolaou procedure. After preparation of the smear, the brush with adhering cells was put into ethanol-carbowax fixation fluid and cytospins were subsequently prepared using a Cyto-Tek centrifuge (Miles Inc, Elkhart, Indiana, USA). The cytospin device deposits the cell material into a well defined square area of the slide, facilitating fully automated measurement. The specimens were stained using the haematoxylin and eosin (H&E) procedure.
Automated cytometric evaluation
Fully automated measurement of H&E stained cytospins was performed by means of the Discovery 2.6 Fluorbance system (Beckton Dickinson Cellular Imaging Systems, Leiden, the Netherlands),8 using a procedure described and evaluated previously.7 In short, cytospins were scanned fully automatically with a ×25 objective (numerical aperture, 0.7) using a motorised stage and automatic focusing. Microscopic fields measuring 550 × 550 μm2 were digitised by a Xillix MicroImager 1400 monochromatic CCD camera (Xillix Technologies Corp, Vancouver, Canada; pixel size 6.8 × 6.8 μm2, 8 bit sampling), resulting in an effective resolving power of 0.54 × 0.54 μm2. Images were acquired using narrow band pass filters suitable for the recognition of cytoplasm and nuclei of H&E stained cells (λ = 540 nm and λ = 580 nm, respectively).
A grey level threshold was applied to the images using fixed threshold values to detect cytoplasmic and nuclear staining in the respective images. Objects were detected based on 8-connectedness of thresholded pixels. All objects with area and contour ratio (1000 × perimeter/(2 × √(π × area)), which reflects the roughness of contour of an object) within certain limits for both cytoplasm and nucleus were considered for measurement. In our previous study, classification results dropped considerably when clumped cells were included in the analysis; therefore, in our present study only single lying cells (that is, cytoplasmic objects encompassing a single nuclear object) were used. Nuclear objects with area > 50 and contour ratio < 1300 were considered to be correctly segmented nuclei; cytoplasmic objects with area > 850 and contour ratio < 1500 were considered to be correctly segmented cytoplasm. Objects detected in this way were classified by two discriminant functions, which have been published previously,7 as either junk (that is, non-cellular object, cellular objects overlapping each other, or poorly segmented cells) or (para)basal, intermediate, or superficial epithelial cells. The recognition of (para)basal epithelial cells was based on the ratio between the area of the nucleus and the area of the cytoplasm. Discrimination between intermediate and superficial epithelial cells was based on the area of the nucleus.7
From these data, the MI and maturation value (MV) were calculated, applying the procedure described previously.6 The MI consists of the percentages of (para)basal (P), intermediate (I), and superficial cells (S) in a specimen. The MV also uses P, I, and S to which the following values are assigned: P = 0, I = 0.5, and S = 1.0. MV values can range from 0, when only (para)basal cells are present (atrophic specimens), to 100 for specimens containing only superficial cells (mature specimens).9 After calculating the MI and MV, for all objects recognised as correctly segmented cells (that is, non-junk objects) in a specimen, we calculated the mean area of cytoplasm and nucleus and the mean area ratio (area of the nucleus divided by the area of the cytoplasm, resulting in values ranging from 0 to 17).
Classic manual assessment
The maturation of the vaginal epithelium was assessed manually, in Papanicolaou stained smears, by an experienced cytotechnologist and cytopathologist. The maturation was expressed as the MI and MV over 300 cells, as described above.
Statistical analysis
All statistics were performed using SPSS for Windows (SPSS Inc, Chicago, Illinois, USA). Women for whom less than 100 countable cells were encountered in any of the manually evaluated specimens or for whom less than 30 cells were recognised by the automated procedure in any specimen were excluded from the respective analysis. Shapiro-Wilks's test for normality showed a significant deviation from normality for several parameters; therefore, the non-parametric Spearman correlation was used to compare the results from the manual assessment and CMA. In addition, linear regression analysis was performed to compare the results from the manual assessment and CMA, using the manual assessment as the dependent variable. The effect of Replens on cytomorphological and cytometrical parameters was studied by paired testing of post treatment versus baseline values, using the Wilcoxon signed ranks test.
RESULTS
For CMA, specimens from 10 of the women contained less than 30 cells suitable for analysis in either the baseline or post treatment specimen, or in both. Thus, only 28 women could be included in our analysis. For manual analysis, one specimen out of 38 contained no cellular material, resulting in 37 women for evaluation. Because of low cell density, in three specimens only 200 cells and in one specimen 120 cells could be counted. In all other specimens 300 cells were counted.
