Abstract
Seven cases of this rare variant of breast carcinoma have been described in three previous publications. This paper describes an additional case, the first following chemotherapy, which in addition had an unfavourable prognosis. It also describes alterations in cell morphology, immunohistochemistry, and ultrastructure following chemotherapy.
Keywords: acinic cell breast carcinoma, chemotherapy, prognosis, cell morphology
A 49 year old woman presented with a lump in her right breast, which was partially cystic, and after draining refilled immediately. A biopsy showed an infiltrating ductal carcinoma grade 3, with the additional feature of unusual cytoplasmic granules. Several courses of cytotoxic chemotherapy were administered which included adriamycin, cyclophosphamide, methotrexate, and 5 fluorouracil. Eight months later the patient underwent mastectomy. A liver ultrasound showed what appeared to be peripherally located cysts, which were too small to biopsy and were considered to be simple cysts. Twelve months later she had massive hepatomegaly as a result of metastases for which she received additional courses of chemotherapy including mitozantrone, mitomycin c, and methotrexate. In addition, she was treated with epirubicin but her liver enlargement continued. Infusional treatment was given but she continued to deteriorate and died three years after her initial diagnosis.
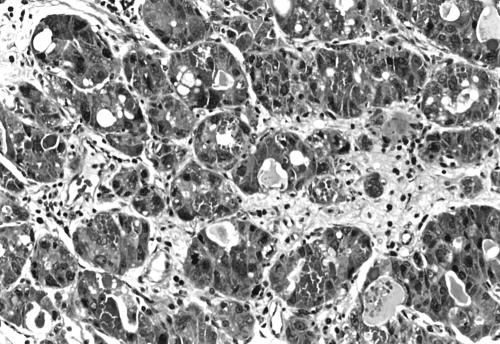
The first biopsy consisted of a piece of tissue measuring 4 × 4.6 × 0.3 cm. Microscopic examination revealed an infiltrating ductal carcinoma grade 3 composed of cells arranged in small tightly packed solid nests and tubular structures. Areas composed of larger solid nests were also noted. The cells showed prominent nuclear pleomorphism with numerous mitotic figures (up to 13/single high power field especially in solid nests). However, the most striking feature was the presence in most of the cells of small and large, brightly eosinophilic cytoplasmic granules (fig 1). Small numbers of cells displayed clear or vacuolated cytoplasm. Secretory material showing less intense eosinophilia was present in many of the tubular structures. Foci of vascular invasion were also present.
Figure 1.
Invasive carcinoma with numerous large and small eosinophilic cytoplasmic granules. Original magnification, ×100.
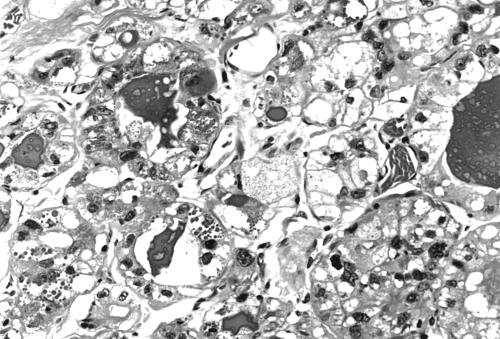
In the mastectomy specimen there was an irregular firm area measuring 2 cm in its maximum dimension. Sections taken from the mastectomy specimen showed patchy residual foci of infiltrating carcinoma. The cells were arranged in small groups and tightly packed tubular structures without the previously described large solid areas. Many of the cells contained clear or multivacuolated cytoplasm, which at times resembled lipoblasts (fig 2). In some areas the cells had intensely eosinophilic cytoplasm. Although some granular cells were present granularity was much less obvious in most cells. This was in contrast to the first biopsy which was composed predominantly of granular cells. Vascular invasion was also present but mitotic activity was low. There were single cells scattered throughout the stroma, a feature sometimes seen after cytotoxic treatment, and some of these cells displayed prominent cytoplasmic granularity. Two of 11 lymph nodes contained small subcapsular metastatic deposits. These cells also showed prominent brightly eosinophilic cytoplasm with greatly diminished granularity.
Figure 2.
Cells with vacuolated cytoplasm and occasional cells with small fine eosinophilic granules. Original magnification, ×250.
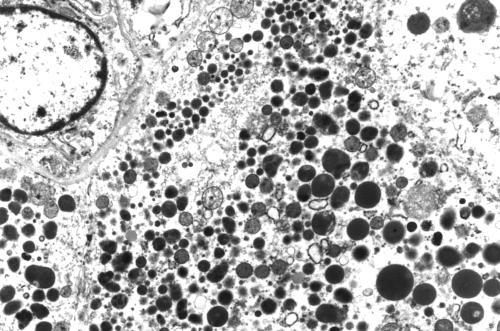
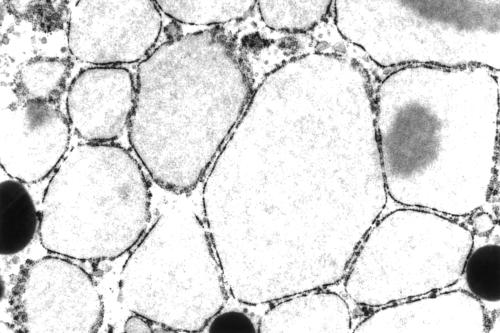
The eosinophilic secretory material was periodic acid Schiff diastase resistant, as were the cytoplasmic granules. The granules were strongly positive with antibodies to salivary amylase, lysozyme, α1 anti-chymotrypsin and α1 anti-trypsin, with fewer numbers of cells staining in the postchemotherapy mastectomy specimen. They were negative for glial fibrillary acidic protein, gross cystic disease fluid protein 15, chromogranin, and synaptophysin. S100 protein showed focal cytoplasmic positivity. A phloxine tartrazine stain for Paneth cell granules was negative. Electron microscopy performed on formalin fixed tissue taken from both specimens demonstrated numerous cytoplasmic electron dense granules of varying size consistent with acinic cell granules (fig 3), and prominent rough endoplasmic reticulum. In addition, many of the cells from the mastectomy specimen contained very few and very small cytoplasmic granules or empty vacuoles, which appeared to be derived from dilatation of the rough endoplasmic recticulum, and a spectrum of rough endoplasmic recticulum change was seen, varying from minimal to extreme dilatation (fig 4). Conversely, this was not seen in the biopsy specimen where cells with cytoplasmic vacuoles were not membrane bound.
