Abstract
Aims: Gluten ingestion in coeliac disease is associated with alterations of the intestinal mucosa, especially the expansion of the lamina propria. Antiendomysium and antireticulin antibodies may result from interactions between gliadin and extracellular matrix components. By behaving as autoantigens, connective tissue proteins could initiate mucosal damage. This study evaluates changes in the distribution of laminin, type IV collagen, and fibronectin in the mucosa of patients with coeliac disease in an attempt to explain the alterations of mucosal morphology.
Methods: Intestinal biopsies were obtained from patients with coeliac disease on admission and while on a gluten free diet. The distribution of type IV collagen, laminin, fibronectin, and α-smooth muscle actin was evaluated by immunofluorescence and by immunogold labelling and electron microscopy.
Results: In patients with coeliac disease, the intensity of type IV collagen, laminin, and fibronectin immunofluorescent staining was decreased and less well defined than in controls, with frequent breaches in the basement membrane; fibronectin staining was weak in the distal third of the elongated crypts and absent under the flat surface. The distribution of smooth muscle fibre in the distal lamina propria of flat mucosae was altered. The distribution of these proteins was normal as assessed by immunoelectron microscopy.
Conclusions: The intensity of staining of some components of the basement membrane is decreased in coeliac disease and the distribution of smooth muscle fibres is altered. These changes may result from interactions between gliadin and components of the extracellular matrix and may play a role in the genesis of mucosal lesions and in the damage to the epithelium.
Keywords: coeliac disease, laminin, fibronectin, type IV collagen
The architecture of small bowel mucosa is altered in coeliac disease, with a flat surface, effacement of villi, and elongation of crypts. The surface epithelium is severely damaged with decreased height, alterations of the brush border, and vacuolisation of cytoplasm with accumulation of fat laden vesicles; the basement membrane is thickened in the flat areas. However, even in the absence of villi the mucosa maintains its normal thickness, and the volume of the lamina propria is increased two to threefold, with a considerable increase of plasma cells, lymphocytes, and some eosinophils. The numbers of intraepithelial lymphocytes are also considerably increased, giving the surface epithelium the appearance of greater cellularity.1,2 The maintenance of normal mucosal morphology requires on the part of the epithelium a delicate balance between proliferation, differentiation, apoptosis, and desquamation.3,4 This implies continuous interactions between the epithelium, the extracellular matrix represented by the basement membrane, and the underlying myofibroblast network. Myofibroblasts and enterocytes synthesise the components of the basement membrane and establish specific gradients of these proteins along the crypt–villus axis and the different segments of the gut,5,6 as has been shown for laminin.7–9 Antireticulin and antiendomysium antibodies are considered pathognomomic of coeliac disease.10 Dieterich et al have recently identified tissue transglutaminase (tTG), an enzyme present in the extracellular matrix, as the endomysial autoantigen.11 It has been proposed that IgA class antibodies against tTG interfere with mesenchymal cell–enterocyte crosstalk, resulting in inhibition of enterocyte differentiation and increased proliferation.12 These antibodies also disturb transforming growth factor β mediated fibroblast–enterocyte crosstalk in the crypt–villus axis.12 This suggests that certain components of the connective tissue may function as autoantigens, and that this may constitute the primary alteration of the mucosa in coeliac disease; in turn, this may be lead to the architectural changes of the mucosa. If coeliac disease results in major morphological changes in the mucosa, are these changes reflected in the extracellular matrix?
“Certain components of the connective tissue may function as autoantigens, and this may constitute the primary alteration of the mucosa in coeliac disease”
The aims of this study were: (1) to evaluate the distribution of three of the main components of the extracellular matrix—laminin, collagen type IV, and fibronectin—in the small intestinal mucosa of patients with coeliac disease at different stages of their evolution, at the time of diagnosis and while on a gluten free diet; and (2) to identify differences in distribution that may explain the changes in mucosal morphology in this disease. The expression of α-smooth muscle actin (αSMA) was evaluated as a marker of the presence and distribution of myofibroblasts and smooth muscle fibres.
PATIENTS, MATERIALS, AND METHODS
Patients
Patients were diagnosed as having coeliac disease on the basis of the criteria established by ESPGAN.13 They had a history of chronic diarrhoea, abdominal distention, short stature, clinical signs of malabsorption, macromicrocytic anaemia, positive antiendomysium antibodies, and histological findings compatible with the diagnosis of coeliac disease. Immediately thereafter, patients were placed on a strict gluten free diet and the presence of antiendomysium antibodies was evaluated periodically; when these became negative the second biopsy was obtained.