Comparing cytomorphometric analysis with manual assessment
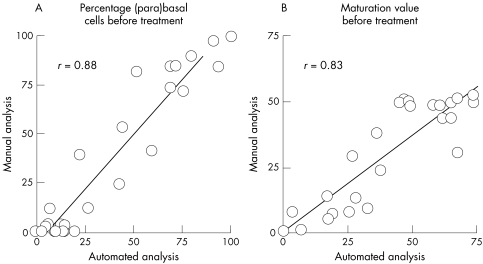
Spearman correlation analysis showed a good and highly significant correlation between data from CMA and manual assessment for the percentages of (para)basal and intermediate cells and the MV (r = 0.79; p < 0.001; table 1; fig 1). For the percentage of superficial cells, the correlation was only moderate (r = 0.41; p < 0.05). Linear regression analysis showed a significant intercept for the percentages of (para)basal and intermediate cells before treatment and the percentage of intermediate cells and the MV after treatment (table 1). The slope of the regression line was close to 1 for the percentages of (para)basal and intermediate cells, whereas for the percentage of superficial cells the slope was almost 0.
Table 1.
Comparison between data from automated cytomorphometric analysis and manual assessment
| Cytomorphometric analysis versus manual assessment | ||
| Baseline | Post-treatment | |
| Percentage (para)basal | ||
| Correlation | 0.88*** | 0.92*** |
| Linear regression | 1.1 (0.1)/−8.0 (3.6)* | 0.91 (0.1)/−5.9 (4.9) |
| Percentage intermediate | ||
| Correlation | 0.79*** | 0.79*** |
| Linear regression | 1.1 (0.2)/22.7 (8.1)** | 1.0 (0.2)/32.0 (8.8)*** |
| Percentage superficial | ||
| Correlation | 0.41* | 0.41* |
| Linear regression | 0.03 (0.01)/−0.2 (0.4) | 0.1 (0.03)/−1.3 (1.0) |
| Maturation value | ||
| Correlation | 0.83*** | 0.86*** |
| Linear regression | 0.8 (0.1)/−0.1 (3.9) | 0.7 (0.1)/9.1 (3.9)* |
Non-parametric (Spearman) correlation coefficients and linear regression data are shown. Linear regression results are shown as slope (SE)/intercept (SE). Intercepts deviating significantly from 0 are indicated with asterisks.
*p<0.05; **p<0.01; ***p<0.001.
Figure 1.
Comparison between automated and manual assessment of baseline values for (A) the percentage of (para)basal cells and (B) the maturation value.
Analysis of the cytological effect of the treatment
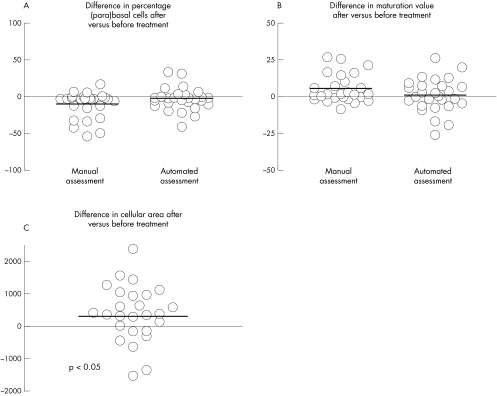
Table 2 lists the mean and SD of the baseline and post treatment values for all the parameters assessed by manual assessment and CMA. Figure 2 shows the difference between baseline and post treatment values for the percentage of (para)basal cells, the MV, and the cellular area. Paired testing showed a significant increase in mean cellular area after 12 weeks of Replens treatment (p = 0.04; fig 2C). No significant effect was found for the percentages of (para)basal, intermediate, and superficial cells and for the MV. In addition, the nuclear area and the ratio between the nuclear and the cellular area showed no significant effects.
Table 2.
Data from both automated cytomorphometric analysis and manual assessment
| Cytomorphometric analysis (N = 28) | Manual assessment (N = 37) | |||
| Baseline | Post treatment | Baseline | Post treatment | |
| Percentage (para)basal | 36.8 (32.5) | 34.1 (29.5) | 35.5 (37.5) | 31.0 (32.2) |
| Percentage intermediate | 36.9 (27.2) | 40.5 (22.3) | 64.1 (37.2) | 68.2 (31.7) |
| Percentage superficial | 26.3 (22.1) | 25.4 (19.4) | 0.4 (1.2) | 0.8 (2.7) |
| Maturation value | 44.7 (24.3) | 45.6 (22.4) | 32.5 (18.9) | 34.9 (16.5) |
| Mean cellular area | 3248 (1371) | 3586 (1418)* | ||
| Mean nuclear area | 335 (97.1) | 346 (79.7) | ||
| Mean area ratio | 0.16 (0.08) | 0.16 (0.08) | ||
The mean and SD values of vaginal smears before (baseline) and after 12 weeks of Replens™ treatment (post-treatment) are shown. Post treatment values significantly different from baseline are indicated by an asterisk.
*p<0.05.
Figure 2.
