Abstract
Aims: Abnormalities involving proliferation, apoptosis, and angiogenesis are important in tumorigenesis. The purpose of this study was to examine these three biological processes, and their relation with the clinical stage and cytological grade in multiple myeloma (MM).
Methods: Fifty four newly diagnosed patients with MM were studied by immunohistochemistry using bone marrow clot sections. Proliferation and apoptosis were evaluated for the proportion of MM cells (indicated by morphology and CD138 reactivity) positive for the Ki67 antigen and single stranded DNA (ssDNA), respectively. Angiogenesis was evaluated by measuring the intratumoral microvessel density (IMVD) and by assessing the immunoreactivity of vascular endothelial growth factor (VEGF).
Results: There were 30 men and 24 women (median age, 65 years; range, 37–84). At initial presentation, 15 (28%) were in Durie stage I, 15 (28%) in stage II, and 24 (44%) in stage III. Advanced clinical stage correlated with high cytological grade (p < 0.03). The medians for Ki67, ssDNA, and IMVD were 4.4% (range, 0–15%), 0.2% (range, 0–2.8%), and 15.5 (range, 0–63), respectively. Among these three continuous parameters, the only significant correlation was that between Ki67 and IMVD (p < 0.0001). Both Ki67 and IMVD also correlated with the clinical stage, cytological grade, and VEGF positivity (p <0.05). No correlation was found between ssDNA and all of the other parameters.
Conclusions: These data suggest that proliferation is associated with angiogenesis in MM. Furthermore, proliferation and angiogenesis, but not apoptosis, may be important in disease progression. Lastly, increased production of VEGF may be one of the contributing factors to the increase in intratumoral vascularity seen in advanced MM.
Keywords: multiple myeloma, proliferation, apoptosis, angiogenesis, clinical stage, cytological grade
Multiple myeloma (MM) is a progressively fatal disease characterised by the accumulation of malignant plasma cells in the bone marrow.1 Previous studies have provided insights into the pathogenesis of this disease, and defects involving proliferation, apoptosis, and angiogenesis are believed to be important.2 Proliferation as measured by bromodeoxyuridine labelling of MM cells has been shown to be an important prognostic factor for MM.3 Proliferation assessed by immunohistochemistry to detect the Ki67 antigen is also associated with advanced pathological stage of MM.4 More recent studies of MM have examined the roles of angiogenesis and apoptosis in this disease. Abnormalities of the apoptosis related proteins have been observed in MM. For instance, overexpression of bcl-2 is present in most patients with MM,5 and this abnormality may mediate the resistance of MM cells to apoptosis induced by dexamethasone and interleukin 6 (IL-6) deprivation.6,7 In addition, mutations of the tumour suppressor gene p53 are present in 5% of patients with MM and 20–40% of patients with acute plasma cell leukaemia. As for angiogenesis, a recent study showed that an increase in the bone marrow microvessel density in MM is associated with a worse prognosis.8
“Proliferation as measured by bromodeoxyuridine labelling of multiple myeloma cells has been shown to be an important prognostic factor for multiple myeloma”
To our knowledge, no reported studies have evaluated proliferation, apoptosis, and angiogenesis at the same time. Vacca et al reported an association between increased intratumoral vascularity and a high plasma cell labelling index.9 Nevertheless, how apoptosis is related to proliferation or intratumoral vascularity is unclear. In addition, little is known about the relation between these biological processes and the clinical stage or cytological grade of MM. Thus, the purpose of our study was to examine proliferation, apoptosis, and angiogenesis in 54 patients with newly diagnosed MM. Specifically, we evaluated whether there is a correlation among these three biological processes, and how these processes are related to the clinical stage or cytological grade.
METHODS
Patient selection
Fifty four newly diagnosed patients with MM were randomly identified and retrieved from the file between 1988 and 2000 in the department of laboratory medicine, Nagoya University School of Medicine.
