Abstract
Aims: Tissue transglutaminase (tTG) is a major autoantigen recognised by IgA anti-endomysial antibodies (IgA EMA). Enzyme linked immunosorbent assays (ELISA) for IgA anti-tissue transglutaminase antibodies (IgA tTG) have therefore been developed as an alternative serological screening test to IgA EMA for coeliac disease (CD). The use of human tTG (h-tTG), as opposed to guinea pig liver tTG (gpl-tTG), in these assays has been reported to produce superior results. This study compared 13 commercial IgA tTG ELISA kits to ascertain their performance characteristics in the diagnosis of CD in patients with biopsy confirmed disease compared with controls. All patients and controls were adults aged 21 years or older.
Methods: Sera from the following groups of patients were tested in each kit: (1) 49 patients with CD confirmed on small bowel biopsies (all IgA EMA positive); (2) 34 patients with small bowel biopsies that were not consistent with CD; and (3) 30 patients with biopsy confirmed inflammatory bowel disease. All controls were negative for IgA EMA and were not IgA deficient. Sensitivities and specificities were determined using both the manufacturers' recommended cut off points and receiver operating characteristic (ROC) analysis derived decision thresholds. The area under the curve (AUC) for each ROC plot was also calculated and compared between kits.
Results: In general, the h-tTG based IgA tTG ELISA kits demonstrated superior performance (especially specificity) compared with the gpl-tTG based kits, although 100% sensitivity and specificity (comparable to the IgA EMA assay) was obtained in only one recombinant h-tTG based kit.
Conclusions: The use of h-tTG in IgA tTG ELISA kits is generally, but not universally, associated with superior performance. Factors other than antigen source are important in determining kit performance.
Keywords: coeliac disease, guinea pig liver, human, tissue transglutaminase
The identification of autoantibodies strongly associated with coeliac disease (CD; also known as gluten sensitive enteropathy), in particular IgA anti-endomysial antibodies (IgA EMA), has enabled the development of non-invasive serological screening tests for this condition.1–3 The IgA EMA indirect immunofluorescence (IIF) assay has, in subjects with untreated CD, a sensitivity of 84–100% and a specificity of 94–100%, which is superior to the IgA anti-reticulin IIF assay and IgA/IgG antigliadin antibody enzyme linked immunosorbent assays (ELISAs).2
“The use of human tissue transglutaminase has been reported to be associated with fewer false negative and false positive results, and an overall performance closely comparable or equal to the “gold standard” IgA anti-endomysial antibody indirect immunofluorescence assay”
Since Dieterich et al described tissue tranglutaminase (tTG), an 82–85 kDa ubiquitous enzyme, as the major autoantigen target of IgA EMA,4 over 30 publications have appeared using this protein as the basis for an alternative assay to the IgA EMA IIF assay.5–38 Most studies used guinea pig liver tTG (gpl-tTG) in ELISA based assays,5–15,18–38 but purified erythrocyte23 and recombinant human tTG (h-tTG)13–17,24,27,29,35,38 have also been used in ELISA,13,23,24,27,29,38 radioimmunoassay,14–17,35 and dot blot27 assays. Because of its ease of use, potential for automation, objectivity in interpretation, and reduced training requirements, there is growing interest in using an ELISA based IgA anti-tTG antibody (IgA tTG) assay as an alternative to the IgA EMA IIF assay.
Although many studies have concluded that the IgA tTG assay has comparable performance to the IgA EMA IIF assay, several have described false negative IgA tTG results in subjects with IgA EMA positive untreated CD,4,10–16,19–21,25,26,28–30,32,33,34,36,38 and false positive IgA tTG results in the absence of IgA EMA and CD. 5,6,9,10,12–16,18,19,22–29,32,33,36,38 However, most of these studies used gpl-tTG, which has only about 81% homology with h-tTG.39 In contrast, the use of h-tTG has been reported to be associated with fewer false negative and false positive results, and an overall performance closely comparable or equal to the “gold standard” IgA EMA IIF assay.13–15,23,24,29,35,38,40 However, because none of these studies has compared gpl-tTG based ELISAs with two or more h-tTG-based ELISAs, it is unclear whether the use of h-tTG alone results in superior performance to the gpl-tTG-based assays.