In total, 32 patients with coeliac disease were studied; of these, 16 were biopsied before the gluten free diet was started and the same number while on a gluten free diet for up to three years.
Sixteen patients who on the basis of the abovementioned criteria were found not to have coeliac disease or who needed surgery for correction of intestinal malformations served as controls.
Tissue samples
Biopsies of jejunal mucosa were obtained by endoscopic or peroral biopsies from patients with coeliac disease at the time of diagnosis and while on a gluten free diet. Tissue samples were fixed in buffered formalin or Bouin's fluid, dehydrated, embedded in paraffin wax, and serially sectioned.
This protocol was approved by the committee on ethics in research in humans (patients older than 16 years or parents or legal guardians for younger individuals provided informed consent for these procedures, which for the patients with coeliac disease were part of their clinical follow up).
Antibodies
The following antibodies were used: (1) monoclonal antibodies 4C7 (M0638; Dako, Carpinteria, California, USA) and LAM-89 (L8271; Sigma Chemical Company, St Louis, Missouri, USA) directed against the α-5 chain of human laminin; (2) monoclonal antibody COL-94 (C1926; Sigma) directed against type IV collagen; (3) polyclonal A0245 (Dako) directed against cellular fibronectin; and (4) monoclonal antibody 1A4 (A2547; Sigma) directed against αSMA. Fluorescein isothiocyanate (FITC) conjugated goat antimouse or antirabbit IgG was used as secondary antibody.
Indirect immunofluorescence
For laminin and type IV collagen detection, sections were treated with pepsin in 0.2N HCl, before incubation with the antibodies against laminin or type IV collagen. The sections were incubated with FITC conjugated antibody. For fibronectin and αSMA, sections were incubated with their specific antisera, and with the FITC conjugated antibody. For all proteins, negative controls were processed omitting the primary antibody. Preparations were viewed using epifluorescence with an Olympus BX-40 microscope equipped with a PM-20 automatic camera.
Analysis of the histological preparations
All sections were examined and evaluated independently by two observers without foreknowledge of the diagnosis of the individual. To describe results the following factors were taken into consideration: presence or absence of label at the crypt or villus levels, location (basement membrane of epithelium, basement membrane of myofibroblasts, capillaries and smooth muscle fibres, diffuse staining in matrix, staining in any other structure), thickness, intensity, and continuity, comparing patients with coeliac disease at their different stages of treatment with controls.
Immunogold labelling for electron microscopy
For immunoelectron microscopy, tissue was fixed in 2% paraformaldehyde with 0.2% glutaraldehyde in phosphate buffer; after washing with buffer it was dehydrated in ethanol/NaCl. The tissue was then transferred to increasing concentrations of LR-Gold (Sigma), which was polymerised at 4°C by the addition of 0.8% (wt/wt) of benzoil peroxide (Sigma). The polymerised blocks were properly oriented and ultrathin sections were cut; the sections were collected on Formvar coated gold grids and stained in Terasaki plates. The primary antibodies were the same as for immunofluorescence and the secondary antibody was Aurion GAM IgG labelled with 10 nm gold particles (Aurion, Wageningen, The Netherlands). After brief counterstaining with uranium and lead citrate, grids were viewed using a Philips CM-100 electron microscope operated at 60 kV.
RESULTS
Immunohistochemistry
Laminin
The distribution of the α-5 laminin chain in the basement membrane was similar in patients with coeliac disease and controls. In healthy individuals, laminin appeared in the crypts as a thin, uninterrupted line, which was thicker and more intensely stained but less well defined in the villi, with occasional 5–7 μm breaches. Subepithelial myofibroblasts, smooth muscle fibres in the axis of villi, and surrounding venules had a laminin sheath around their perimeter. In the lamina propria of the villi there were some lightly stained areas of irregular size and shape with diffuse edges. The distribution of laminin remained unchanged in the patients with coeliac disease; however, its staining was consistently less intense, the thickness and the definition of the basement membrane was decreased, and it was interrupted by wider, more frequent breaches in the crypts and the blunt villi, and particularly under the epithelium of the flat surface. In biopsies of patients with coeliac disease who were on a gluten free diet, as the morphology improved, the appearance of the laminin reverted to normal.
