Abstract
Aims: To compare commercially available Ki-67 equivalent antibodies with regard to qualitative and quantitative immunohistochemical staining characteristics.
Methods: The following antibodies were used: monoclonal MIB-1 (Immunotech), monoclonal MM1 (Novocastra), polyclonal NCL-Ki-67p (Novocastra), and polyclonal Rah Ki-67 (Dako). All immunostainings were evaluated in squamous epithelium from formalin fixed and paraffin wax embedded pharyngeal tonsils. Labelling indices (LIs) were recorded twice to test their reproducibility.
Results: By application of all four antibodies the nuclear staining could be either diffuse, granular, or a combination of both (classified as granular in this study). The diffuse pattern generally showed a strong or moderate staining intensity, whereas the granular pattern displayed a continuum from strong to very weak, making it difficult to discriminate between positive and negative nuclei. The diffuse staining pattern was seen in approximately 59% of the nuclei with the MIB-1 antibody and in 35–45% when the other antibodies were used. The following mean LIs were recorded: MIB-1, 31%; NCL-Ki-67p, 21%; Rah Ki-67, 17%; and MM1, 14%. The reproducibility was excellent for all four antibodies, with the mean of differences between the two runs of counts ranging from 1.1% to 1.5%.
Conclusions: The four tested Ki-67 equivalent antibodies revealed differences in qualitative and quantitative staining characteristics, which resulted in considerable variations in registered LIs. The MIB-1 antibody appears to have a higher sensitivity for detecting the Ki-67 antigen than the other three tested antibodies. These differences are important to consider when proliferative activity is determined by the Ki-67 LI.
Keywords: Ki-67, proliferative activity, immunohistochemistry
Counting of mitoses is the classic method used to determine proliferative activity in normal and neoplastic tissues by light microscopy. However, this method registers only the M phase of the cell cycle and the number of identifiable mitoses also depends upon the period of time between surgical removal and fixation of the specimen.1,2 Furthermore, strict morphological criteria for mitoses have to be used to avoid confusion with nuclear pyknosis and karyorrhexis.3–6 Lack of such standard criteria, in addition to heterogeneous distribution of mitoses,4,7 may explain the often poor reproducibility of mitotic counts.3,8,9 Therefore, there has been a need for alternative methods to determine proliferative activity. However, several of the methods that have been developed have never been used in routine surgical pathology because they are not practical or are time consuming; examples of such methods are the determination of nucleolar organiser regions and 5-bromodeoxyuridine and tritiated thymidine labelling.
An important landmark was reached in 1983 when Gerdes et al reported the monoclonal antibody Ki-67, which recognises a proliferation specific nuclear antigen expressed during the G1, S, G2, and M phases, but not in the G0 phase.10 Determination of the Ki-67 labelling index (LI) soon became widely used in surgical pathology. In most types of cancer (for example, carcinomas, sarcomas, lymphomas, and gliomas), the Ki-67 LI was found to correlate with tumour grade and clinical course.11–14 However, a major drawback of this prototypic Ki-67 antibody is that it can be used on frozen sections only. Therefore, the discovery of the proliferating cell nuclear antigen (PCNA), which could be detected in paraffin wax sections after microwave heating, was met with much enthusiasm. However, it was soon realised that PCNA is not at all proliferation specific,15–18 and many studies revealed a poor correlation between this antigen and other proliferation markers, in addition to clinical parameters.19–22 Consequently, PCNA staining is no longer recommended for use in surgical pathology.20,23,24
During the past decade, several monoclonal and polyclonal antibodies against peptides from recombinant fragments of the Ki-67 antigen have been produced.25–29 The epitopes detected by these Ki-67 equivalent antibodies resist formalin fixation and the immunostainings can thus be performed on paraffin wax embedded sections after antigen retrieval. The most frequently used antibody of this kind is MIB-1, which was first reported by Cattoretti et al in 1992.25 The proliferative activity determined by MIB-1 has generally confirmed the findings obtained with the original Ki-67 antibody; that is, correlation with tumour grade,30–32 clinical course,33–36 and other methods for the evaluation of growth rate.30,31,37
“A major drawback of the prototypic Ki-67 antibody is that it can be used on frozen sections only”
It is expected that the application of the various Ki-67 equivalent antibodies should produce similar staining results, although to our knowledge comparisons of the qualitative and quantitative staining properties of Ki-67 equivalent antibodies under standardised conditions have not been reported previously. Thus, our present study was undertaken to determine and compare the staining properties of four commercially available Ki-67 equivalent antibodies with special emphasis on the LIs.