Figure 3.
Numerous electron dense cytoplasmic granules. Original magnification, ×4000.
Figure 4.
Dilated rough endoplasmic reticulum in the cytoplasm of clear cells. Original magnification, ×7000.
We also examined four other postchemotherapy breast carcinomas ultrastructurally and found dilated rough endoplasmic reticulum in one case.
DISCUSSION
The first case of acinic cell carcinoma of the breast was described by Roncaroli and colleagues1 and this case, together with five additional cases, was subsequently included in an immunohistochemical and ultrastructural study published by Damiani et al.2 A single additional case was recently reported by Schmitt and colleagues3 who, on the basis of their case and the initial case reported by Roncaroli, suggested that this breast carcinoma variant might be associated with a good prognosis. Their patient was alive and well 21 months after surgery and of the six patients described by Damiani, one was alive and well after five years, three were alive and well after one year, one had a recurrence after four years, and one was lost to follow up. At least some of the cases would appear to have been in the grade 2 and 3 categories. This case demonstrates that primary acinic cell carcinoma of the breast can be associated with a poor prognosis, particularly when of high grade and with additional adverse prognostic features, such as vascular invasion.
Take home messages .
Primary acinic cell carcinoma of the breast is a rare variant of breast carcinoma that has been suggested to carry a good prognosis
This paper describes a case of primary acinic cell carcinoma of the breast following chemotherapy, which had an unfavourable prognosis, demonstrating that this lesion can be associated with a poor prognosis, particularly when of high grade and with additional adverse prognostic features, such as vascular invasion
Alterations in cell morphology, immunohistochemistry, and ultrastructure were seen and might be the result of chemotherapy
“Cytoplasmic vacuolation following chemotherapy is a documented feature, although we are unaware of any studies that specifically examined or included carcinomas with granular cytoplasm”
Carcinomas more frequently arising in other organs or tissues but also occurring as primary carcinomas in the breast may be difficult to diagnose. It may also be difficult to determine whether they are primary or metastatic.4 As Damiani et al point out,2 primary acinic cell carcinoma is a new addition to the list of carcinomas that can arise in both the salivary gland and the breast, a list that includes mucoepidermoid carcinoma, myoepithelial/adenomyoepithelial carcinomas, and the recently described malignant oncocytoma.5 The differential diagnosis, discussed by Damiani et al, includes other breast carcinomas containing granular cells including neuroendocrine and apocrine carcinomas, and metastatic carcinoma from the salivary gland, kidney, and pancreas.2
In our case, the cytoplasmic granularity was greatly reduced and the number of clear and vacuolated cells greatly increased in the postchemotherapy mastectomy specimen. Cytoplasmic vacuolation following chemotherapy is a documented feature,6 although we are unaware of any studies that specifically examined or included carcinomas with granular cytoplasm. Clear cells in acinic cell carcinoma of the salivary gland are thought to be caused by tissue processing artefact or changes in organelles, including dilated rough endoplasmic reticulum.7 In our case, the prominent cytoplasmic vacuolation may merely mirror the even rarer variant of clear cell salivary acinic cell carcinoma. Alternatively, some of the morphological alterations might reasonably be ascribed to the effects of chemotherapy.
Acknowledgments
We wish to thank Mrs S Thompson for excellent secretarial assistance, Dr A Curry for electron and photomicrographs, and Mrs L Griffin-Davis for immunohistochemistry.
REFERENCES
- 1.Roncaroli F, Lamovec J, Zidar A, et al. Acinic cell-like carcinoma of the breast. Virchows Arch 1996;429:69–74. [DOI] [PubMed] [Google Scholar]
- 2.Damiani S, Pasquinelli G, Lamovec J, et al. Acinic cell carcinoma of the breast: an immunohistochemical and ultrastructural study. Virchows Arch 2000;437:74–81. [DOI] [PubMed] [Google Scholar]
- 3.Schmitt FC, Ribeiro CA, Alvarenga S, et al. Primary acinic cell-like carcinoma of the breast – a variant with a good prognosis? Histopathology 2000;36:286–9. [DOI] [PubMed] [Google Scholar]
- 4.Coyne JD, Slevin N, Barr L. Mucoepidermoid carcinoma metastatic to the breast. Breast 2000;9:293–5. [Google Scholar]
- 5.Damiani S, Eusebi V, Losi L, et al. Oncocytic carcinoma (malignant oncocytoma) of the breast. Am J Surg Pathol 1998;22:221–30. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy S, Merino MJ, Swain SM, et al. The effects of hormonal and chemotherapy on tumoral and non neoplastic breast tissue. Hum Pathol 1990;21:192–8. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhry AP, Cutler LS, Leifer C, et al. Histogenesis of acinic cell carcinoma of the major and minor salivary glands. An ultrastructural study. J Pathol 1986;148:307–20. [DOI] [PubMed] [Google Scholar]