We compared 13 commercial IgA tTG ELISA kits, seven gpl-tTG based and six h-tTG based (four recombinant h-tTG), in 49 IgA EMA positive adult patients with CD and 64 adult disease controls to establish the sensitivity and specificity of each kit, and thus determine whether the h-tTG based kits consistently outperformed the gpl-tTG based kits, and produced comparable results to the IgA EMA IIF assay.
METHODS
Patients
One hundred and thirteen sera were selected from samples submitted to: Division of Immunology, Queensland Health Pathology Services, Royal Brisbane and Princess Alexandra Hospitals; Central Sydney Immunology Laboratory; and Department of Immunology, Sullivan Nicolaides Pathology. These comprised sera from the following patients who were aged 21 years or older: (1) 49 patients with typical histological changes of CD on small bowel biopsy,3,41 who had previously been found to have a positive IgA EMA, 38 of whom had never been on a gluten free diet, and 11 of whom were poorly compliant or non-compliant with the diet and had an abnormal small bowel biopsy close to the time of blood sampling; (2) 34 subjects who had been investigated with upper gastrointestinal fibreoptic endoscopy and small bowel biopsy for possible CD and were found not to have histological changes consistent with CD (non-CD controls, with the following results on small bowel biopsy (no evidence of villous atrophy in all cases): normal duodenum (n = 27), duodenal ulcer (n = 3), dilated Brunner's glands (n = 1), non-specific duodenitis (n = 1), fibrotic and thickened small bowel (n = 1), and gastric atrophy (n = 1)); and (3) 30 subjects with biopsy confirmed inflammatory bowel disease (IBD controls).
All sera were retested for IgA EMA at the start of the study to ensure that the sera from patients with CD had not degraded during storage at −70°C. Total serum IgA values were also measured in all 64 non-CD and IBD control sera by nephelometry (Behring Diagnostics, Frankfurt, Germany). All 64 controls had values within the normal range for adults (1.24–4.16 g/litre), thus excluding IgA deficiency as a potential cause for negative results.
IgA EMA IIF assay
The IgA EMA assay was performed by IIF using cryostat sections of monkey oesophagus (The Binding Site, Birmingham, UK), as described previously2 at a screening dilution of 1/4. All slides were viewed by two independent observers and a positive or negative result was determined by consensus.
IgA tTG ELISA
The manufacturer's instructions (table 1) were followed for all 13 IgA tTG ELISA kits. All specimens were tested in duplicate.
Table 1.
IgA tTG commercial enzyme linked immunosorbent assay kits: manufacturers' kit details
| Manufacturer | Source of tTG | Calcium activated tTG | Serum dilution | Incubation times (serum, conjugate) in minutes | Conjugate | Substrate |
| AESKULISA/Aesku.Lab Diagnostica (Wendelsheim, Germany) | Recombinant human | NS | 1/100 | 30, 15 | HRP antihuman IgA | TMB |
| The Binding Site (Birmingham, UK) | Guinea pig liver | Yes | 1/100 | 30, 30 | Rabbit HRP antihuman IgA | TMB |
| Recombinant human | No | 1/100 | 30, 30 | Rabbit HRP antihuman IgA | TMB | |
| Eurospital S.p.A (Trieste, Italy) | Guinea pig liver | NS | 1/25 | 60, 60 | Sheep HRP antihuman IgA | TMB |
| Recombinant human | NS | 1/25 | 60, 60 | Sheep HRP antihuman IgA | TMB | |
| Genesis Diagnostics (Littleport, UK) | Guinea pig liver | Yes | 1/100 | 30, 30 | Rabbit HRP antihuman IgA | TMB |
| ImmuLisa/Immco Diagnostics Inc (Buffalo, New York, USA) | Guinea pig liver | NS | 1/50 | 60, 30 | Alkaline phosphatase antihuman IgA | PNPP |
| Immunopharmacology Research Diagnostics (Catania, Italy) | Guinea pig liver | NS | 1/100 | 30, 30 | HRP antihuman IgA† | TMB |
| QUANTA Lite/Inova (Diagnostics Inc, San Diego, California, USA) | Guinea pig liver | NS | 1/100 | 30, 30 | Goat HRP antihuman IgA | TMB |
| Purified human erythrocyte | NS | 1/100 | 30, 30 | Goat HRP antihuman IgA | TMB | |
| Medizyme/Medipan Diagnostica GmbH (Selchow, Germany) | Guinea pig liver | NS | 1/50 | 60, 30 | Sheep HRP antihuman IgA | TMB |
| Orgentec Diagnostika GmbH (Mainz, Germany) | Purified human | NS | 1/100 | 30,15 | Rabbit HRP antihuman IgA | TMB |
| Varelisa/Pharmacia & Upjohn Diagnostics GmbH & Co (Freiburg, Germany) | Recombinant human | NS | 1/100 | 30, 30 | HRP antihuman IgA | TMB |
†In the manufacturer's kit insert, alkaline phosphatase was mentioned under “Principle”, but HRP was mentioned under “Reagent supplied”.