MATERIALS AND METHODS
We used pharyngeal tonsils obtained at tonsillectomy from 10 patients aged 32–63 years (four men and six women). Clinical indications for surgery were tonsillar hyperplasia, inflammation, or suspicion of malignancy. However, we only included in our study those tonsils without malignancy or inflammation involving the surface squamous epithelium. The specimens were immediately fixed in 4% phosphate buffered formaldehyde. Eight tonsils were fixed for approximately 24 hours, one for five days, and one for seven days before further processing and paraffin wax embedding.
For immunohistochemical stainings, 4 μm sections were incubated with the four Ki-67 equivalent antibodies listed in table 1 after microwave antigen retrieval. For information concerning antibody production details the reader is referred to the data sheets handed out by the suppliers. The optimal working dilution of each antibody was determined by titrations. An automatic immunohistostainer (TechMate 500; Dako, Glostrup, Denmark) was used with a standard avidin–biotinylated immunoperoxidase technique. The lymphoid tissue of the tonsils was used as an internal positive control in each case. In the negative controls the primary antibodies were omitted. All four antibodies produced an immune staining that should be regarded as specific for the Ki-67 antigen, with staining of nuclei at known proliferative locations, such as germinal centres and the basal half of the squamous epithelium, and with no background staining. In serial sections stained with the four antibodies, corresponding areas of the squamous epithelium were identified and marked with ink. Only areas with intact epithelium were evaluated and areas with leucocyte infiltration and prominent stromal papillae were omitted.
Table 1.
Survey of the Ki-67 equivalent antibodies used in our study
| Antibody | Clonality | Dilution | Manufacturer |
| MIB-1 | Monoclonal | 1/100 | Immunotech, Marseille, France |
| NCL-Ki-67-MM1 (MM1) | Monoclonal | 1/100 | Novocastra Laboratories, Newcastle upon Tyne, UK |
| NCL-Ki-67p | Polyclonal | 1/100 | Novocastra |
| Rabbit antihuman Ki-67 antigen (Rah Ki-67) | Polyclonal | 1/150 | Dako AS, Glostrup, Denmark |
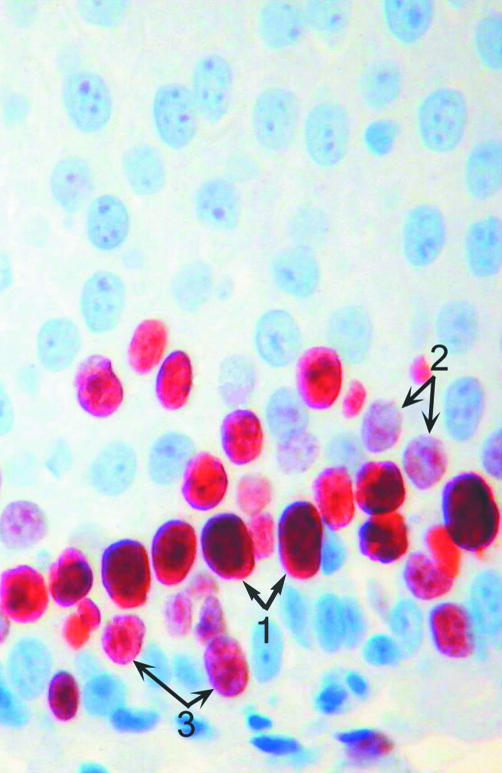
All sections were evaluated with regard to the distribution and intensity of the immunohistochemical reaction product. The following types of staining pattern were seen (fig 1): a diffuse staining of the nucleoplasm and a granular pattern, which stained nucleoli or granules of different size dispersed throughout the nucleoplasm. Some nuclei showed a mixed pattern; that is, strongly stained granules against a diffusely positive background of a lower staining intensity. We classified these nuclei as granular. The staining intensity of the diffuse and granular patterns was classified as strong, moderate, or weak.