Fibronectin
In the crypts of Lieberkühn of the controls, fibronectin appeared as a sharp, well defined structure underlining the epithelium. The intensity of the staining decreased progressively distally along the crypts–villus axis to such a degree that in the distal third of the villi it appeared as a thin, lightly stained, discontinuous line. As with laminin, there were areas of weak fluorescence in the connective tissue between the crypts. In the patients with coeliac disease, the overall distribution of fibronectin was similar to the controls, although the staining was always less intense. Under the epithelium of the flat surface, fibronectin staining was usually absent, whereas near the opening of the crypts it appeared as a succession of dots; this same punctate distribution was apparent in the basement membrane surrounding the myofibroblasts around the crypts. Staining reverted to normal after a gluten free diet.
Type IV collagen
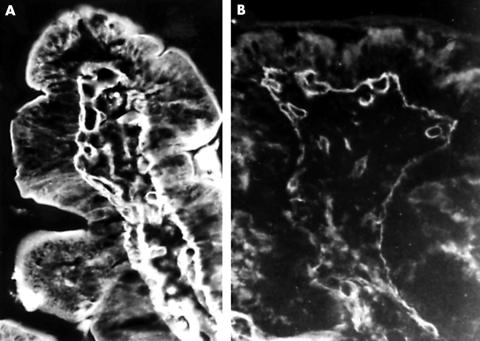
A thick, well defined band of type IV collagen was present in the basement membrane of the controls; it occasionally presented interruptions 5–7 μm in width. Type IV collagen was also present in the endomysium of the smooth muscle fibres and the basement membrane of blood capillaries in the lamina propria (fig 1A) In the patients with coeliac disease, type IV collagen was thinner under the epithelium of both the crypts and the villi, and it showed frequent interruptions; these were also present in the basement membrane of blood capillaries (fig 1B). Like the other two proteins, type IV collagen staining reverted to normal as the histology of the mucosa improved with the gluten free diet.
Figure 1.
Immunofluorescent staining for type IV collagen. (A) In healthy individuals, type IV collagen stains in the villi as a thick, well defined structure in the basement membrane. (B) In patients with coeliac disease, the distribution of type IV collagen in the basement membrane is similar to that seen in normal controls, although it stains much less intensely and has many interruptions; original magnification, ×200.
α-SMA
This protein was studied as a marker of myofibroblast and smooth muscle fibre distribution. In normal controls, the cytoplasm of myofibroblasts at the crypt and villus level was lightly stained. Compact bundles of smooth muscle fibres were present between the crypts of Lieberkühn and in the axis of villi. In the cytoplasm of these smooth muscle fibres, myofibrils appeared as closely packed, very thin lines, parallel to the main axis of the cells (fig 2A).
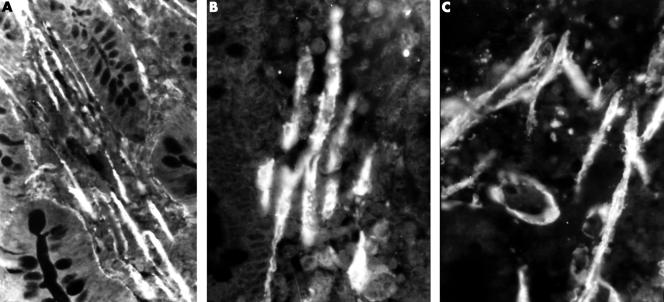
Figure 2.
Immunofluorescent staining for α-smooth muscle actin. (A) In healthy individuals, smooth muscle fibres appear as bundles of parallel elements between the crypts and in the axis of the villi; original magnification, ×100. (B) As the mucosa is damaged and villi become short and blunt the smooth muscle bands become less compact; original magnification, ×250. (C) When the mucosa is flat the smooth muscle fibres become disorganised and single fibres can be seen in any direction in the distal third of the mucosa; original magnification, ×200.
In untreated patients with coeliac disease, in those areas of the mucosa where villi were reduced to thick, short remnants, the normal arrangement of muscle fibres began to appear distorted—they were organised into less compact, although still parallel, bundles and the thickness of individual fibres was irregular (fig 2B).