Difference between post treatment and baseline values for (A) the percentage of (para)basal cells, (B) the maturation value, and (C) the cellular area. The solid lines indicate the median values over all cases.
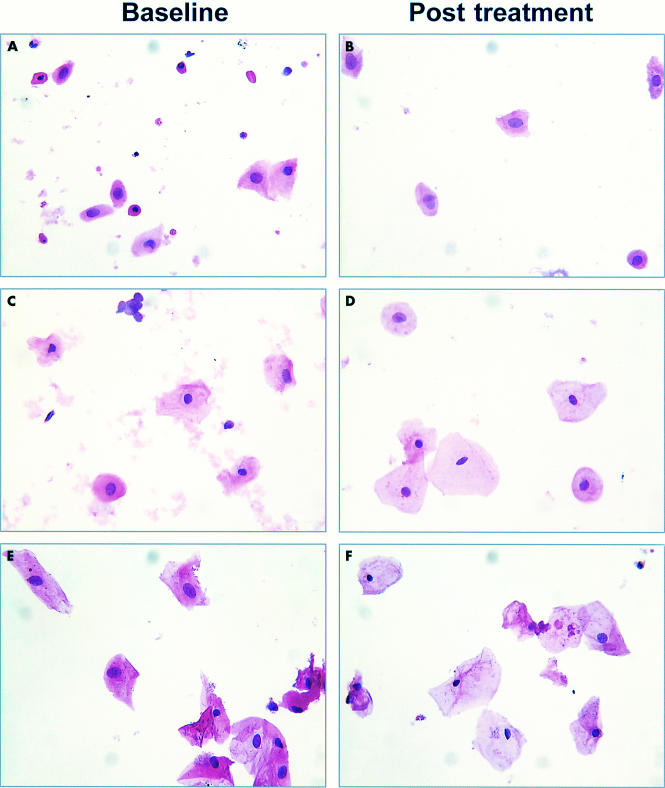
Figure 3 shows examples of microscopical images of H&E stained cells before and after treatment. Microscopical fields of view were randomly selected from three patients who showed an increase in mean cellular area, as assessed by CMA.
Figure 3.
Randomly selected microscopical images of haematoxylin and eosin stained smears from three women with a pronounced increase in cellular area, as assessed by cytomorphometric analysis. Patients 1 (A, B), 2 (C, D), and 3 (E, F) at baseline (A, C, E) and post treatment (B, D, F).
DISCUSSION
In our study, the effect of a non-hormonal treatment for postmenopausal vaginal atrophy on vaginal cytology was evaluated. A group of 38 postmenopausal women, suffering from diverse complaints related to vaginal atrophy, completed 12 weeks of Replens treatment. Data on the clinical effects of Replens in our study have been described separately (LMT Beijers-de Bie et al. Presented at the 11th annual meeting of the North American Menopause Society, 7–9 September 2000, Orlando, USA). Overall, a positive effect of Replens on atrophy related complaints was seen This agrees with previous findings (VV Ragavan et al. Presented at the international congress on the menopause, Sydney, Australia, 3–7 November 1996).2,3,5 Whereas the clinical effect of Replens has been studied extensively, only sparse data regarding the cytological effect are available. The treatment of postmenopausal atrophy by oestrogen replacement restores the maturation of the vaginal epithelium to its premenopausal state.10 This is expressed in the MV, which increases from an average value of 40 in atrophic epithelium to values over 70 after treatment.10,11
“The increase in vaginal maturation observed by other authors could only partly be confirmed in our study”
In previous studies, a similar increase in the maturation of the vaginal epithelium was seen after treatment with Replens. Increased numbers of intermediate and superficial cells were observed, even after a single application of Replens, in the vaginal epithelium of baboons with decreased ovarian function.4 Nachtigall2 reported a reversal of atrophy in 60% of Papanicolaou stained smears of menopausal women after 12 weeks of treatment with Replens. The most convincing data were found by Ragavan et al (VV Ragavan et al. Presented at the international congress on the menopause, Sydney, Australia, 3–7 November 1996), who described a highly significant (p < 0.001) decrease of atrophic, crowded, (para)basal cells in vaginal smears of atrophic patients with breast cancer, with cells appearing to be larger after treatment. The use of the maturation index is recommended for the assessment of the degree of maturation of the vaginal epithelium in vaginal smears.12 No other studies have assessed MI and MV after Replens treatment.