Figure 1.
MIB-1 stain of squamous epithelium displaying three nuclear staining patterns: 1, diffuse; 2, granular; and 3, combined diffuse and granular (avidin–biotin–peroxidase complex method).
The Ki-67 LIs were determined by light microscopy at ×400 magnification using an ocular grid. Two observers performed the counts independently of each other from the same areas of the epithelium. First, at least 1000 nuclei were counted throughout the whole thickness of the epithelium, and then all positively stained nuclei within the same area were counted. The LI was defined as the percentage of immunoreactive nuclei. The first observer counted all positive nuclei regardless of staining quality (granular and diffuse), whereas the second observer registered diffusely stained nuclei only. Both observers performed their counts twice from exactly the same area without any delay, keeping the ocular grid in place.
The statistical analysis of the data was done using the SPSS for Windows software (SPSS, Chicago, Illinois, USA). Spearman's rank correlation was used to assess the correlation between the different Ki-67 antibodies. Comparisons of the repeated countings and of the LIs were performed with one way and two way analysis of variance tests. p Values less than 0.05 were regarded as significant.
RESULTS
The intensities of the diffuse nuclear staining pattern were generally classified as strong or moderate, making it easy to discriminate between positive and negative nuclei. The granular pattern, on the other hand, displayed a continuum from a strong to a very weak staining intensity, often making it difficult to discern between weakly positive and negative nuclei.
The relative proportions of nuclei with granular and diffuse staining patterns can be calculated from the figures in table 2. Thus, by staining with the MIB-1 antibody, more than half of the nuclei (mean, 59%) showed a diffuse staining pattern, whereas this pattern was seen in only 35–45% of the nuclei when the other three stains were used.
Table 2.
Ki-67 labelling indices in tonsillar squamous epithelium from 10 cases stained with four different Ki-67 equivalent antibodies
| First observer: granular and diffuse staining pattern | Second observer: diffuse staining pattern only | |||||||||||||||||||||||
| MIB-1 | MM1 | NCL-Ki-67p | Rah Ki-67 | MIB-1 | MM1 | NCL-Ki-67p | Rah Ki-67 | |||||||||||||||||
| Case no. | 1st | 2nd | Diff | 1st | 2nd | Diff | 1st | 2nd | Diff | 1st | 2nd | Diff | 1st | 2nd | Diff | 1st | 2nd | Diff | 1st | 2nd | Diff | 1st | 2nd | Diff |