In the areas where the mucosa was severely damaged and flat, the distribution of muscle fibres was comparable to that seen in controls only in the deeper parts; in the distal half of the mucosa, in the vicinity of the intestinal lumen, fibres were oriented in all directions, very unlike that seen in normal control villi. The myofilaments were less numerous and their appearance resembled a bead chain, as a result of their uneven thickness (fig 2C).
During the gluten free diet the alterations to the smooth muscle fibres persisted until the normal architecture of the mucosa was restored.
Immunoelectron microscopy
Although the density of labelling for type IV collagen, laminin, and fibronectin was not very high, it was easy to identify the gold particles and to study there location and distribution.
Type IV collagen was detected only in the basement membrane of the epithelium (fig 3) and the blood capillaries and in the cytoplasm and the surface of subepithelial myofibroblasts. Type IV collagen was not detected in the enterocytes. Fibronectin was seen in the cytoplasm of myofibroblasts and in the basement membrane but, in contrast to type IV collagen, it was easily detected in the matrix of the lamina propria, in the cytoplasm of enterocytes, and occasionally in the lumen of blood capillaries (fig 4). The distribution of laminin was similar to that seen for fibronectin (fig 5).
Figure 3.
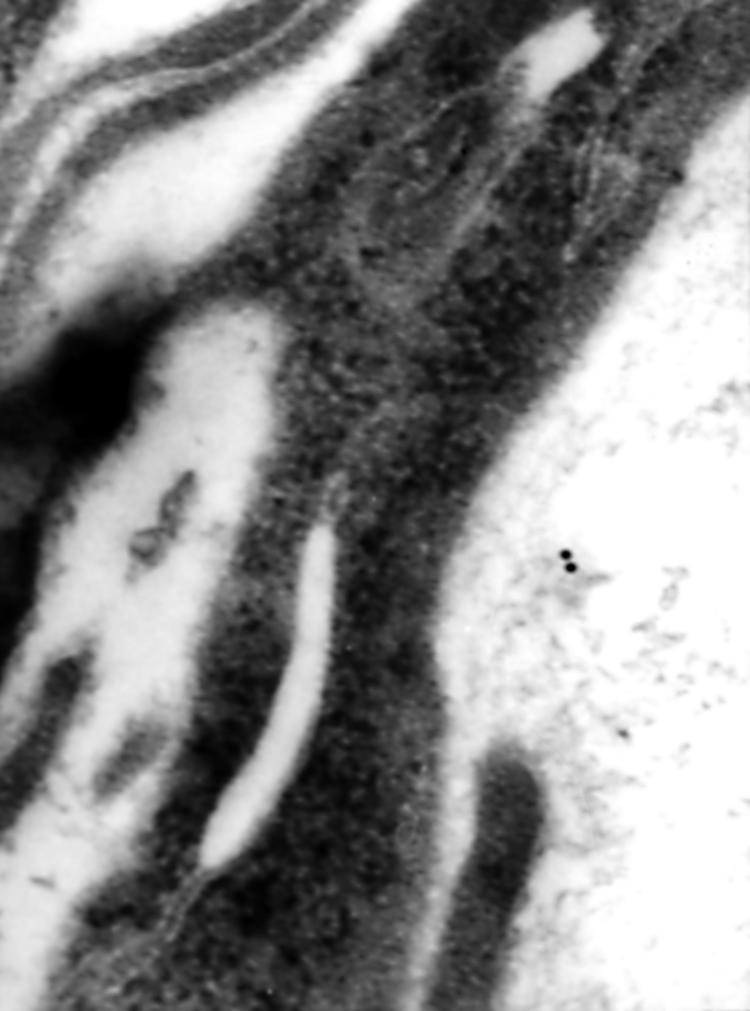
Immunogold staining of type IV collagen for electron microscopy. High magnification view of staining in the basement membrane of the surface epithelium in untreated coeliac disease; original magnification, ×90 000.
Figure 4.
