Abstract
Aims: Cyclo-oxygenases 1 and 2 (COX-1 and COX-2) are key enzymes in prostaglandin biosynthesis. COX-2 is induced by a wide variety of stimuli, and present during inflammation. COX-2 overexpression has been observed in colon, head and neck, lung, prostate, stomach, and breast cancer. In colon and gastric cancer, COX-2 expression was associated with angiogenesis. The aim of this study was to determine the relation between COX-2 expression and angiogenesis in breast cancer, and to correlate the expression of this enzyme with classic clinicopathological parameters.
Methods: COX-2 expression was investigated by immunohistochemistry and western blotting analysis. The expression of COX-2 was then related to age, histological grade, nodal status, oestrogen receptor status, p53 expression,c-erb-B2 overexpression, mitotic counts, MIB-1 labelling index, apoptotic index, sialyl-Tn expression, transforming growth factor α expression, microvessel density, and disease free survival in 46 patients with invasive ductal breast carcinoma.
Results: By means of immunohistochemistry, COX-2 expression was detected in eight of the 46 carcinomas studied. Western blotting showed COX-2 protein expression in the same breast tumours, but not in normal adjacent tissues. The density of microvessels immunostained with anti-F-VIII related antigen was significantly higher in patients with COX-2 expression than in those without expression (p = 0.03). In addition, COX-2 was significantly associated with the presence of sialyl-Tn expression (p = 0.02), lymph node metastasis (p = 0.03), a high apoptotic index (p = 0.03), and a short disease free survival (p = 0.03) in univariate analyses.
Conclusions: These data suggest that COX-2 expression is associated with angiogenesis, lymph node metastasis, and apoptosis in human breast cancer. Moreover, these results warrant further studies with larger series of patients to confirm the association with short disease free survival in patients with breast cancer.
Keywords: cyclo-oxygenase 2, angiogenesis, breast cancer
A rachidonic acid metabolites, such as prostaglandins, thromboxanes, and various lipoxygenase products, are produced in many tissues and are responsible for a wide variety of biological responses. They are potent mediators, affecting several signal transduction pathways that modulate cellular adhesion, growth, and differentiation.1,2
Cyclo-oxygenase (COX) (otherwise known as prostaglandin endoperoxide synthase) catalyses the conversion of arachidonic acid to prostaglandin endoperoxide (prostaglandin H2). This is the rate limiting step in prostaglandin and thromboxane biosynthesis. Two isoforms of prostaglandin synthase have been identified and are often referred to as COX-1 and COX-2.3 COX-1 is constitutively expressed in most tissues and is thought to be involved in maintaining cellular homeostasis.4 In contrast, COX-2 is frequently undetectable at baseline in normal tissues, but can undergo rapid induction in response to a variety of stimuli, including mitogens, hormones, cytokines, and growth factors. The effects of both cyclo-oxygenases are mediated via tyrosine kinase, protein kinase C, and/or protein kinase A signal transduction pathways.5
Overexpression of COX-2 and high concentrations of prostaglandins have been associated with chronic inflammatory diseases, such as rheumatoid arthritis,6,7 and several types of human cancer, including colon, head and neck, lung, bladder, prostate, stomach, and breast cancer.8–18 During cancer progression, prostaglandins can mediate several mechanisms, including cell proliferation,19 apoptosis,20,21 modulation of the immune system,22,23 and angiogenesis.24,25 Angiogenesis or neovascularisation, is the formation of new blood vessels from pre-existing ones.26,27 In physiological processes, angiogenesis plays an important role in the female reproductive cycle (ovulation, menstruation) and in tissue growth and repair. In chronic inflammatory diseases, the formation of new blood vessels maintain the inflammatory state, by transporting inflammatory cells and supplying oxygen and nutrients to the proliferative inflamed tissue.28
“Increased concentrations of prostaglandins E2, a major product of cyclo-oxygenase 2, have been reported in human breast cancer and in experimental murine mammary tumour models”
During tumorigenesis, angiogenesis is important for tumour growth and metastasis. Any increase in a tumour mass must be preceded by an increase in the vascular supply, to deliver nutrients and oxygen to the tumour. Angiogenesis is mediated by a change in the local equilibrium between positive and negative angiogenic factors. Among the most important proangiogenic factors are vascular endothelial growth factor (VEGF), fibroblast growth factor, platelet derived growth factor, and angiopoietin 1.26,27
Although the mechanisms underlying the contribution of COX-2 to tumorigenesis are still unclear, an association between COX-2 overexpression and angiogenesis has been demonstrated in two types of cancer—colorectal and gastric cancer.23,24 Overexpression of COX-2 in a colorectal cancer cell line was shown to stimulate the production of angiogenic factors, such as VEGF.24 In a recent study, COX-2 expression was associated with increased angiogenesis in gastric cancer.25
Increased concentrations of prostaglandins E2, a major product of COX-2, have been reported in human breast cancer and in experimental murine mammary tumour models.29,30 Several studies with murine mammary tumour cells indicate that prostaglandins E2 may have a multifunctional role in controlling growth, metastasis, and the host immune response in breast cancer.29–31 The COX-2 expression profile has also been evaluated in human breast cancer samples,32,33 but the causal role and molecular mechanisms of the enzyme during breast tumorigenesis are still not well defined.