| 1 | 28.9 | 30.7 | 1.8 | 12.0 | 11.1 | 0.9 | 18.0 | 18.5 | 0.5 | 15.4 | 15.2 | 0.2 | 19.6 | 19.2 | 0.4 | 4.3 | 4.4 | 0.1 | 6.5 | 6.8 | 0.3 | 6.9 | 7.2 | 0.3 |
| 2 | 37.5 | 39.0 | 1.5 | 16.8 | 16.5 | 0.3 | 17.0 | 15.9 | 1.1 | 18.4 | 16.7 | 1.7 | 22.5 | 22.5 | 0.0 | 5.6 | 5.2 | 0.4 | 4.9 | 5.3 | 0.4 | 11.2 | 11.2 | 0.0 |
| 3 | 34.8 | 36.1 | 1.3 | 16.9 | 15.3 | 1.6 | 19.8 | 19.8 | 0.0 | 26.1 | 23.8 | 2.3 | 21.0 | 22.1 | 1.1 | 8.5 | 8.3 | 0.2 | 10.9 | 11.3 | 0.4 | 7.9 | 8.6 | 0.7 |
| 4 | 38.6 | 38.2 | 0.4 | 18.0 | 19.4 | 1.4 | 29.1 | 26.6 | 2.5 | 22.4 | 22.1 | 0.3 | 23.7 | 24.9 | 1.2 | 6.4 | 6.7 | 0.3 | 9.4 | 9.9 | 0.5 | 9.4 | 9.9 | 0.5 |
| 5 | 27.7 | 26.1 | 1.6 | 12.5 | 10.5 | 2.0 | 26.9 | 27.3 | 0.4 | 16.5 | 14.3 | 2.2 | 16.8 | 15.3 | 1.5 | 4.8 | 5.1 | 0.3 | 8.7 | 8.6 | 0.1 | 8.8 | 8.8 | 0.0 |
| 6 | 26.5 | 29.6 | 3.1 | 14.9 | 16.5 | 1.6 | 26.7 | 22.7 | 4.0 | 15.7 | 16.3 | 0.6 | 17.6 | 16.1 | 1.5 | 10.2 | 10.9 | 0.7 | 8.7 | 8.4 | 0.3 | 9.9 | 9.7 | 0.2 |
| 7 | 23.4 | 22.9 | 0.5 | 9.4 | 9.8 | 0.4 | 11.7 | 11.5 | 0.2 | 20.3 | 17.4 | 2.9 | 9.7 | 11.3 | 1.6 | 4.0 | 3.8 | 0.2 | 4.6 | 4.7 | 0.1 | 5.8 | 5.8 | 0.0 |
| 8 | 33.5 | 30.7 | 2.8 | 15.6 | 18.1 | 2.5 | 23.4 | 23.4 | 0.0 | 20.3 | 17.4 | 2.9 | 17.7 | 17.7 | 0.0 | 7.0 | 6.6 | 0.4 | 5.9 | 5.7 | 0.2 | 7.5 | 8.0 | 0.5 |
| 9 | 35.5 | 35.6 | 0.1 | 13.3 | 13.3 | 0.0 | 24.3 | 22.9 | 1.4 | 16.0 | 15.4 | 0.6 | 18.5 | 18.3 | 0.2 | 3.1 | 2.8 | 0.3 | 6.7 | 6.7 | 0.0 | 6.1 | 6.4 | 0.3 |
| 10 | 22.0 | 20.1 | 1.9 | 9.1 | 9.9 | 0.8 | 14.7 | 13.6 | 1.1 | 7.7 | 7.6 | 0.1 | 14.6 | 15.8 | 1.2 | 4.3 | 4.1 | 0.2 | 5.3 | 5.2 | 0.1 | 3.5 | 2.8 | 0.7 |
| Mean | 30.8 | 30.9 | 1.5 | 13.9 | 14.0 | 1.2 | 21.2 | 20.2 | 1.1 | 17.9 | 16.6 | 1.4 | 18.2 | 18.3 | 0.9 | 5.8 | 5.8 | 0.3 | 7.2 | 7.3 | 0.2 | 7.7 | 7.8 | 0.3 |
Diff, difference between the 1st and 2nd readings.
Table 2 shows the Ki-67 LIs (both granular and diffuse staining patterns) obtained by staining with the four antibodies. As can be seen, there are considerable differences in the mean LIs, with the highest figure for MIB-1 (∼ 31%), followed by NCL-Ki-67p (∼ 21%), Rah Ki-67 (∼ 17%), and MM1 (∼ 14%). The MIB-1 LIs were significantly higher than those obtained with the other three antibodies, as was the case for NCL-Ki-67p compared with MM1. The correlation between the LIs of the four antibodies was variable. However, MM1 correlated positively with all the other antibodies and MIB-1 with Rah Ki-67 and MM1. There were no significant differences in LIs between the first and second counting.