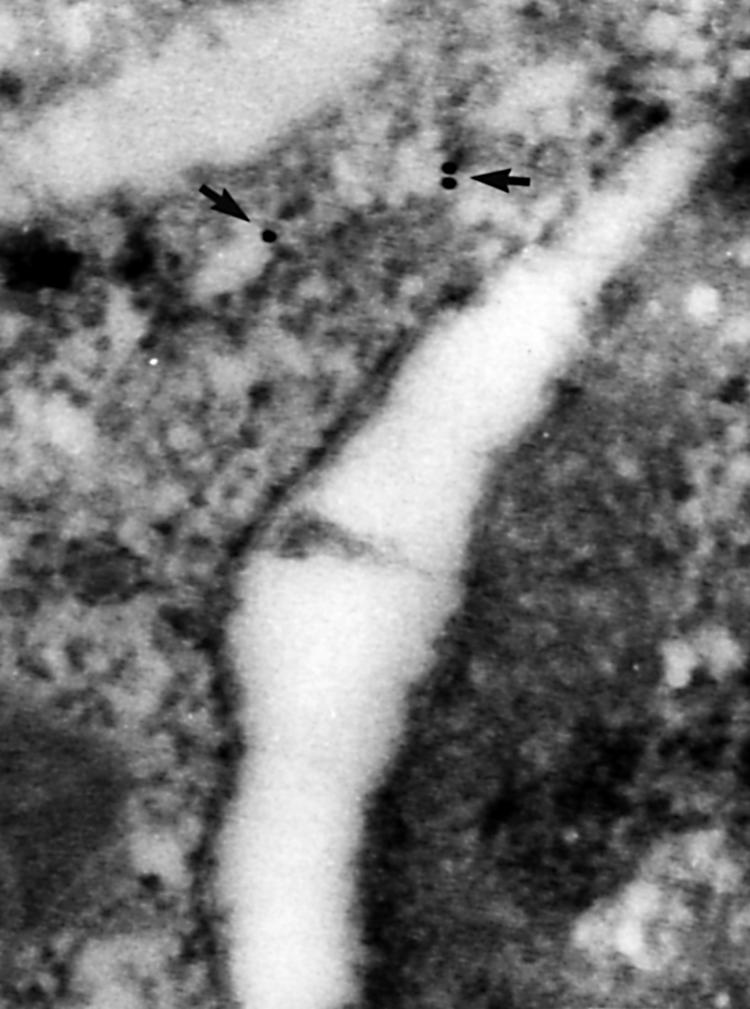
Immunogold staining of fibronectin for electron microscopy. High magnification view of fibronectin staining in the endoplasmic reticulum of an epithelial cell at the surface of the mucosa in untreated coeliac disease; original magnification, ×110 000.
Figure 5.
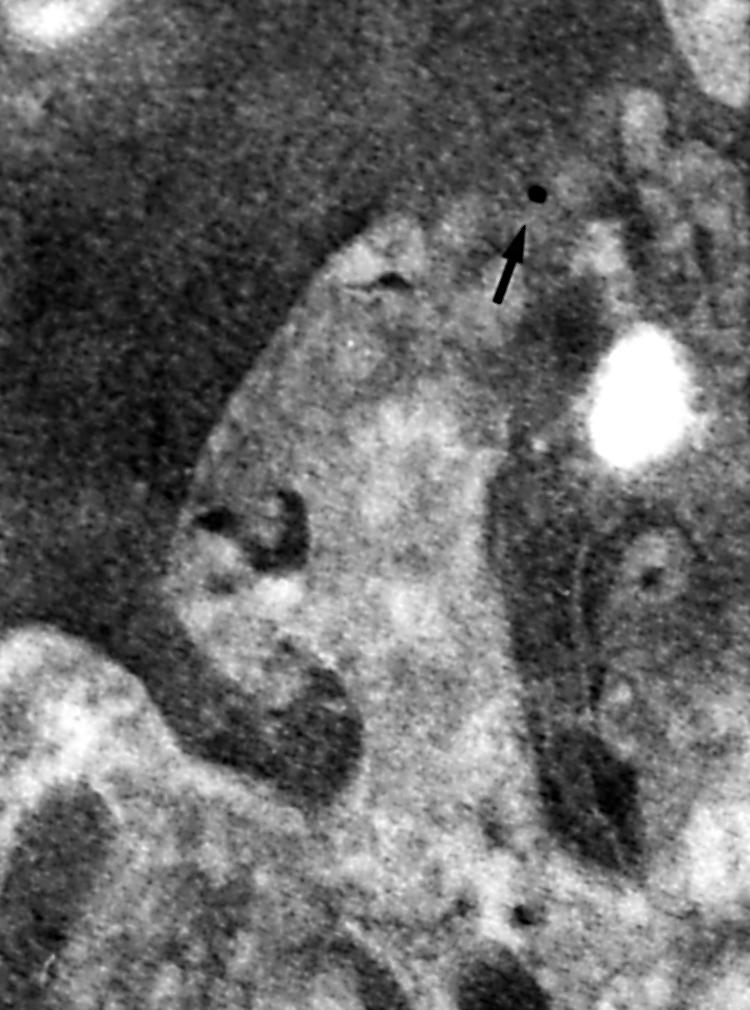
Immunogold staining of laminin for electron microscopy. High magnification view of laminin staining in a tangential section of the surface of a myofibroblast in the vicinity of the lumenal opening of a crypt in untreated coeliac disease; original magnification, ×125 000.