Therefore, the aim of our present study was to investigate whether there is an association between COX-2 expression and angiogenesis, in addition to other clinicopathological parameters of aggressiveness in human breast cancer.
MATERIAL AND METHODS
Case selection
Our study was carried out in 46 women, aged 28–77 years (mean, 56; SE, 1.3), with primary breast carcinomas diagnosed in our institute. Surgical specimens obtained by mastectomy were 10% buffered formalin fixed and paraffin wax embedded; 4 μm sections were cut and stained with haematoxylin and eosin. The histological grading was performed using the modified criteria of Bloom and Richardson, as described by Elston and Ellis.34 The disease free survival was defined as the period between the diagnosis and death of the patient. Only patients with at least a 24 month follow up period were included in the survival analysis. Frozen tumour material was obtained from 19 of the 46 patients, in addition to two matched normal breast tissue samples, and these samples were used for western blotting.
Mitotic counts and the evaluation of apoptosis
Mitotic counts and the evaluation of the apoptotic index were performed according to the method described and validated by Liu et al.35 Briefly, to assess the mitotic counts we only considered those mitotic cells in which (1) the nuclear membrane was absent, (2) the condensed chromosomes showed hair-like extensions of nuclear material, (3) there was no central clear zone, and (4) there was a basophilic staining of the cytoplasm. To evaluate the apoptotic index, we considered only those apoptotic cells that showed the following morphological criteria: cell shrinkage, chromatin condensation, nuclear fragmentation, and lack of inflammatory cells.
To obtain the mitotic counts, we counted the number of mitotic figures in 10 high power fields (HPFs) using a ×40 objective and ×10 ocular, numerical aperture 0.74 mm2 in the hot spot areas of the tumours, which were defined as the areas with the highest cellularity and number of mitotic figures.
To calculate the apoptotic index, in each tumour we assessed only the areas with higher cellularity without necrosis and estimated the number of cells for each field as described by Liu et al.35 After that, we counted the number of typical apoptotic cells in 10 consecutive HPFs, as described by these authors, and calculated the apoptotic index, which was defined as the number of apoptotic cells in each 1000 cell.
Immunohistochemistry
The streptavidin–biotin peroxidase complex method was used for immunohistochemistry. Briefly, 4 μm sections were cut from paraffin wax blocks, dewaxed, and hydrated. Factor VIII related antigen (F-VIII) immunostaining was performed with antigen retrieval by means of pepsin digestion, at room temperature for 30 minutes. Oestrogen receptor (ER) (Dakopatts, Glostrup, Denmark), p53 (Immunotech, Marseille, France), and MIB-1 (Novocastra, Newcastle, UK) immunostainings were performed, preceded by antigen retrieval with incubation in 10mM citrate buffer for 7.5 minutes at 750 W (domestic microwave), in a thermoresistant container. Distilled water and buffer were added every 1.5 minutes to the container to prevent drying during the incubation process. Immunostaining for c-erbB2 (Dakopatts), transforming growth factor α (TGF-α) (Serotec, Oxford, UK), and sialyl Tn (STn; Dakopatts) was carried out with no previous antigen retrieval step. Induced epitope retrieval using a target retrieval solution was used for COX-2. The slides were treated with 3% peroxide (H2O2), in methanol for 10 minutes to quench the endogenous peroxidase activity. ER, p53, c-erbB2, STn, TGF-α, and MIB-1 immunostaining was carried out as described previously.36 Polyclonal antibodies for F-VIII (Dakopatts) and COX-2 (Santa Cruz Biotechnology, San Diego, California, USA) were applied to the sections at a dilution of 1/150 (with a 30 minute incubation at room temperature) and 1/10 with an overnight incubation at 4°C, respectively. The anti-COX-2 antibody is specific for COX-2, and does not crossreact with COX-1; it recognises an epitope mapping to the C-terminus of human COX-2.