Monoclonal antibodies and immunohistochemistry
Monoclonal antibodies used in our study were directed against the following antigens: Ki67, (1/100 dilution; Immunotech, Marseille, France), CD34 (1/100 dilution; Immunotech), single stranded DNA (ssDNA) (1/100 dilution; Dako, Kyoto, Japan), and vascular endothelial growth factor (VEGF; 1/200 dilution; Santa Cruz Biotechnology, Santa Cruz, California, USA). Immunohistochemistry was performed on 3 μm tissue sections of formalin fixed, paraffin wax embedded bone marrow aspirate clots. After dewaxing in xylene and dehydration through graded concentrations of ethanol, the tissue sections were subjected to microwave antigen retrieval (750 W; citrate buffer, 0.01 mol/litre, pH 6.0) for five minutes. The tissue sections were subsequently put into an automated immunostainer (Ventana Medical System, Tucson, Arizona, USA). For each case, double marker analysis was performed combining CD138 with Ki67 and ssDNA. Reactivity for Ki67 and ssDNA was detected by a streptavidin–alkaline phosphatase system with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphatase as the chromogen. After washing in phosphate buffered saline (0.1 mol/litre, pH 7.5) three times, immunohistochemical staining for CD138 (1/40 dilution; Serotec, Kidlington, UK) was performed using a streptavidin–biotin–peroxidase linking system and diaminobenzidine as the chromogen. Counterstaining was then performed using haematoxylin.
Evaluation of immunoreactivity
Two thousand CD138 positive MM cells from a minimum of five representative areas were evaluated microscopically under ×400 magnification. The reactivity for Ki67 and ssDNA is expressed as a percentage of CD138 positive MM cells positive for these two markers; the staining intensity of Ki67 and ssDNA was irrelevant in the scoring. The assessment of VEGF staining was based on the sum of the points scored for the VEGF staining intensity (0, negative; 1, slight staining; 2, moderate staining; 3, strong staining) and the points scored for the percentage of MM cells positive for VEGF (0, no positive cells; 1, 1–25% positive cells; 2, 26–50% positive cells; 3, > 50% positive cells). A final score of > 2 points was considered to be VEFG positive, whereas a final score of ≤ 2 points was considered to be VEGF negative.10
Intratumoral vascularity was assessed using the intratumoral microvessel density (IMVD), which was based on a modified method described previously.11 Briefly, the five most vascular areas were selected under a microscope using low power scanning. Vessels highlighted by CD34 immunoreactivity were counted under light microscopy with ×400 magnification. IMVD was the average of the number of vessels derived from the five areas.
Statistical analysis
The Mann-Whitney U test was used to determine the significance of the differences in Ki67, IMVD, and ssDNA among the three clinical stages (Durie's) and the three cytological grades (mature, immature, blastic). Pairs of numerical variables including Ki67, ssDNA, and IMVD were analysed using the Spearman correlation. Correlation between the categorical (clinical stage, pathological stage, and VEGF) and continuous variables (Ki67, ssDNA, and IMVD) was done by the Kruskall-Wallis method and the Cox hazard proportional model. A p value of < 0.05 was considered to be significant. The Wilcoxon rank sum test was used to determine whether there was a significant difference between Ki67 or IMVD in VEGF positive versus VEGF negative MM.
RESULTS
Patients, clinical stage, and cytological grade
There were 30 men and 24 women (median age, 65 years; range, 37–84). At initial presentation, 15 (28%) were in Durie stage I, 15 (28%) in stage II, and 24 (44%) in stage III. According to Bartl's pathological stage, there were 13 (24%) in plasmablastic cell type, 21 (39%) in immature cell type, and 20 (37%) in mature cell type. Plasmablastic was considered to be high grade. Advanced stage disease correlated with high cytological grade (p < 0.05).
Expression of the Ki67 antigen
An illustration of Ki67 immunostaining is shown in fig 1A, and the results are summarised in table 1. The proportion of Ki67 positive myeloma cells was variable from case to case, ranging from 0% to 15%, with an overall median of 4.4%. Ki67 labelled MM cells increased with advanced clinical stage. As summarised in table 1, the mean percentages of Ki67 positive MM cells in Durie's clinical stage I, II, and III were 0.72%, 3.72%, and 7.15%, respectively. This positive correlation between Ki67 and clinical stage is significant (p < 0.0001; Mann-Whitney U test). Similarly, the mean percentages of Ki67 positive MM cells increased with the cytological grade: 1.37% in the mature cell type, 4.64% in the immature cell type, and 7.28% in the plasmablastic cell type. This correlation was significant (p < 0.001, Mann-Whitney U test; table 2).