It was difficult to establish differences in the density and distribution of the labelling with this technique when comparing healthy controls and patients with coeliac disease in all stages of treatment of this condition.
DISCUSSION
Although the mechanism(s) causing the lesion of the small intestinal mucosa in coeliac disease remain unknown, it has been thought for many years that an alteration in the epithelium is present at its inception. In patients with coeliac disease, antibodies against an exogenous protein, gliadin, and against proteins of the extracellular matrix synthesised by the myofibroblasts of the lamina propria can be detected.14,15 These immune responses have been interpreted as a consequence of the damage to the epithelium and the access of gliadin to the lamina propria, which results in the secondary expression of hidden epitopes present in connective tissue.14,16
The protein components of the basement membrane in the interface between the enterocytes and the connective tissue have a dual origin because they are synthesised by both the epithelium and the myofibroblasts. Their distribution along the crypt–villus axis establishes gradients that are involved in the regulation of multiplication and differentiation of the absorptive epithelium.5,7,17 It is possible that the injury causing coeliac disease affects the matrix of connective tissue and that the changes in the architecture of the mucosa result, at least in part, from this damage to the matrix. The antiendomysium antibodies, directed against tissue transglutaminase, alter the exchange of information between myofibroblasts and enterocytes, and at the same time induce the activation of tissue metalloproteinases, which results in the changes in mucosal architecture seen in coeliac disease. For this reason, we tried to answer the following question: are there any gross changes in the extracellular matrix or the basement membrane of the epithelium which could be associated with the lesion of the small intestinal mucosa of coeliac patients?
Changes of some components of the basement membrane cause morphological and functional alterations of the intestinal mucosa. Goulet et al suggested that inborn defects of laminin are associated with a flattening of the mucosal surface that resembles that of coeliac disease.18 Other authors, using non-specific stains, described deposits of subepithelial collagen below the basement membrane of the epithelium in coeliac disease and in other conditions of the gastrointestinal tract.19–21 In our study, type IV collagen was not increased and, on the contrary, it consistently stained less intensely than in the normal controls. This suggests that the thickening of the basement membrane demonstrated by other investigators19–21 may be caused by other forms of collagen, not by type IV.
Our study shows that in coeliac disease, the distribution of type IV collagen, laminin, and fibronectin is similar to the controls but that these proteins consistently stain less intensely and there are increased numbers of breaches. Similar changes are detected in the subepithelial blood capillaries, which suggests that there may be a generalised alteration of basement membranes in coeliac disease.
“Our results support the idea that in coeliac disease gliadin sets in motion mechanisms that damage the small intestinal mucosa by acting primarily on elements of the lamina propria and that the epithelium is secondarily affected”
Korhonen et al recently published the results of a similar study on laminin, fibronectin, and tenascin. Their results show that laminin distribution is maintained in coeliac disease but that the proportion between the different zones of the mucosa varies—there were differences in the distribution of laminin isoforms along the crypt–villus axis.22 In our study, we only evaluated the α5 laminin isoform and, like Korhonen et al, we found that its distribution followed a gradient along the crypt–villus axis, with staining more intense in the villi and the flat surface of the mucosa in untreated patients with coeliac disease. The breaches in the continuity of the basement membrane may be caused by impairment of the capacity to repair damage to this structure or by the increased migration of lymphocytes in and out of the epithelial compartment. In our study, the results for fibronectin were similar to those described by Korhonen et al—fibronectin was normally distributed in coeliac disease, but staining was more intense in the crypts; however, in our study staining of the basement membrane proteins was less intense in patients with coeliac disease than in the controls. In coeliac disease, mRNA for matrix metalloproteinases is increased and the increased enzymatic activity may explain the less intense staining of the basement membrane proteins that we observed.22,23
It was difficult to establish differences when comparing the density and distribution of the immunogold labelling between healthy controls and patients with coeliac disease at all stages of the disease. This probably results from the magnifications that had to be used to identify the 10 nm gold label and the fact that the density of the labelling is not high. This technique corroborated most of our findings by immunofluorescence, but it also showed the location of fibronectin and laminin in the cytoplasm of enterocytes and myofibroblasts.