Negative controls were carried out by omission of the primary antibody. As positive controls, sections from previously studied cases of breast cancer known to express ER, p53, MIB-1, c-erbB2, TGF-α, and STn were used. Sections from highly angiogenic breast carcinomas and colon carcinomas known to express COX-2 were used as positive controls for F-VIII and COX-2, respectively.
Evaluation of the immunohistochemical data
ER, p53, TGF-α, c-erbB2, MIB-1, and STn were evaluated as described previously.36,37 COX-2 positivity was indicated by the presence of brown cytoplasmic staining. Immunoreactivity was evaluated in epithelial cells, in neighbouring stroma, and in endothelial cells. All cases with any unequivocal staining of the cytoplasm of neoplastic cells for COX-2 were considered positive.33 Angiogenesis was evaluated by immunohistochemical staining of intratumoral microvessels for F-VIII. Any positively staining single cell, or cluster of cells, clearly separated from adjacent clusters and background, with or without lumen, was considered an individual vessel, as recommended in previous studies.38,39 Areas of fibrosis, necrosis, and inflammation, in addition to vessels with a muscle wall, were excluded from the counting. Microvessels were counted in the three most vascularised areas in a 200× field (0.74 mm2) by four observers simultaneously. Because there was no significant difference between the highest count and the average vessel count in each case, we used the results obtained in the average vessel count in each case.
Western blotting analysis
Total protein was extracted from frozen tissues using the Tripure isolation reagent (Boehringer, Mannhein, Germany) and measured by spectrophotometry. Equal amounts of protein were denatured by boiling for two minutes, and then electrophoresed in a 10% polyacrylamide gel with a 5% stacking gel. After electrophoresis, proteins were electrotransferred to a Hybond nitrocellulose membrane (Amersham, Little Chalfont, Buckinghamshire, UK). Membranes were incubated with antibodies against COX-2 (Santa Cruz Biotechnology) and β actin (Santa Cruz Biotechnology) at a 1/2000 dilution overnight at 4°C. After several washing cycles the membranes were incubated with horseradish peroxidase conjugated secondary antibody at a 1/2000 dilution, for 45 minutes at room temperature. Western blotting protein products were visualised using the ECL chemiluminescence system (Amersham), after membrane exposure to an autoradiography film (Hyperfilm; Amersham). The highly invasive breast cancer cell line Hs578T, which is known to express COX-2, was used as a positive control40 and β actin was used as the internal control.
Statistical analysis
Statistical analysis was carried out using a Statview program. Statistical differences between COX-2 expression and histological grade, node status, TGF-α expression, ER content, p53 expression, c-erbB2 expression, and STn expression were calculated using the χ2 test. Analysis of variance (ANOVA) with Yates correction was used to compare COX-2 expression and mean values for age, proliferative index, mitotic counts, apoptotic index, and microvessel density. A value of p < 0.05 was considered significant. As a further validation of the apoptotic index and mitotic counts, we compared these two continuous variables with the MIB-1 proliferation index using Spearman's rank correlation.
To perform the disease free survival analysis, patient groups were defined according to positive or negative immunoreactivity for COX-2 and were compared for disease free survival using the Cox-Mantel non-parametric test. The test was performed unilaterally for all of the comparisons; the null hypothesis was that both groups were equal in terms of time under evaluation, and the alternative hypothesis was that one of the groups evolved better than the other.
RESULTS
Immunohistochemical analysis of breast tumour tissues for COX-2
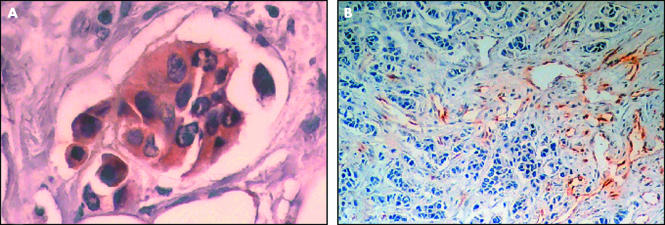
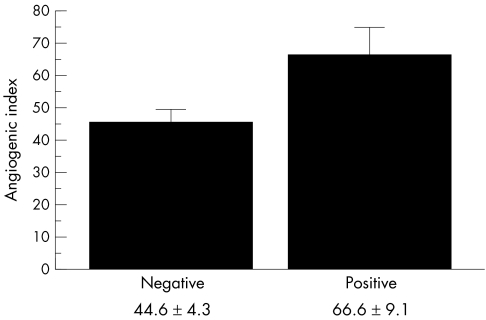
Immunohistochemical staining of the formalin fixed, paraffin wax embedded tumour tissue revealed COX-2 cytoplasmic immunoreactivity in eight of the 46 patients with invasive ductal breast carcinoma studied. COX-2 expression was restricted to tumour cells in all the positive samples (fig 1A). Neighbouring stromal cells, such as fibroblasts and endothelial cells, showed no immunoreactivity for COX-2. Table 1 summarises the associations between COX-2 expression in malignant cells and other clinicopathological features studied. Tumour expression of COX-2 was significantly associated with microvessels count evaluated by F-VIII expression (table 1; figs 1B,2). COX-2 expression was also significantly associated with lymph node metastases, apoptotic index, and STn expression in neoplastic cells. No significant relation was found between COX-2 immunostaining in tumour cells and the other clinicopathological characteristics analysed (table 1).