HRP, horseradish peroxidase; NS, not stated; PNPP, paranitrophenyl phosphate; TMB, 3,3`,5,5` tetramethylbenzidine; tTG, tissue transglutaminase.
Bovine serum albumin and gelatin coated ELISA plates
To investigate the possibility of IgA anti-bovine serum albumin (BSA) antibodies producing false positive IgA tTG results, ELISA plates (Costar, Corning Inc, New York, USA) were coated with 250 μl of 5% BSA (Sigma Chemical Co, St Louis, Missouri, USA) or 1% gelatin (Bio-Rad, Hercules, California, USA). Serum diluted 1/100 in Tween/phosphate buffered saline was incubated for one hour at room temperature. After three washes, horseradish peroxidase (HRP) labelled goat antihuman IgA (Silenus Labs, Melbourne, Australia), at a dilution of 1/500, was added and the plates were incubated for one hour (room temperature). ABTS (2.2`-azino-bis-3-ethylbenzthiazolin-6-sulphonic acid) substrate (Medical Innovations, Sydney, Australia) was added for 15 minutes, and absorbances read at 405 nm.
Cut off values
Both the manufacturers' recommended cut off values and decision thresholds determined by receiver operating characteristic (ROC) plots (see below) were used to calculate the sensitivity and specificity of each assay/kit. The IBD controls were not used in the calculation of specificity because some had not undergone small bowel biopsy to exclude CD.
ROC plot analysis
ROC plot analysis was performed on each kit using the Accuroc software package (Accumetric Corporation, McGill University Health Centre, Montreal, Quebec, Canada) to determine a decision threshold and area under curve (AUC) estimation. The IBD controls were not included in the ROC analysis because some had not undergone small bowel biopsy to exclude CD. The AUC was calculated using the trapezoid rule.42,43 Comparisons between the AUCs of each kit were performed by the non-parametric method for correlated samples, as previously described by DeLong et al.44
RESULTS
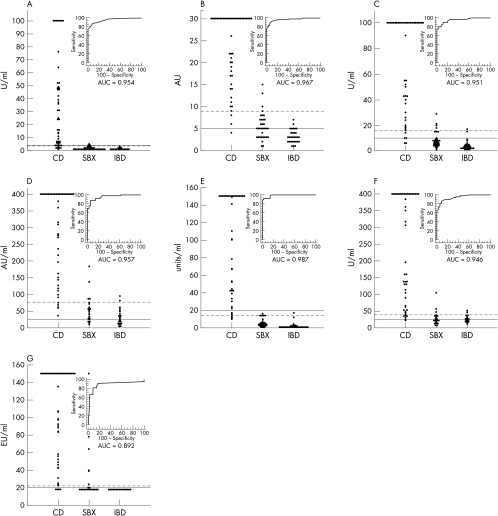
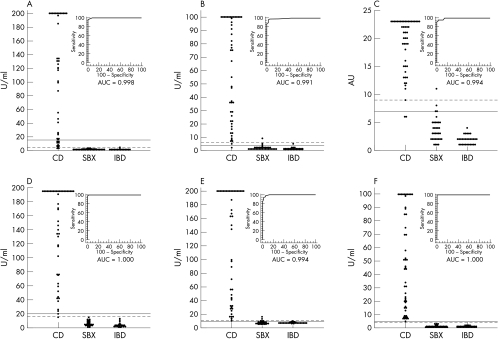
The IgA tTG values of the patients with CD and the non-CD and IBD controls measured with the 13 kits are shown in fig 1 (gpl-tTG based kits) and fig 2 (h-tTG based kits) with corresponding ROC curves and AUC estimations. The numbers of sera from patients with CD, and the non-CD and IBD controls that were positive in each assay, using both the manufacturers' and ROC analysis derived decision thresholds, are shown in table 2 (gpl-tTG based kits) and table 3 (h-tTG based kits), with corresponding sensitivities and specificities. Table 4 shows the AUC comparisons between kits, with a significant difference denoted by a p value of < 0.05.