It has been proposed that in the small intestinal mucosa there are at least two populations of myofibroblasts that are involved in the regulation of the proliferation, differentiation, and function of the epithelium. Myofibroblasts from the villi, which stain for αSMA, stimulate the differentiation of crypt cells and play a role in the development of a mature cover of villus enterocytes, whereas the myofibroblast population surrounding the crypts, which do not stain for αSMA, stimulates epithelial stem cell proliferation and crypt development.24 The equilibrium between these two populations of myofibroblasts may shift as a result of alterations in mucosal cytokine patterns, favouring the preferential development of either crypts or villi and inducing differences in the amounts of laminin and type IV collagen synthesised.24,25 Gliadin induces changes in the pattern of intestinal cytokines of patients with coeliac disease,26,27 and this may modulate the morphology and function of the small intestinal mucosa. In our study, αSMA staining enabled us to see the changes in the organisation of smooth muscle in the axis of the villi, and perhaps in the myofibroblasts of the areas where the mucosa is flat.
Our results support the idea that in coeliac disease gliadin sets in motion mechanisms that damage the small intestinal mucosa by acting primarily on elements of the lamina propria and that the epithelium is secondarily affected; the functional derangement of the epithelium is a consequence of the primary effect of gliadin and antibodies directed against tTG, reticulin, and endomysium on the myofibroblasts and the extracellular matrix of the connective tissue.
Take home messages.
The intensity of staining of type IV collagen, laminin, and fibronectin is decreased in coeliac disease and the distribution of smooth muscle fibres is altered
These changes may result from interactions between gliadin and components of the extracellular matrix and may play a role in the genesis of mucosal lesions and in the damage to the epithelium
Acknowledgments
Supported by Fondecyt Project 198–1089. The authors are grateful to Drs J Wenger, F Ossandon, and JJ Latorre of Hospital Luis Calvo Mackenna; Drs E Chávez and S Cruchet of Hospital Clínico San Borja Arriarán; and Dr A Csendes of Hospital Clínico Universitario JJ. Aguirre, Santiago, Chile for their generous cooperation. We acknowledge the Fellowship of the Nestlé Scholarship Programme for Dr S Verbeke.
Abbreviations
FITC, fluorescein isothiocyanate
αSMA, α-smooth muscle actin
tTG, tissue transglutaminase
REFERENCES
- 1.Trier JS. Celiac sprue. N Engl J Med 1991;325:1709–19. [DOI] [PubMed] [Google Scholar]
- 2.Arato A, Hacsek G, Savilahti E. Immunohistochemical findings in the jejunal mucosa of patients with coeliac disease. Scand J Gastroenterol 1998;33(suppl):S3–10. [PubMed] [Google Scholar]
- 3.Kedinger M. What triggers intestinal cells to move or stay and to proliferate or differentiate?. Gastroenterology 1994;107:885–7. [DOI] [PubMed] [Google Scholar]
- 4.Simon-Assmann P, Kedinger M. Heterotypic cellular co-operation in gut morphogenesis and differentiation. Cell Biol 1993;4:221–30. [DOI] [PubMed] [Google Scholar]
- 5.Simon-Assmann P, Bouziges F, Vigny M, et al. Origin and deposition of basement membrane heparan sulfate proteoglycan in the developing intestine. J Cell Biol 1989;109:1837–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simo P, Simon-Assmann P, Arnold C, et al. Mesenchyme-mediated effect of dexamethasone on laminin in cocultures of embryonic gut epithelial cells and mesenchyme-derived cells. J Cell Sci 1992;101:161–71. [DOI] [PubMed] [Google Scholar]