Figure 1.
Immunohistochemical analysis of breast tumour tissue using antibodies to (A) cyclo-oxygenase 2 and (B) factor VIII related antigen to evaluate angiogenesis.
Table 1.
Cyclo-oxygenase 2 (COX-2) expression in tumour cells in relation to other clinicopathological features
| COX-2 expression in the neoplastic cells | ||||
| Clinicopathological features | No. of cases | Positive | Negative | p Value |
| Age in years (mean (SE)) | 46 | 49.8 (5.8) | 57.4 (2.2) | 0.82 |
| Histological grade | 0.16 | |||
| I | 8 | 0/8 | 8/8 | |
| II | 19 | 3/19 | 16/19 | |
| III | 16 | 5/16 | 11/16 | |
| Nodal status | 0.03 | |||
| Positive | 22 | 7/22 | 15/22 | |
| Negative | 20 | 1/20 | 19/20 | |
| Transforming growth factor α | 0.38 | |||
| Positive | 34 | 7/34 | 27/34 | |
| Negative | 11 | 1/11 | 10/11 | |
| Oestrogen receptor content | 0.46 | |||
| Positive | 27 | 4/27 | 23/27 | |
| Negative | 17 | 4/17 | 13/17 | |
| p53 expression | 0.82 | |||
| Positive | 20 | 2/20 | 18/20 | |
| Negative | 23 | 4/23 | 19/23 | |
| c-erbB2 expression | 0.39 | |||
| Positive | 11 | 3/11 | 8/11 | |
| Negative | 32 | 5/32 | 27/32 | |
| Sialyl-Tn expression | 0.02 | |||
| Positive | 14 | 6/14 | 8/14 | |
| Negative | 21 | 2/21 | 19/21 | |
| Microvessel count (mean (SE)) | 46 | 66.6 (9.0) | 44.6 (4.3) | 0.03 |
| Apoptotic index (mean (SE)) | 7.2 (1.3) | 3.9 (0.5) | 0.03 | |
| Mitotic count (mean (SE)) | 43 | 17.7 (2.3) | 17.7 (2.7) | 0.99 |
| MIB-1 expression (mean (SE)) | 35 | 22.2 (4.8) | 24.1 (4.2) | 0.88 |
Figure 2.
Association between cyclo-oxygenase 2 expression and angiogenic index (mean ± SE) evaluated by immunohistochemistry (p = 0.03).
Western blotting analysis of COX-2 in breast tumour tissues and matched normal tissues
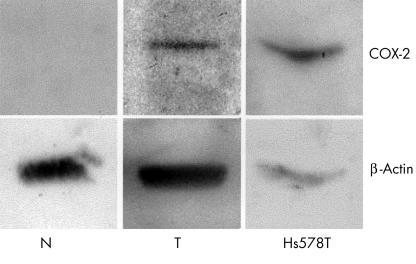
COX-2 expression was also analysed by western blotting in tumour specimens from 19 of the invasive ductal breast carcinoma frozen samples and two matched normal breast tissue samples. COX-2 expression was detected in eight of the 19 cases studied. In all cases in which COX-2 was detected by western blotting, immunohistochemistry was also positive for COX-2. In normal tissue there was no expression of COX-2. Figure 3 illustrates the western blotting results from a tumour and its matched normal tissue.
Figure 3.
Western blotting analysis for adjacent normal tissue (N) and a breast tumour (T). The Hs578T breast cancer cell line was used as a positive control for cyclo-oxygenase 2 (COX-2) expression. Note the presence of a low intense band for COX-2 in the tumour and the absence of COX-2 in the adjacent normal tissue.