Figure 1.
IgA anti-tissue transglutaminase (tTG) antibody values of the patients with coeliac disease (CD), and the non-CD (SBX) and inflammatory bowel disease (IBD) controls in the seven purified guinea pig liver tTG based enzyme linked immunosorbent assay (ELISA) kits, with corresponding receiver operating characteristic (ROC) curves and area under curve (AUC) estimations. The solid lines represent the manufacturers' recommended cut off values and the broken lines represent the ROC plot analysis derived decision thresholds. (A) The Binding Site, (B) Eurospital, (C) Genesis Diagnostics, (D) Immunopharmacology Research Diagnostics, (E) QUANTA Lite (Inova), (F) Medizyme (Medipan Diagnostica), (G) ImmuLisa (Immco).
Figure 2.
IgA anti-tissue transglutaminase (tTG) antibody values of the patients with coeliac disease (CD), and the non-CD (SBX) and inflammatory bowel disease (IBD) controls in the six human tTG based enzyme linked immunosorbent assay (ELISA) kits, with corresponding receiver operating characteristic (ROC) curves and area under curve (AUC) estimations. The solid lines represent the manufacturers' recommended cut off values and the broken lines represent the ROC plot analysis derived decision thresholds. (A) AESKULISA (Aesku.Lab), (B) The Binding Site, (C) Eurospital, (D) QUANTA Lite (Inova), (E) Orgentec, (F) Varelisa (Pharmacia & Upjohn).
Table 2.
IgA EMA and IgA tTG results in patients with CD and controls using the manufacturers' cut off points and ROC plot analysis derived decision thresholds for the seven guinea pig liver tTG based ELISA kits
| Manufacturer's cut off point | ROC plot analysis | |||||||
| Assay type/Manufacturer | Cut off | CD (sensitivity) | Non-CD controls (specificity) | IBD controls | Threshold | CD (sensitivity) | Non-CD controls (specificity) | IBD controls |
| IgA EMA IIF/The Binding Site | 1/4 | 49/49 (100%) | 0/34 (100%) | 0/30 | NA | NA | NA | NA |
| Guinea pig liver tTG based ELISA/The Binding Site | 4 U/ml | 43/49 (88%) | 3/34 (91%) | 0/30 | 3.5 U/ml | 43/49 (88%) | 3/34 (91%) | 0/30 |
| Guinea pig liver tTG based ELISA/Eurospital | 5 AU | 48/49 (98%) | 22/34 (35%) | 7/30 | 9 AU | 46/49 (94%) | 4/34 (88%) | 0/30 |
| Guinea pig liver tTG based ELISA/Genesis Diagnostics | 10 U/ml | 47/49 (96%) | 8/34 (76%) | 2/30 | 15.3 U/ml | 44/49 (90%) | 4/34 (88%) | 1/30 |
| Guinea pig liver tTG based ELISA/ImmuLisa | 20 EU/ml | 45/49 (92%) | 8/34 (76%) | 0/30 | 22.2 EU/ml | 44/49 (90%) | 6/34 (82%) | 0/30 |
| Guinea pig liver tTG based ELISA/ Immunopharmacology Research Diagnostics | 25 AU | 49/49 (100%) | 30/34 (12%) | 16/30 | 76.4 AU | 43/49 (88%) | 4/34 (88%) | 2/30 |
| Guinea pig liver tTG based ELISA/QUANTA Lite | 20 units/ml | 42/49 (86%) | 0/34 (100%) | 0/30 | 14.1 units/ml | 45/49 (92%) | 1/34 (97%) | 1/30 |
| Guinea pig liver tTG based ELISA/Medizyme | 25 U/ml | 48/49 (98%) | 16/34 (53%) | 15/30 | 38.5 U/ml | 43/49 (88%) | 4/34 (88%) | 2/30 |
Results equal to or greater than the cut off/threshold were considered positive. Specificity was calculated using only the non-CD controls (see text).