- 7.Beaulieu JF, Vachon P. Reciprocal expression of laminin a-chain isoforms along the crypt–villus axis in the human small intestine. Gastroenterology 1994;106:829–39. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu JF. Differential expression of the VLA family of integrins along the crypt–villus axis in the human small intestine. J Cell Sci 1992;102:427–36. [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez ED, Grapperhaus KJ, Rubin DC. Ontogenic regulation of spatial differentiation in the crypt–villus axis of normal and isografted small intestine. Am J Physiol 1995;269:G500–11. [DOI] [PubMed] [Google Scholar]
- 10.Mäki M. Coeliac disease and autoimmunity due to unmasking of cryptic epitopes? Lancet 1996;348:1046–7. [DOI] [PubMed] [Google Scholar]
- 11.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of coeliac disease. Nat Med 1997;3:797–801. [DOI] [PubMed] [Google Scholar]
- 12.Halttunen T, Mäki M. Serum immunoglobulin A from patients with celiac disease inhibits human T84 intestinal crypt epithelial cell differentiation. Gastroenterology 1999;116:566–72. [DOI] [PubMed] [Google Scholar]
- 13.Walker-Smith JA, Guandalini S, Schmitz J. Revised criteria for diagnosis of coeliac disease. Report of working group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child 1990;65:909–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mäki M. The humoral immune system in coeliac disease. In: Howdle PD, ed. Coeliac disease. Ballieres Clin Gastroenterol 1995;9:231–49. [DOI] [PubMed] [Google Scholar]
- 15.Mäki M. Autoantibodies as markers of autoimmunity in coeliac disease pathogenesis. In: Feighery C, O'Farrely C, eds. Gastrointestinal immunology and gluten-sensitive disease. Dublin: Oak Tree Press, 1994:246–52.
- 16.Mäki M. Celiac disease. In: Dervin EE, Lentze MJ, eds. Gastrointestinal functions. Nestlé Nutrition Workshop Series, Pediatric Program, Vol. 46. Nestec Ltd, Vevey/Lippincott. Philadelphia: William & Wilkins, 2001:257–74.
- 17.Pujuguet P, Hammann A, Martin F, et al. Abnormal basement membrane in tumors induced by rat colon cancer cells. Gastroenterology 1994;107:701–11. [DOI] [PubMed] [Google Scholar]
- 18.Goulet O, Kedinger M, Brousse N, et al. Intractable diarrhea of infancy with epithelial and basement membrane abnormalities. J Pediatr 1995;127:212–19. [DOI] [PubMed] [Google Scholar]
- 19.Weinstein WM, Saunders DR, Tytgat GN, et al. Collagenous sprue: an unrecognized type of malabsorption. N Engl J Med 1970;283:1207–301. [DOI] [PubMed] [Google Scholar]
- 20.Zeroogian JM, Chopra S. Collagenous colitis and lymphocytic colitis. Annu Rev Med 1994;45:105–18. [DOI] [PubMed] [Google Scholar]
- 21.Bossart R, Henry K, Booth CC, et al. Subepithelial collagen in intestinal malabsorption. Gut 1975;16:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korhonen M, Ormio M, Burgeson RE, et al. Unaltered distribution of laminins, fibronectin and tenascin in celiac intestinal mucosa. J Histochem Cytochem 2000;48:1011–20. [DOI] [PubMed] [Google Scholar]
- 23.Daum S, Bauer U, Foss H-D, et al. Increased expression of mRNA for matrix metalloproteinases-1 and -3 and tissue inhibitor of metalloproteinases-1 in intestinal biopsy samples from patients with coeliac disease. Gut 1999;44:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell DW, Mifflin RC, Valentich JD, et al. Myofibroblast. II. Intestinal subepithelial myofibroblasts. Am J Physiol 1999;277:C193–201. [DOI] [PubMed] [Google Scholar]
- 25.Fritsch C, Simon-Assmann P, Kedinger M, et al. Cytokines modulate fibroblast phenotype and epithelial–stroma interactions in rat intestine. Gastroenterology 1997;112:826–38. [DOI] [PubMed] [Google Scholar]
- 26.Chowers Y, Marsh MN, De Granpre L, et al. Increased proinflammatory cytokine gene expression in the colon of celiac disease patients in the early period after gluten challenge. Clin Exp Immunol 1997;107: 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsen EM, Jahnsen FL, Lundin KEA, et al. Gluten induces an intestinal cytokine response strongly dominated by interferon-gamma in patients with celiac disease. Gastroenterology 1998;115:551–63. [DOI] [PubMed] [Google Scholar]