Mitotic counts and apoptotic index
The mean (SE) values of the mitotic counts in 10 HPFs and the apoptotic index in each 1000 cells were 17.74 (2.30) and 4.51(0.57), respectively. There was a highly significant correlation between mitotic counts and apoptotic index (p < 0.0001). In addition, an equally strong significant association was found between the mitotic index and the MIB-1 proliferation index (p < 0.0001).
COX-2 positive cases showed a significantly higher apoptotic index (p = 0.031); however, no association was found between mitotic counts or MIB-1 and COX-2 expression (p = 0.995 and p = 0.883, respectively).
Disease free survival analysis
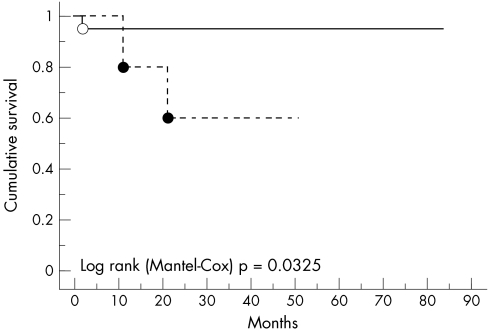
For the disease free survival analysis, we only included the 26 patients who attained a minimum 24 month follow up period. The relation between COX-2 expression and disease free survival is shown using Kaplan-Meier survival curves (fig 4). Using the log rank Cox-Mantel test, patients with breast cancer whose tumours were COX-2 positive had a significantly shorter disease free survival (p = 0.0325).
Figure 4.
Cyclo-oxygenase 2 (COX-2) positive and COX-2 negative breast carcinomas plotted against disease free survival.
DISCUSSION
Our study revealed that the COX-2 protein was expressed in cases of human invasive ductal breast carcinoma, analysed by both immunohistochemistry and western blotting, whereas the COX-2 protein was undetectable in normal breast tissue. These results are in accordance with the data reported by Parrett and colleagues32 and Hwang et al.33 Both these studies, which used different analytical methods, showed increased COX-2 expression in human breast carcinomas. Our figures (17.4% of the cases) were similar to those reported by Parrett et al,32 although they were lower than those described by Huang et al,33 who showed COX-2 expression in 68.18% of breast cancer samples.
Although the role of COX-2 during tumorigenesis is not yet well defined, an association between tumour COX-2 expression and angiogenic status was seen in our study. Angiogenesis is required during tumorigenesis for the supply of oxygen and nutrients. At the same time, angiogenesis helps tumour cells to migrate through blood vessels and metastasise to distant organs. In our study, the expression of COX-2 was only seen in tumour cells and not in stromal or endothelial cells. However, the expression of COX-2 in tumour cells was related to increased endothelial cell proliferation. COX-2 is one of the enzymes that catalyses the synthesis of prostaglandins, which are local hormones mainly involved in the inflammatory response. Recently, several reports have suggested a role for prostaglandins in angiogenesis. Arakawa et al showed that ulcer healing was accelerated when prostaglandins are synthesised by COX-2, but not by COX-1.41 These findings emphasise the putative stimulation of angiogenesis by a COX-2 dependent mechanism. Accordingly, other studies suggest that prostaglandins exhibit vasoactive and mitogenic properties and are important mediators of increased vascular permeability in the endometrium.42 COX-2 has also been proposed to reduce the adherence of tumour cells to the extracellular matrix, promoting angiogenesis and the development of metastasis. Similarly, COX-2 overexpression has been seen in several pathological states, such as cancer and rheumatoid arthritis, in which angiogenesis is strongly required. All these findings are in agreement with our results, suggesting that COX-2 may also play a role in angiogenesis. The involvement of COX-2 in angiogenesis has already been reported in other cancers, such as colorectal and gastric cancer.24,25 However, to our knowledge this is the first study to suggest that COX-2 might also play a role in angiogenesis in breast cancer.
Despite the various reports of the involvement of COX-2 in angiogenesis, little is known with regard to its mechanism. Tsujii et al showed that tumours expressing COX-2 often produce VEGF and basic fibroblast growth factor, two growth factors associated with angiogenesis.24 VEGF is a specific endothelial cell promotor because it acts by binding to its receptors (VEGFR-I and VEGFR-II) that exist in the cytoplasmic membrane of endothelial cells. VEGF is also called vascular permeability factor because of its ability to increase vessel permeability, which is important during both angiogenesis and inflammation processes. Because COX-2 and its products (prostaglandins) are mainly regarded as inflammatory factors, it is probable that they stimulate VEGF to induce permeability. COX-2 is overexpressed in several cancer types and it seems to upregulate angiogenesis; therefore, the use of COX inhibitors (such as non-steroidal anti-inflammatory drugs or preferentially specific COX-2 inhibitors) might be useful in antiangiogenic treatment.