AU, arbitrary units; CD, coeliac disease; ELISA, enzyme linked immunosorbent assay; IBD, inflammatory bowel disease; IgA EMA, IgA anti-endomysial antibody; IgA tTG, IgA anti-tissue transglutaminase antibody; IIF, indirect immunofluorescence; NA, not applicable; tTG, tissue transglutaminase; ROC, receiver operating characteristic.
Table 3.
IgA EMA and IgA tTG results in patients with CD and controls using manufacturers' cut offs points and ROC plot analysis derived decision thresholds for the six human tTG based ELISA kits
| Manufacturer's cut off point | ROC plot analysis | |||||||
| Assay type/Manufacturer | Cut off | CD (sensitivity) | Non-CD controls (specificity) | IBD controls | Threshold | CD (sensitivity) | Non-CD controls (specificity) | IBD controls |
| IgA EMA IIF/The Binding Site | 1/4 | 49/49 (100%) | 0/34 (100%) | 0/30 | NA | NA | NA | NA |
| Human tTG based ELISA/Aesku.Lab | 15 U/ml | 35/49 (71%) | 0/34 (100%) | 0/30 | 4 U/ml | 47/49 (96%) | 0/34 (100%) | 1/30 |
| Human tTG based ELISA/The Binding Site | 4 U/ml | 48/49 (98%) | 3/34 (91%) | 1/30 | 6 U/ml | 47/49 (96%) | 1/34 (97%) | 0/30 |
| Human tTG based ELISA/Eurospital | 7 AU | 47/49 (96%) | 4/34 (88%) | 0/30 | 9 AU | 47/49 (96%) | 1/34 (97%) | 0/30 |
| Human tTG based ELISA/QUANTA Lite | 20 U/ml | 48/49 (98%) | 0/34 (100%) | 0/30 | 16 U/ml | 48/49 (98%) | 0/34 (100%) | 0/30 |
| Human tTG based ELISA/Orgentec | 10 U/ml | 49/49 (100%) | 5/34 (85%) | 1/30 | 11 U/ml | 48/49 (98%) | 2/34 (94%) | 0/30 |
| Human tTG based ELISA/Varelisa | 5 U/ml | 49/49 (100%) | 0/34 (100%) | 0/30 | 4 U/ml | 49/49 (100%) | 0/34 (100%) | 0/30 |
Results greater than or equal to the cut off/threshold are considered positive. Specificity was calculated using only the non-CD controls (see text).
AU, arbitrary units; CD, coeliac disease; ELISA, enzyme linked immunosorbent assay; IBD, inflammatory bowel disease; IgA EMA, IgA anti-endomysial antibody; IgA tTG, IgA anti-tissue transglutaminase antibody; IIF, indirect immunofluorescence; NA, not applicable; tTG, tissue transglutaminase; ROC, receiver operating characteristic.
Table 4.