DISCUSSION
Determination of the proliferative activity by the use of the Ki-67 LI depends on several factors, the most obvious being interobserver variations in cut off values between weakly positive and unstained nuclei. Before commencing our present study, we met to agree upon qualitative and quantitative staining criteria. Furthermore, we have previously cooperated on immunohistochemical studies of proliferative activity38,39 and therefore feel confident that our evaluations are in agreement with one another. In addition, several factors related to tissue handling and histotechnical procedures are important.39,40 The aim of our present study was to evaluate the importance of the choice of primary antibody by comparing qualitative and quantitative staining characteristics of four commercially available Ki-67 equivalent antibodies. It might be expected that these antibodies would have a similar sensitivity and specificity, but their recognition of different epitopes of the Ki-67 antigen makes this questionable.
The nuclear distribution of the immunohistochemical staining product was found to be granular, diffuse, or a combination of both. This finding is in agreement with previous reports in which the staining sites have been accurately determined at an ultrastructural level and which have also demonstrated variations of the granular and diffuse staining patterns in the various phases of proliferation.24,41–44 We found that the diffuse staining pattern was generally strongly or moderately intense, whereas the granular pattern could show the whole spectrum of intensities. Staining with the MIB-1 antibody revealed a predominance of diffusely stained nuclei (mean, 59%), in contrast to staining with the other three antibodies, in which this staining pattern was seen in 35–45% of the nuclei. Therefore, it is possible that the various Ki-67 equivalent antibodies recognise epitopes that are expressed differently throughout the various phases of proliferation. However, the relative proportions of the two staining patterns had no influence on the reproducibility of the LIs.
“The large differences in Ki-67 labelling indices obtained by staining with various antibodies necessitate the exact identification of the antibody used in every case”
The most important finding of our present study is undoubtedly the considerable differences in Ki-67 LIs obtained with the four antibodies. Thus, the MIB-1 antibody stained more than twice as many nuclei as the MM1 antibody (means, 31% and 14%, respectively). The NCL-Ki-67p (mean, 21%) and Rah Ki-67 (mean, 17%) antibodies produced intermediate results, although they were closer to the MM1 values than to those of MIB-1. This indicates that the MIB-1 antibody has a higher sensitivity than the other three antibodies, and it also gives the best visual staining, with more diffusely and strongly stained nuclei. However, as mentioned above, this qualitative staining property does not improve the reproducibility of the LI, which is equally good for all four examined antibodies.
There have been many reports of correlations between Ki-67 equivalent antibodies and other proliferation markers. Of particular interest in this context is the demonstration of considerably higher LIs by staining with the MIB-1 and Rah Ki-67 antibodies compared with the prototypic Ki-67 antibody.38,45,46 One explanation for this finding could be better preservation of epitopes after formaldehyde fixation and antigen retrieval than by freezing of the tissue. Rose et al found a qualitatively similar immune staining using MIB-1 and polyclonal Ki-67 antibodies, but quantitative comparisons were not performed.23 To our knowledge, the only previous study that has presented relevant quantitative data is the study of Torp in 1997.47 He determined Ki-67 LIs in 11 glioblastomas by staining with the prototypic Ki-67 antibody in addition to the same four Ki-67 equivalent antibodies that were used in our study. The reproducibility was tested by performing two runs of counts for two of the antibodies (MIB-1 and Rah Ki-67). Torp found the following mean LIs: prototypic Ki-67, 5.7%; MIB-1, 6.1/9.4%; MM1, 7.9%; NCL-Ki-67p, 5.9%; and Rah Ki-67, 10.9/5.2%. Thus, Torp's study did not reveal higher LIs using the MIB-1 antibody, and the differences between the two runs of counts were larger than those between each of the antibodies. However, it should be emphasised that Torp counted at random in a neoplasm with known heterogeneity, whereas we counted systematically in constant areas. Therefore, the findings of these two studies cannot be compared directly.
Take home messages.