“To our knowledge this is the first study to suggest that cyclo-oxygenase 2 might also play a role in angiogenesis in breast cancer”
We found no significant association between COX-2 expression and age, tumour grade, TGF-α, ER receptor content, p53, c-erbB2, or MIB-1. Despite the observations by Lui and Rose43 in two human breast cancer cell lines, where COX-2 expression is regulated by hormonal status, no significant association between COX-2 and ER status was found in our series. This might be because of the low sensitivity of the immunohistochemical analysis performed or because of the fact that there are two ERs: ERα and ERβ.44 In our study, we only analysed ERα; thus, our results may have been an underestimation. Although COX-2 is an inflammatory mediator, no association was found between COX-2 expression and tumour inflammatory infiltration (data not shown). Sawaoka et al,45 studying normal gastric epithelial cells, showed that COX-2 was induced by TGF-α and thereafter enhanced cell proliferation. In a previous study, we found that TGF-α was present in 75.1% of the breast cancer cases studied and was associated significantly with angiogenesis.46 However, in that study we found no association between TGF-α and COX-2.
Despite the fact that COX-2 positive cases did not exhibit higher proliferative indices, as evaluated both by MIB-1 immunoexpression and mitotic counting, we found a strong association between COX-2 expression and the apoptotic index. These findings provide further evidence for the putative role of COX-2 in the control of apoptosis in neoplastic cells of several types of human cancer,21 including human breast cancer cells.
In our present study, we also found an association between COX-2 expression, lymph node status, and STn expression. STn is a simple mucin-type carbohydrate antigen, which is also associated with lymph node metastases, as described previously by our group.37
We found a significant association between COX-2 expression and a reduced disease free survival of patients with breast cancer in univariate analysis. In view of the small number of patients included in our study, these findings warrant larger studies with multivariate analysis to clarify the association of COX-2 and poor prognosis in patients with breast cancer.
Take home messages.
Cyclo-oxygenase 2 (COX-2) expression appears to be associated with angiogenesis, lymph node metastasis, and apoptosis in human breast cancer
However, our results indicate that COX-2 expression in human breast cancer might be a late event in tumour progression and not involved in tumorigenesis
Further studies with larger series of patients are needed to confirm the association with short disease free survival in patients with breast cancer
In conclusion, we found a significant association between COX-2 expression, microvessel density, apoptotic index, STn expression, and lymph node status. These findings could indicate that COX-2 expression in human breast cancer might be a late event in tumour progression, in contrast to colorectal and gastric cancer, in which COX-2 plays a role in tumorigenesis and is considered to be an early event.
New JCP online submission and review system
We are pleased to inform authors and reviewers of the new online submission and review system at JCP. Developed by HighWire Press (CA, USA), Bench Press is a fully integrated electronic system that utilises the web to allow rapid and efficient submission of manuscripts. It also allows the peer review process to be conducted entirely online. We are one of the first journals in the BMJ Special Journals group to go online in this way. The aim, apart from saving trees, is to speed up the often frustratingly slow process (for both authors and editors) from submission to publication. Many reviewers might appreciate this too. Authors may submit their manuscript in any standard word processing software. Acceptable standard graphic formats include: jpeg, tiff, gif, and eps. The text and graphic files are automatically converted to PDF for ease of distribution and reviewing purposes. Authors are asked to approve their submission before it formally enters the reviewing process. On approval by the authors, the submission is passed to the editor and/or reviewers via the web. All transactions are secure. To access the system click on “SUBMIT YOUR MANUSCRIPT HERE” on the JCP homepage: HYPERLINK http://www.jclinpath.com, or you can access Bench Press directly at HYPERLINK http://submit-jcp.bmjjournals.com. We are very excited with this new development and would encourage authors and reviewers to use the online system whenever possible. As editors, we will use it all the time, the up side being lack of need to travel to the editorial office to deal with papers, the down side having no more excuses to postpone decisions on papers because we are “at a meeting”! The system is very easy to use and should be a big improvement on the current peer review process. Full instructions can be found on Bench Press http://submit-jcp.bmjjournals.com and JCP online at http://www.jclinpath.com. Please contact Natalie Davies, Project Manager, HYPERLINK mailto:ndavies@bmjgroup.com for any further information.