Comparisons between AUC estimations44
| Guinea pig liver tTG | Binding Site | ||||||||||||
| 0.406 | Eurospital | ||||||||||||
| 0.908 | 0.494 | Genesis | |||||||||||
| 0.073 | 0.014* | 0.128 | Immco | ||||||||||
| 0.034* | 0.166 | 0.026* | 0.013* | Quanta Lite | |||||||||
| 0.900 | 0.518 | 0.831 | 0.044* | 0.109 | IPR | ||||||||
| 0.711 | 0.286 | 0.816 | 0.115 | 0.020* | 0.616 | Medizyme | |||||||
| Human tTG | 0.027* | 0.093 | 0.043* | 0.008* | 0.317 | 0.051 | 0.023* | Orgentec | |||||
| 0.023* | 0.058 | 0.022* | 0.006* | 0.102 | 0.027* | 0.014* | 0.198 | Varelisa | |||||
| 0.022* | 0.057 | 0.026* | 0.006* | 0.123 | 0.030* | 0.016* | 0.249 | 0.264 | Aesku.Lab | ||||
| 0.056 | 0.071 | 0.063 | 0.007* | 0.597 | 0.067 | 0.024* | 0.678 | 0.195 | 0.254 | Binding Site | |||
| 0.033* | 0.089 | 0.047* | 0.006* | 0.427 | 0.056 | 0.024* | 0.900 | 0.186 | 0.301 | 0.738 | Eurospital | ||
| 0.023* | 0.057 | 0.023* | 0.006* | 0.106 | 0.027* | 0.014* | 0.195 | 0.480 | 0.278 | 0.194 | 0.200 | Quanta Lite |
*p<0.05.
AUC, area under curve; tTG, tissue transglutaminase.
The recombinant h-tTG based Varelisa (Pharmacia & Upjohn Diagnostics, GmbH & Co, Freiburg, Germany) and purified erythrocyte h-tTG based QUANTA Lite (Inova Diagnostic Inc, San Diego, California, USA) kits performed best, with sensitivities of 100% and 98%, specificities of 100% and 100% (using the manufacturers' cut off values), and AUC estimations of 1.000 and 1.000, respectively (fig 2; table 3).
Of the seven gpl-tTG based kits (fig 1; table 2), the QUANTA Lite kit performed best, with 86% sensitivity and 100% specificity using the manufacturer's cut off value of 20 arbitrary units/ml, and an AUC of 0.987. Applying the ROC analysis derived decision threshold of 14.1 arbitrary units/ml improved sensitivity to 92% but reduced specificity to 97%.
To exclude the possibility that some reactions to tTG were really reactions to blocking agents used in the ELISA kits, anti-BSA and antigelatin antibodies were determined (data not shown). Sera from one IBD control and two patients with CD reacted significantly on the BSA coated ELISA plates, suggesting the presence of IgA anti-BSA antibodies. However, none of the non-CD controls reacted significantly on the BSA coated plates and no sera reacted on the gelatin coated plates.
DISCUSSION
In this comparison of 13 commercial IgA tTG ELISA kits, we found that the human tTG based kits tested generally demonstrated superior performance (especially specificity) to the gpl-tTG based kits (tables 2,3). However, the use of h-tTG alone was insufficient to confer performance equal to the IgA EMA IIF assay, because only two h-tTG based kits (recombinant h-tTG based Varelisa and purified erythrocyte h-tTG based QUANTA Lite) produced closely comparable results to the IgA EMA IIF assay. Furthermore, two of the gpl-tTG based kits (QUANTA Lite and Eurospital (Trieste, Italy)) had AUC estimations that were not significantly different from the h-tTG kits (figs 1, 2; tables 2, 3). This demonstrates that factors other than antigen source are important in determining kit performance.
Using the manufacturers' cut off values, false positive results were found in three of the six h-tTG based and six of the seven gpl-tTG based IgA tTG kits in our study (table 4). This has also been reported in other studies.5,6,9,10,12–16,18,19,22–29,32,33,36,38 However, as in our study, most of these false positive results were detected in gpl-tTG based ELISAs.5,6,9,10,12–15,18,19,22–26,28,29,32–34,36,38 These findings raise the important issue of contaminants in gpl-tTG,24,29,38 which may contain other hepatic proteins.29,38 On sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the gpl-tTG extract (Sigma T5398; Sigma Chemical Co) used in several gpl-tTG based ELISAs5,6,9–11,13–15,18,20–25,29,32,33,36,38 contains multiple bands in addition to the 82–83 kDa tTG band,24,29,38 which only accounted for about 30% of the total protein.29,38 This may be partially overcome by further purification steps, and should be less of an issue with recombinant h-tTG.24,29,38
However, three of the six h-tTG based kits (two recombinant h-tTG based) evaluated also produced false positive results in the absence of IgA EMA and CD, as previously reported.13,14,16,23,24,27 Therefore, other explanations for false positive results are required.