The MIB-1 antibody had a higher sensitivity than the other three antibodies, and also gave the best visual staining, with more diffusely and strongly stained nuclei, although the reproducibility of the labelling index (LI) was equally good for all four examined antibodies
Obviously, these differences are important to consider when proliferative activity is determined by the Ki-67 LI, and the exact antibody used should be identified in all cases studied
The large differences in Ki-67 LIs obtained by staining with various antibodies necessitate the exact identification of the antibody used in every case. Based on our study, MIB-1 seems to have a higher sensitivity for the Ki-67 antigen than the other three tested antibodies. However, our findings should be confirmed by others and extended to various tissues. Obviously, our findings have serious implications when the Ki-67 LI is used as a criterion for tumour grading or for clinical prognostic indication.
Abbreviations
LI, labelling index
PNCA, proliferating cell nuclear antigen
REFERENCES
- 1.Graem N, Helweg-Larsen K. Mitotic activity and delay in fixation of tumour tissue. The influence of delay in fixation on mitotic activity of a human osteogenic sarcoma grown in athymic nude mice. Acta Pathol Microbiol Immunol Scand [A] 1979;87:375–8. [PubMed] [Google Scholar]
- 2.Donhuijsen K, Schmidt U, Hirche H, et al. Changes in mitotic rate and cell cycle fractions caused by delayed fixation. Hum Pathol 1990;21:709–14. [DOI] [PubMed] [Google Scholar]
- 3.Donhuijsen K. Mitosis counts: reproducibility and significance in grading of malignancy. Hum Pathol 1986;17:1122–5. [DOI] [PubMed] [Google Scholar]
- 4.Montironi R, Collan Y, Scarpelli M, et al. Reproducibility of mitotic counts and identification of mitotic figures in malignant glial tumors. Appl Pathol 1988;6:258–65. [PubMed] [Google Scholar]
- 5.Woosley JT. Measuring cell proliferation. Arch Pathol Lab Med 1991;115:555–7. [PubMed] [Google Scholar]
- 6.van Diest PJ, Baak JPA, Matze-Cok P, et al. Reproducibility of mitosis counting in 2469 breast cancer specimens: results from the multicenter morphometric mammary carcinoma project. Hum Pathol 1992;23:603–7. [DOI] [PubMed] [Google Scholar]
- 7.Jannink I, Risberg B, van Diest PJ, et al. Heterogeneity of mitotic activity in breast cancer. Histopathology 1996;29:421–8. [DOI] [PubMed] [Google Scholar]
- 8.Silverberg SG. Reproducibility of the mitosis count in the histologic diagnosis of smooth muscle tumors of the uterus. Hum Pathol 1976;7:451–4. [DOI] [PubMed] [Google Scholar]
- 9.Rudolph P, Peters J, Lorenz D, et al. Correlation between mitotic and Ki-67 labeling indices in paraffin-embedded carcinoma specimens. Hum Pathol 1998;29:1216–22. [DOI] [PubMed] [Google Scholar]
- 10.Gerdes J, Schwab U, Lemke H, et al. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 1983;31:13–20. [DOI] [PubMed] [Google Scholar]
- 11.Hall PA, Richards MA, Gregory WM, et al. The prognostic value of Ki67 immunostaining in non-Hodgkin's lymphoma. J Pathol 1988;154:223–35. [DOI] [PubMed] [Google Scholar]
- 12.Zuber P, Hamou M-F, de Tribolet N. Identification of proliferating cells in human gliomas using the monoclonal antibody Ki-67. Neurosurgery 1988;22:364–8. [DOI] [PubMed] [Google Scholar]
- 13.Ueda T, Aozasa K, Tsujimoto M, et al. Prognostic significance of Ki-67 reactivity in soft tissue sarcomas. Cancer 1989;63:1607–11. [DOI] [PubMed] [Google Scholar]
- 14.Veronese SM, Gambacorta M, Gottardi O, et al. Proliferation index as a prognostic marker in breast cancer. Cancer 1993;71:3926–31. [DOI] [PubMed] [Google Scholar]