H Holzel, P van Diest
Abbreviations
COX, cyclo-oxygenase
ER, oestrogen receptor
F-VIII, factor VIII related antigen
HPF, high power field
STn, sialyl Tn
TGF-α, transforming growth factor α
VEGF, vascular endothelial growth factor
REFERENCES
- 1.Xie WL. Mitogen-inducible prostaglandin G/H synthase: a target for nonsteroidal antiinflammatory drugs. Drug Dev Res 1991;25:249–65. [Google Scholar]
- 2.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 1995;83:493–501. [DOI] [PubMed] [Google Scholar]
- 3.Williams CW, DuBois RN. Prostaglandin endoperoxide synthase: why two isoforms? Am J Physiol 1996;270:G393–400. [DOI] [PubMed] [Google Scholar]
- 4.O'Neill G, Hutchinson AF. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett 1993;330:156–60. [DOI] [PubMed] [Google Scholar]
- 5.Herschman HR. Regulation of prostaglandin synthase-1 and prostaglandin synthase-2. Cancer Metastasis Rev 1994;13:241–56. [DOI] [PubMed] [Google Scholar]
- 6.Simon LS. Role and regulation of cyclooxygenase-2 during inflammation. Am J Med 1999;106:37S–42S. [DOI] [PubMed] [Google Scholar]
- 7.Kargman SL, O'Neill GP, Vickers PJ, et al. Expression of prostaglandin G/H synthase-1 and -2 protein in human colon cancer. Cancer Res 1995;55:2556–9. [PubMed] [Google Scholar]
- 8.Lim HY, Joo HJ, Choi JH, et al. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res 2000;6:519–25. [PubMed] [Google Scholar]
- 9.Bennett A, Charlier EM, McDonald AM, et al. Prostaglandins and breast cancer. Lancet 1977;2:624–6. [DOI] [PubMed] [Google Scholar]
- 10.Wolff H, Saukkonen K, Anttila S, et al. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res 1998;58:4997–5001. [PubMed] [Google Scholar]
- 11.Yip-Schneider MT, Barnard DS, Billings SD, et al. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis 2000;21:139–46. [DOI] [PubMed] [Google Scholar]
- 12.Mohammed SI, Knapp DW, Bostwick DG, et al. Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res 1999;59:5647–50. [PubMed] [Google Scholar]
- 13.Hixson L, Alberts D, Krutzsch M, et al. Antiproliferative effect of nonsteroidal anti-inflammatory drugs (NSAIDs) against human colon cancer cells. Cancer Epidemiol Biomarkers Prev 1994;3:433–8. [PubMed] [Google Scholar]
- 14.Chan G, Boyle JO, Yang EK, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res 1999;59:991–4. [PubMed] [Google Scholar]
- 15.Hida T, Yatabe Y, Achiwa H, et al. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res 1998;58:3761–4. [PubMed] [Google Scholar]
- 16.Wolff H, Saukkonen K, Anttila S, et al. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res 1998;58:4997–5001. [PubMed] [Google Scholar]
- 17.Tucker ON, Dannenberg AJ, Yang EK, et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res 1999;59:987–90. [PubMed] [Google Scholar]
- 18.Tanji N, Kikugawa T, Yokoyama M. Immunohistochemical study of cyclooxygenases in prostatic adenocarcinoma; relationship to apoptosis and Bcl-2 protein expression. Anticancer Res 2000;20:2313–19. [PubMed] [Google Scholar]
- 19.Hanif R, Pittas A, Feng Y, et al. Effects of non-steroidal anti-inflammatory drugs on proliferation and induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol 1996;52:237–425. [DOI] [PubMed] [Google Scholar]
- 20.Sheng H, Shao J, Morrow JD, et al. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res 1998;58:362–6. [PubMed] [Google Scholar]
- 21.Prescott SM, Fitzpatrick FA. Cyclooxygenase-2 and carcinogenesis. Biochem Biophys Acta 2000;1470:M69–78. [DOI] [PubMed] [Google Scholar]
- 22.Fulton AM, Heppner GH. Relationships of prostaglandin E and natural killer sensitivity to metastatic potential in murine mammary adenocarcinomas. Cancer Res 1985;45:4779–84. [PubMed] [Google Scholar]
- 23.Hla T, Ristimaki A, Appleby S, et al. Cyclooxygenase gene expression in inflammation and angiogenesis. Ann N Y Acad Sci 1993;696:197–204. [DOI] [PubMed] [Google Scholar]