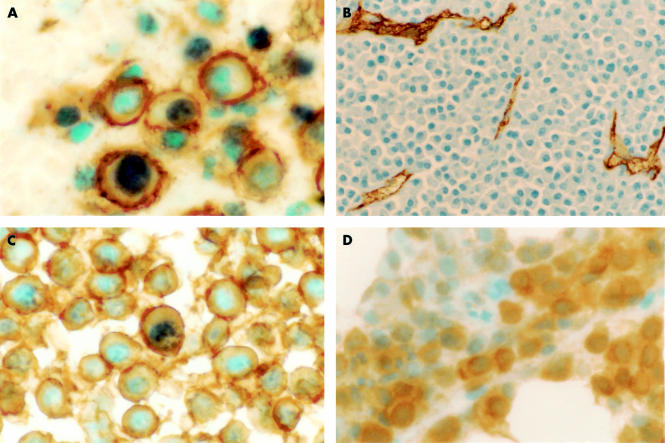
Figure 1.
(A) Ki67 immunoreactivity (blue nuclear staining) in CD138 positive (brown cell surface staining) cells in a bone marrow clot section from a patient with multiple myeloma (original magnification, ×1000). (B) measurement of intratumoral vascular density using CD34 immunostaining to highlight intratumoral blood vessels (original magnification, ×200). (C) Single stranded DNA immunostaining (blue nuclear staining) in CD138 positive (brown cell surface staining) cells in a bone marrow clot section from a patient with multiple myeloma (original magnification, ×1000). (D) Immunostaining of vascular endothelial growth factor in a patient with multiple myeloma (original magnification, ×400).
Table 1.
Correlation between clinical stage and proliferation index, apoptosis index, and intratumour microvessel density in 54 multiple myelomas
| Stage I (N=15) Mean (SD) (%) | Stage II (N=15) Mean (SD) (%) | Stage III (N=24) Mean (SD) (%) | p Value | |
| Ki67 | 0.72 (0.88) | 3.72 (3.94) | 7.15 (3.70) | <0.0001 |
| Intratumour microvessel density | 5.29 (4.38) | 14.47 (8.07) | 22.20 (14.15) | <0.0001 |
| Single stranded DNA | 0.05 (0.12) | 0.17 (0.25) | 0.24 (0.60) | >0.05 |
Table 2.
Correlation between Bartl's pathological stage and proliferation index, apoptosis index, and intratumor microvessel density in 54 multiple myelomas
| Plasmablast (N=13) Mean (SD) (%) | Immature (N=21) Mean (SD) (%) | Mature (N=20) Mean (SD) (%) | p Value | |
| Ki67 | 7.28 (4.00) | 4.64 (3.80) | 1.37 (1.54) | <0.001 |
| Intratumour microvessel density | 23.39 (17.96) | 15.10 (9.91) | 9.56 (7.76) | <0.05 |
| Single stranded DNA | 0.12 (0.19) | 0.23 (0.60) | 0.25 (0.64) | >0.05 |
IMVD in MM
Figure 1B shows typical results for IMVD. The median IMVD was 5.29 vessels/high power field in stage I, 14.47 vessels/high power field in stage II, and 22.20 vessels/high power field in stage III (table 1). This correlation was significant (p < 0.0001; Mann-Whitney U test). IMVD also increased with the cytological grade: 9.56 vessels/high power field in the mature cell type, 15.1 vessels/high power field in the immature cell type, and 23.39 vessels/high power field in the plasmablastic cell type. This correlation was also significant (p < 0.05; Mann-Whitney U test; table 2).
Expression of ssDNA
The results of ssDNA are summarised in table 1, and illustrated in fig 1C. The percentages of ssDNA positive MM cells were less variable than those of Ki67 or IMVD, ranging form 0% to 2.8%, with a median of 0.2%. As shown in table 1 and table 2, there was no significant correlation between the percentage of ssDNA positive cells and the clinical stage or cytological grade (p > 0.05; Mann-Whitney U test).