- 15.Hall PA, McKee PH, Menage HD, et al. High levels of p53 protein in UV-irradiated normal human skin. Oncogene 1993;8:203–7. [PubMed] [Google Scholar]
- 16.Hall PA, Coates PJ, Goodlad RA, et al. Proliferating cell nuclear antigen expression in non-cycling cells may be induced by growth factors in vivo. Br J Cancer 1994;70:244–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donzelli M, Negri C, Mandarino A, et al. Poly(ADP-ribose) synthesis: a useful parameter for identifying apoptotic cells. Histochem J 1997;29:831–7. [DOI] [PubMed] [Google Scholar]
- 18.Prosperi E. Multiple roles of the proliferating cell nuclear antigen: DNA replication, repair and cell cycle control. Progress in Cell Cycle Research 1997;3:193–210. [DOI] [PubMed] [Google Scholar]
- 19.Louis DN, Edgerton S, Thor AD, et al. Proliferating cell nuclear antigen and Ki-67 immunohistochemistry in brain tumors: a comparative study. Acta Neuropathol 1991;81:675–9. [DOI] [PubMed] [Google Scholar]
- 20.Leonardi E, Girlando S, Serio G, et al. PCNA and Ki67 expression in breast carcinoma: correlations with clinical and biological variables. J Clin Pathol 1992;45:416–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bromley M, Rew D, Becciolini A, et al. A comparison of proliferation markers (BrdUrd, Ki-67, PCNA) determined at each cell position in the crypts of normal human colonic mucosa. Eur J Histochem 1996;40:89–100. [PubMed] [Google Scholar]
- 22.Sittel C, Ruiz S, Kvasnicka HM, et al. Zur prognostischen Relevanz der Proliferationsmarker Ki-67 (MIB 1), PCNA und p53 bei kombiniert chirurgisch und radiologisch therapierten Karzinomen des Oropharynx und der Mundhöhle. Laryngorhinootologie 2000;79:86–92. [DOI] [PubMed] [Google Scholar]
- 23.Rose DSC, Maddox PH, Brown DC. Which proliferation markers for routine immunohistology? A comparison of five antibodies. J Clin Pathol 1994;47:1010–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholzen T, Gerdes J. The Ki -67 protein: from the known and the unknown. J Cell Physiol 2000;182:311–22. [DOI] [PubMed] [Google Scholar]
- 25.Cattoretti G, Becker MH, Key G, et al. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol 1992;168:357–63. [DOI] [PubMed] [Google Scholar]
- 26.Key G, Becker MHG, Baron B, et al. New Ki-67-equivalent murine monoclonal antibodies (MIB 1–3) generated against bacterially expressed parts of the Ki67 cDNA containing three 62 base pair repetitive elements encoding for the Ki-67 epitope. Lab Invest 1993;68:629–36. [PubMed] [Google Scholar]
- 27.Kubbutat MHG, Key G, Duchrow M, et al. Epitope analysis of antibodies recognising the cell proliferation associated nuclear antigen previously defined by the antibody Ki-67 (Ki-67 protein). J Clin Pathol 1994;47:524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudolph P, Lappe T, Schubert C, et al. Diagnostic assessment of two novel proliferation-specific antigens in benign and malignant melanocytic lesions. Am J Pathol 1995;147:1615–25. [PMC free article] [PubMed] [Google Scholar]
- 29.Rudolph P, Kellner U, Chassevent A, et al. Prognostic relevance of a novel proliferation marker, Ki-S11, for soft-tissue sarcoma. A multivariate study. Am J Pathol 1997;150:1997–2007. [PMC free article] [PubMed] [Google Scholar]
- 30.Keshgegian AA, Cnaan A. Proliferation markers in breast carcinoma. Mitotic figure count, S-phase fraction, proliferating cell nuclear antigen, Ki-67 and MIB-1. Am J Clin Pathol 1995;104:42–9. [DOI] [PubMed] [Google Scholar]