- 24.Tsujii M, Kawano S, Tsuji S, et al. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 1998;93:705–16. [DOI] [PubMed] [Google Scholar]
- 25.Uefuji K, Ichikura T, Mochizuki H. Cyclooxygenase-2 expression is related to prostaglandin biosynthesis and angiogenesis in human gastric cancer. Clin Cancer Res 2000;6:135–8. [PubMed] [Google Scholar]
- 26.Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell 1996;87:1153–5. [DOI] [PubMed] [Google Scholar]
- 27.Risau W. Mechanisms of angiogenesis. Nature 1997;386:671–4. [DOI] [PubMed] [Google Scholar]
- 28.Jackson JR, Seed MP, Kircher CH, et al. The codependence of angiogenesis and chronic inflammation. FASEB J 1997;11:457–65. [PubMed] [Google Scholar]
- 29.Schrey MP, Patel KV. Prostaglandin E2 production and metabolism in human breast cancer cells and breast fibroblasts. Regulation by inflammatory mediators. Br J Cancer 1995;72:1412–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rolland PH, Martin PM, Jacquemier J, et al. Prostaglandin in human breast cancer: evidence suggesting that an elevated prostaglandin production is a marker of high metastatic potential for neoplastic cells. J Natl Cancer Inst 1980;64:1061–70. [PubMed] [Google Scholar]
- 31.Fulton AM, Heppner GH. Relationships of prostaglandin E and natural killer sensitivity to metastatic potential in murine mammary adenocarcinomas. Cancer Res 1985;45:4779–84. [PubMed] [Google Scholar]
- 32.Parrett ML, Harris RL, Joarder FS, et al. Cyclooxygenase-2 gene expression in human breast cancer. Int J Oncol 1997;10:503–8. [DOI] [PubMed] [Google Scholar]
- 33.Hwang D, Scollard D, Byrne J, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst 1998;90:455–60. [DOI] [PubMed] [Google Scholar]
- 34.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403–10. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Edgerton SM, Moore DH, 2nd, et al. Measures of cell turnover (proliferation and apoptosis) and their association with survival in breast cancer. Clin Cancer Res 2001;7:1716–23. [PubMed] [Google Scholar]
- 36.Marinho A, Soares R, Ferro J, et al. Angiogenesis in breast cancer is related to age but not to other prognostic parameters. Pathol Res Pract 1997;193:267–73. [DOI] [PubMed] [Google Scholar]
- 37.Soares R, Marinho A, Schmitt F. Expression of sialyl-Tn in breast cancer. Correlation with prognostic parameters. Pathol Res Pract 1996;192:1181–6. [DOI] [PubMed] [Google Scholar]
- 38.Soares R, Botelho M, Silva C, et al. Expression of TGFα and EGFR in breast cancer and its relation to angiogenesis. Breast J 2000;6:171–7. [DOI] [PubMed] [Google Scholar]
- 39.Weidner N, Folkman J, Pozza F, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 1992;84:1875–87. [DOI] [PubMed] [Google Scholar]
- 40.Gilhooly EM, Rose DP. The association between a mutated ras gene and cyclooxygenase-2 expression in human breast cancer cell lines. Int J Oncol 1999;15:267–70. [PubMed] [Google Scholar]
- 41.Arakawa T, Higuchi K, Fukuda T, et al. Prostaglandins in the stomach: an update. J Clin Gastroenterol 1998;27(suppl 1):S1–11. [DOI] [PubMed] [Google Scholar]
- 42.Lim H, Paria B, Das S, et al. Multiple female reproductive failures in cyclooxygenase 1-deficient mice. Cell 1997;91:197–208. [DOI] [PubMed] [Google Scholar]
- 43.Liu XH, Rose DP. Differential expression and regulation of cyclooxygenase-1 and -2 in two human breast cancer cell lines. Cancer Res 1996;56:5125–7. [PubMed] [Google Scholar]
- 44.Gustafsson JA. Estrogen receptor β—a new dimension in estrogen mechanism of action. J Endocrinol 1999;163:379–83. [DOI] [PubMed] [Google Scholar]
- 45.Sawaoka H, Tsuji S, Tsuji M, et al. Involvement of cyclooxygenase-2 in proliferation and morphogenesis induced by transforming growth factor alpha in gastric epithelial cells. Prostaglandins Leukot Essent Fatty Acids 1999;61:315–22. [DOI] [PubMed] [Google Scholar]
- 46.Schmitt F, Soares R. TGFα and angiogenesis. Am J Surg Pathol 1999;23:358–9. [DOI] [PubMed] [Google Scholar]