Expression of VEGF
To evaluate whether IMVD is related to VEGF values, the expression of VEGF was assessed in all 54 cases. The immunostaining for VEGF is shown in fig 1D. Six cases were VEFG negative and 48 cases were VEGF positive. There was no significant correlation between VEFG positivity and either the clinical stage or the cytological grade.
Correlation between Ki67, IMVD, VEGF, and ssDNA
Ki67 correlated with IMVD (p < 0.05; Spearman). Using the Wilcoxon rank sum test, we found that Ki67 was also significantly higher in the VEGF positive than the VEGF negative cases (4.67% v 2.40%; p < 0.001). Similarly, IMVD was also significantly higher in VEGF positive than VEGF negative cases (16.23 v 10; p < 0.001). There was no significant correlation identified between ssDNA and the other three parameters.
DISCUSSION
In summary, we identified a significant correlation between Ki67 and IMVD, and between these two continuous variables with the clinical stage, cytological grade, and VEFG positivity. Thus, patients with MM who have a high clinical stage/cytological grade tend to have a higher rate of proliferation, higher intratumoral vascularity, and increased VEFG in the neoplastic cells. In contrast, apoptosis as assessed by ssDNA labelling appears to be an independent parameter.
With regard to the correlation between Ki67 and the clinical stage, our findings are in keeping with those of an earlier study,12 although another study that examined Ki67 by immunohistochemistry applied to bone marrow biopsy specimens showed no significant correlation between Ki67 and the clinical stage.13 Although this discrepancy may be related to the use of different staining techniques, it is also possible that the difference may be related to the inclusion of non-myeloma cells during the measurement of Ki67 labelling. This may occur because erythroid precursors can be morphologically confused with MM cells in bone marrow biopsy sections. Because erythroid precursors have the highest proliferative activity in the bone marrow,14 erroneous inclusion of these cells during the estimation of Ki67 will lead to falsely high results. To avoid this potential problem, we used CD138 immunostaining to enhance our specificity during Ki67 staining.
The observation that Ki67 correlates with IMVD is interesting. Vacca et al demonstrated this correlation in MM previously. To explain the underlying mechanism for this correlation, the authors have suggested that proliferating MM cells may promote angiogenesis through autocrine and paracrine release of IL-6, a potent angiogenic factor.9
The increase in IMVD in MM of advanced clinical stage may also be related to the release of additional angiogenic cytokines, such as IL-8 and granulocyte–macrophage colony stimulating factor, by the host bone marrow cells. In addition, as shown in our study, increased production of VEGF by MM cells may contribute to the increased intratumoral vascularity seen in advanced stages. Similar to our finding, others have found a correlation between angiogenesis and VEGF expression in some types of solid tumours15,16 and acute myeloid leukaemia.17 Bellamy et al also showed that VEGF is overexpressed by MM cells, but not by benign plasma cells from normal bone marrow.18 Similarly, Dankbar et al demonstrated the expression of VEGF by myeloma cell lines and MM patient samples. Because the receptors for VEFG (Flt-1 and KDR) are expressed in normal myeloid and monocyte cells,19 it is possible that VEGF plays a role in the angiogenesis and growth of MM through a paracrine or autocrine mechanism. Taken together, the correlation between the expression of VEGF in MM cells and IMVD in our study suggests that VEGF may directly contribute to the angiogenesis of MM through an autocrine pathway.
“Patients with multiple myeloma who have a high clinical stage/cytological grade tend to have a higher rate of proliferation, higher intratumoral vascularity, and increased vascular endothelial growth factor in the neoplastic cells”
Although we found a correlation between angiogenesis and proliferation in MM, this correlation is not seen uniformly in solid tumours. For instance, IMVD does not correlate with tumour cell proliferation in epidermoid lung carcinoma,10 breast ductal carcinoma,20,21 and carcinomas of the oesophagus.22 In contrast, Vermeulen et al found an association between Ki67 labelling and intratumour vascularity in colorectal adenocarcinomas.16 Thus, the relation between the IMVD and proliferation of tumour cells is probably specific to the tumour type. Furthermore, the mechanisms underlying the correlation between IMVD and proliferation seen in MM and some of the solid tumours may also be different.