- 31.Jensen V, Høyer M, Sørensen FB, et al. MIB-1 expression and iododeoxyuridine labelling in soft tissue sarcomas: an immunohistochemical study including correlations with p53, bcl-2 and histological characteristics. Histopathology 1996;28:437–44. [DOI] [PubMed] [Google Scholar]
- 32.Hsu DW, Louis DN, Efird JT, et al. Use of MIB-1 (Ki-67) immunoreactivity in differentiating grade II and grade III gliomas. J Neuropathol Exp Neurol 1997;56:857–65. [DOI] [PubMed] [Google Scholar]
- 33.Enestrøm S, Vavruch L, Franlund B, et al. Ki-67 antigen expression as a prognostic factor in primary and recurrent astrocytomas. Neurochirurgie 1998;44:25–30. [PubMed] [Google Scholar]
- 34.Jansen RL, Hupperets PS, Arends JW, et al. MIB-1 labelling index is an independent prognostic marker in primary breast cancer. Br J Cancer 1998;78:460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geisler JP, Geisler HE, Miller GA, et al. MIB-1 in endometrial carcinoma: prognostic significance with 5-year follow-up. Gynecol Oncol 1999;75:432–6. [DOI] [PubMed] [Google Scholar]
- 36.Prayson RA, Mohan DS, Song P, et al. Clinicopathologic study of forty-four histologically pure supratentorial oligodendrogliomas. Ann Diagn Pathol 2000;4:218–27. [DOI] [PubMed] [Google Scholar]
- 37.Moriki T, Takahashi T, Kataoka H, et al. Proliferation marker MIB-1 correlates well with proliferative activity evaluated by BrdU in breast cancer: an immunohistochemical study including correlation with PCNA, p53, c-erbB-2 and estrogen receptor status. Pathol Int 1996;46:953–61. [DOI] [PubMed] [Google Scholar]
- 38.Torp SH, Johannesen E, Lindboe CF. Comparison of different Ki67 antibodies in human glioblastomas. Mol Pathol 1995;48:M191–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindboe CF, Johannesen E, Torp SH. Immunostaining of human glioblastomas with various PCNA antibodies. Oncol Rep 1997;4:85–8. [PubMed] [Google Scholar]
- 40.Linden MD, Torres FX, Kubus J, et al. Clinical application of morphologic and immunocytochemical assessments of cell proliferation. Am J Clin Pathol 1992;97(suppl 1):S4–13. [PubMed] [Google Scholar]
- 41.Verheijen R, Kuijpers HJ, van Driel R, et al. Ki-67 detects a nuclear matrix-associated proliferation-related antigen. II. Localization in mitotic cells and association with chromosomes. J Cell Sci 1989;4:531–40. [DOI] [PubMed] [Google Scholar]
- 42.Starborg M, Gell K, Brundell E, et al. The murine Ki-67 cell proliferation antigen accumulates in the nucleolar and heterochromatic regions of interphase cells and at the periphery of the mitotic chromosomes in a process essential for cell cycle progression. J Cell Sci 1996;109:143–53. [DOI] [PubMed] [Google Scholar]
- 43.Endl E, Gerdes J. The Ki-67 protein: fascinating forms and an unknown function. Exp Cell Res 2000;257:231–7. [DOI] [PubMed] [Google Scholar]
- 44.MacCallum DE, Hall PA. The location of pKi67 in the outer dense fibrillary compartment of the nucleolus points to a role in ribosome biogenesis during the cell division cycle. J Pathol 2000;190:537–44. [DOI] [PubMed] [Google Scholar]
- 45.Barbareschi M, Girlando S, Mauri FM, et al. Quantitative growth fraction evaluation with MIB1 and Ki67 antibodies in breast carcinomas. Am J Clin Pathol 1994;102:171–5. [DOI] [PubMed] [Google Scholar]
- 46.Mazerolles C, Rishmann P, Chopin D, et al. Usefulness of MIB1 monoclonal antibody in assessing the proliferative index in human bladder carcinoma: comparison with Ki-67 antibody. Histopathology 1994;25:563–8. [DOI] [PubMed] [Google Scholar]
- 47.Torp SH. Proliferative activity in human glioblastomas: evaluation of different Ki67 equivalent antibodies. Mol Pathol 1997;50:198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]