In our present study, the cytomorphological changes were analysed in detail, using both classic manual evaluation and automated cytomorphometric analysis. The increase in vaginal maturation observed by other authors could only partly be confirmed in our study. The mean cellular area, assessed by CMA, was significantly increased. This corresponds to Ragavan's findings and may be indicative of an oestrogen-like effect of Replens on the maturation of the vaginal epithelium. However, the increase in nuclear area seems to contradict such an effect. Classic manual assessment of maturation in terms of percentages of (para)basal, intermediate, and superficial epithelial cells did not reveal a cytomorphological effect of Replens. Manually classifying cells in one of three classes has limited accuracy.7 Moreover, because maturation of the vaginal epithelium is a continuous process, this classification is an artificial one. The positive clinical effect of Replens could not be explained by improvement in MI or MV, either assessed manually or by the automated procedure. In contrast to this, morphometric parameters assessed using CMA did show such an effect. The increase in cellular area was found to be significant. This shows that the use of CMA may reveal subtle differences, not discernible by classic manual analysis. The need for CMA to assess the classic parameters of MI and MV is doubtful, because morphometric parameters are available that offer higher precision.
A good correlation exists between data from manual assessment and CMA, indicating that both methods assess comparable information. However, linear regression analysis revealed a bias for several of the parameters. Manual assessment identified high percentages of intermediate cells and almost no superficial cells. The automated procedure, in contrast, also found a substantial number of superficial cells in many smears. This may be attributed to the subjectivity of the manual assessment, as has been shown in a previous study.7
In conclusion, in our study we found a significant effect on the morphology of epithelial cells in vaginal smears in patients with symptoms of vaginal atrophy being treated with Replens. This effect could be detected using automated cytomorphometric analysis. A good correlation was found between manual and automated assessment of the degree of maturation in vaginal smears. The use of automated cytomorphometric analysis is recommended for the assessment of treatment related parameters in smear specimens because it is highly accurate and because it provides additional information, which cannot be acquired by manual assessment.
Acknowledgments
The authors wish to thank Mr WN Mäkel and Mr OR Leeuwenkamp for their cooperation. We thank Ms E Delforterie, Ms T Heijnen-Wijnen, Mr HMJ Kerstens, and Mr MMM Pahlplatz for their valuable contributions. This study was sponsored by Columbia Laboratories France, Paris (COL-1003-NL01).
Abbreviations
CMA, cytomorphometric analysis
H&E, haematoxylin and eosin
I, intermediate cells
MI, maturation index
MV, maturation value
P, (para)basal cells
S, superficial cells
Footnotes
Preliminary results of this work were presented as a poster at the 14th International Congress of Cytology, 27–31 May 2001, Amsterdam, The Netherlands.
REFERENCES
- 1.Kaufman RH, Friedrich EG, Gardner HL. Atrophic, desquamative, and postradiation vulvovaginitis. In: Benign diseases of the vulva and vagina. Chicago: Year book medical publishers, 1989:419–24.
- 2.Nachtigall LE. Comparative study: Replens versus local estrogen in menopausal women. Fertil Steril 1994;61:178–80. [DOI] [PubMed] [Google Scholar]
- 3.Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas 1996;23:259–63. [DOI] [PubMed] [Google Scholar]
- 4.Hubbard GB, Carey KD Levine H, et al. Evaluation of a vaginal moisturizer in baboons with decreasing ovarian function. Lab Anim Sci 1997;47:36–9. [PubMed] [Google Scholar]
- 5.Gelfand MM, Wendman E. Treating vaginal dryness in breast cancer patients: results of applying a polycarbophil moisturizing gel. J Womens Health 1994;3:427–34. [Google Scholar]
- 6.Wied GL, Bibbo M. Hormonal cytology. In: Bibbo M, ed. Comprehensive cytopathology. Philadelphia: WB Saunders Company, 1991:85–114.
- 7.van der Laak JAWM, Schijf CP, Kerstens HMJ, et al. Development and validation of a computerized cytomorphometric method to assess the maturation of vaginal epithelial cells. Cytometry 1999;35:196–202. [PubMed] [Google Scholar]
- 8.Schipper NW, Nauwelaers FA, Ploem JS. The DISCOVERY system. In: Grohs HK, Husain OAN, eds. Automated cervical cancer screening. New York-Tokyo: Igaku-Shoin, 1994:270–8.
- 9.Meisels A. The maturation value. Acta Cytol 1967;11:249. [PubMed] [Google Scholar]
- 10.Kicovic PM, Cortes-Prieto J, Milojevic S, et al. The treatment of postmenopausal vaginal atrophy with Ovestin vaginal cream or suppositories: clinical, endocrinological and safety aspects. Maturitas 1980;2:275–82. [DOI] [PubMed] [Google Scholar]
- 11.Trevoux R, van der Velden WH, Popovic D. Ovestin vaginal cream and suppositories for the treatment of menopausal vaginal atrophy. Reproduccion 1982;6:101–6. [PubMed] [Google Scholar]
- 12.Nilsson K, Risberg B, Heimer G. The vaginal epithelium in the postmenopause—cytology, histology and pH as methods of assessment. Maturitas 1995;21:51–6. [DOI] [PubMed] [Google Scholar]