Apoptosis is an important factor in tumorigenesis,23 and previous studies have shown that spontaneous apoptosis occurs more frequently in tumours of high cytological grade and advanced clinical stage of disease.24,25 Defects in apoptosis have been documented in MM.26 Nevertheless, no comprehensive study has been performed to evaluate apoptotic activity in untreated MM. The rate of apoptosis is relatively low in untreated MM when compared with highly proliferative haematological disorders (such as acute leukaemia), but it is comparable to low grade haematological disorders (such as chronic lymphocytic leukaemia).27 Importantly, there was no significant correlation between apoptosis and clinical stage, Ki67 labelling, or ssDNA labelling. Its independence from clinical stage may be related to the fact that bcl-2 overexpression also does not correlate with clinical stage. It is possible that defects in apoptosis in MM occur early in its pathogenesis and persist through the course of the disease, and may not be important in disease progression.
To conclude, our data suggest that proliferation is associated with angiogenesis in MM. Furthermore, proliferation and angiogenesis, but not apoptosis, may be important in disease progression. Lastly, increased production of VEGF may be one of the contributing factors to the increase in intratumoral vascularity seen in advanced MM.
Take home messages .
In multiple myeloma (MM) proliferation appears to be associated with angiogenesis
Proliferation and angiogenesis may be important in disease progression in MM
However, apoptosis as assessed by ssDNA labelling appears to be an independent parameter
Increased production of vascular endothelial growth factor may be a contributing factor to the increase in intratumoral vascularity seen in advanced MM
Abbreviations
IL, interleukin
IMVD, intratumoral microvessel density
MM, multiple myeloma
ssDNA, single stranded DNA
VEGF, vascular endothelial growth factor
REFERENCES
- 1.Bataille R, Harousseau JL. Multiple myeloma. N Engl J Med 1997;336:1657–64. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Bergsagel PL, Anderson K. Multiple myeloma: increasing evidence for a multistep transformation process. Blood 1998;91:3–21. [PMC free article] [PubMed] [Google Scholar]
- 3.Rajkumar SV, Greipp PR. Prognostic factors in multiple myeloma. Hematol Oncol Clin North Am 1999;13:1295–314. [DOI] [PubMed] [Google Scholar]
- 4.Lai R, Medeiros LJ, Wilson CS, et al. Expression of the cell-cycle-related proteins E2F-1, P53, mdm-2, P21waf-1, and Ki-67 in multiple myeloma: correlation with cyclin-D1 immunoreactivity. Mod Pathol 1998;11:642–7. [PubMed] [Google Scholar]
- 5.Ong F, Nieuwkoop JA, De Groot-Swings GMJS, et al. Bcl-2 protein expression is not related to short survival in multiple myeloma. Leukemia 1995;9:1282–4. [PubMed] [Google Scholar]
- 6.Schwarze MMK, Hawley RG. Prevention of myeloma cell apoptosis by ectopic BCL expression or interleukin-6 mediated up-regulation of BCL-X-L. Cancer Res 1995;55:2262–9. [PubMed] [Google Scholar]
- 7.Tian E, Gazitt Y. The role of p53, bcl-2 and bax network in dexamethasone induced apoptosis in multiple myeloma cell lines. Int J Oncol 1996;8:719–26. [DOI] [PubMed] [Google Scholar]
- 8.Sezer O, Niemoller K, Eucker J, et al. Bone marrow microvessel density is a prognostic factor for survival in patients with multiple myeloma. Ann Hematol 2000;79:574–7. [DOI] [PubMed] [Google Scholar]
- 9.Vacca A, Ribatti D, Roncali L, et al. Bone marrow angiogenesis and progression in multiple myeloma. Br J Haematol 1994;87:503–8. [DOI] [PubMed] [Google Scholar]
- 10.Mattern J, Koomagi R, Volm M. Association of vascular endothelial growth factor expression with intratumoral microvessel density and tumour cell proliferation in human epidermoid carcinoma. Br J Cancer 1996;73:931–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu C, Tanigawa N. Spontaneous apoptosis is inversely related to intratumoral microvessel density in gastric carcinoma. Cancer Res 1997;57:221–4 [PubMed] [Google Scholar]
- 12.Drach J, Gattringer C, Glassl H, et al. The biological and clinical significance of the Ki-67 growth fraction in multiple myeloma. Hematol Oncol 1992;10:125–34. [DOI] [PubMed] [Google Scholar]
- 13.Girino M, Riccardi A, Luoni R, et al. Monoclonal antibody Ki-67 as a marker of proliferative activity in monoclonal gammopathies. Acta Haematol 1991;85:26–30. [DOI] [PubMed] [Google Scholar]
- 14.Pellegrini W, Facchetti F, Marocolo D, et al. Assessment of cell proliferation in normal and pathological bone marrow biopsies: a study using double sequential immunophenotyping on paraffin sections. Histopathology 1995;27:397–405. [DOI] [PubMed] [Google Scholar]
- 15.Tipoe GL, Jin Y, White FH. The relationship between vascularity and cell proliferation in human normal and pathological lesions of the oral cheek epithelium. Eur J Cancer 1996;1:24–31. [DOI] [PubMed] [Google Scholar]
- 16.Vermeulen PB, Verhoeven D, Hubens G, et al. Microvessel density, endothelial cell proliferation and tumour cell proliferation in human colorectal adenocarcinomas. Ann Oncol 1995;6:59–64. [DOI] [PubMed] [Google Scholar]
- 17.Hussong JW, Geroge MR, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood 2000;95:309–13. [PubMed] [Google Scholar]
- 18.Bellamy WT, Richter L, Frutiger Y, et al. Expression of vascular endothelial growth factor and its receptors in hematopoietic malignancies. Cancer Res 1999;59:728–33. [PubMed] [Google Scholar]
- 19.Dankbar B, Padro T, Leo R, et al. Vascular endothelial growth factor and interleukin-6 in paracrine tumor–stromal cell interactions in multiple myeloma. Blood 2000;95:2630–6. [PubMed] [Google Scholar]
- 20.Fox SB, Gatter KC, Bicknell R, et al. Relationship of endothelial cell proliferation to tumour vascularity in human breast cancer. Cancer Res 1993;53:4161–3. [PubMed] [Google Scholar]
- 21.Vartanian RK, Weidner N. Correlation of intratumoral endothelial cell proliferation with microvessel density (tumor angiogenesis) and tumour cell proliferation in breast carcinoma. Am J Pathol 1994;144:1188–94. [PMC free article] [PubMed] [Google Scholar]
- 22.Porschen R, Classen S, Pointek M, et al. Vascularization of carcinomas of the esophagus and its correlation with tumour proliferation. Cancer Res 1994;54:587–91. [PubMed] [Google Scholar]
- 23.Carson DA, Ribeiro JM. Apoptosis and disease. Lancet 1993;341:1251–4. [DOI] [PubMed] [Google Scholar]
- 24.Tatebe S, Ishida M, Kasagi, N, et al. Apoptosis occurs more frequently in metastatic foci than in primary lesions of human colorectal carcinomas: analysis by terminal-deoxynucleotidyl-transferase-mediated dUTP-biotin nick end labeling. Int J Cancer 1996;65:173–7. [DOI] [PubMed] [Google Scholar]
- 25.Aihara M, Truong LD, Dunn JK, et al. Frequency of apoptotic bodies positively correlates with Gleason grade in prostate cancer. Hum Pathol 1994;25:797–801. [DOI] [PubMed] [Google Scholar]
- 26.Shima Y, Nishimoto N, Yoshizaki K, et al. Fas antigen/APO-1 (CD95) expression on myeloma cells. Leuk Lymphoma 1996;23:521–31. [DOI] [PubMed] [Google Scholar]
- 27.Binet JL, Mentz F, Merle-Beral H. Apoptosis in blood diseases. Hematol Cell Ther 1996;38:253–64. [DOI] [PubMed] [Google Scholar]