“The methods used to extract and purify tissue derived tissue transglutaminase (tTG), produce and process recombinant tTG, and then coat tTG on to ELISA wells may lead to alterations in the tertiary structure of tTG”
More false positive IgA tTG results were detected in the non-CD controls compared with the IBD controls. A possible explanation may be the presence of IgA anti-BSA antibodies in some of the non-CD control sera, reacting with the BSA used as a blocking agent in some kits.10 However, Lock and colleagues10 did not detect significant IgA anti-BSA antibodies in two disease controls tested, and significant IgA anti-BSA antibodies were not demonstrated in our non-CD controls.
False negative results were found in six of the seven gpl based and five of the six h-tTG based kits in IgA EMA positive patients with CD (figs 1, 2; tables 2, 3), in agreement with previous reports.4,10–16,19–21,25,26,28–30,32–34,36,38 The methods used to extract and purify tissue derived tTG, produce and process recombinant tTG, and then coat tTG on to ELISA wells may lead to alterations in the tertiary structure of tTG. Therefore, conformational epitopes may be lost or formed, with a loss leading to a reduced ability of tTG to bind IgA tTG, thus explaining some of the false negative results.25 Furthermore, the formation of conformational neoepitopes may also result in false positive results (see above).
Take home messages .
In general, the human tissue transglutaminase (h-tTG) based kits tested demonstrated superior performance (especially specificity) to the guinea pig liver tTG (gpl-tTG) based kits
Because this was a general and not a universal funding, factors other than antigen source are important in determining kit performance
Most of the kits performed significantly better when the cut off values/decision thresholds were adjusted via receiver operating characteristic plot analysis, which emphasises the importance of cut off point revalidation by laboratories, using appropriate samples from their referral population
The function and tertiary structure of tTG is also altered by the presence of ionised calcium.45 It has been suggested that antibody binding epitopes may be formed or hidden by the presence of ionised calcium in the coating buffer of the IgA tTG ELISA.10,46 Sulkanen and colleagues6 reported that the pretreatment of tTG with calcium (“calcium activation”) dramatically improved the separation between CD and non-CD sera in a gpl-tTG based ELISA, and also increased the binding affinity of tTG to CD sera. However, in our study, the two kits in which the use of “calcium activation” of tTG is recorded (Binding Site gpl-tTG kit and Genesis) did not clearly demonstrate superior performance to the other kits. Furthermore, Lock and colleagues10 found that the addition of calcium to the coating buffer increased both the signal and background values, and therefore produced no overall improvement in the performance of their in house gpl-tTG based IgA tTG ELISA. Nakachi and colleagues46 also reported that the autoantibody binding sites of tTG were formed in a manner that was essentially calcium independent.
Finally, we found that the performances of most of the IgA tTG ELISA kits were significantly improved by adjusting the cut off values/decision thresholds via ROC plot analysis. These discrepancies between the ROC analysis derived decision thresholds and manufacturers' recommended cut off values illustrate the importance of cut off point revalidation by laboratories, using appropriate samples from their referral population. However, the adjustment of the cut off values/decision thresholds via ROC plot analysis would not compensate for less than satisfactory kit performance. Therefore, in selecting an IgA tTG ELISA kit for diagnostic purposes, a laboratory should consider not only the source of tTG antigen, but also the performance of the kit using locally derived cut off values.
Supplementary Material
Acknowledgments
We acknowledge K Smithers (Central Sydney Immunology Laboratory, Royal Prince Alfred Hospital) for excellent technical assistance; the Australian distributors of the commercial IgA tTG kits for the generous provision of their kits for assessment in this study; and P Hobson (Department of Immunology, Sullivan Nicolaides Pathology, Brisbane, Australia) for providing some of the sera for the study and performing some of the assays.
Abbreviations
ABTS, 2.2`-azino-bis-3-ethylbenzthiazolin-6-sulphonic acid
AU, arbitrary units
AUC, area under curve
BSA, bovine serum albumin
CD, coeliac disease
ELISA, enzyme linked immunosorbent assay
gpl-tTG, guinea pig liver tissue transglutaminase
HRP, horseradish peroxidase
h-tTG, human tissue transglutaminase
IBD, inflammatory bowel disease
IgA EMA, IgA anti-endomysial antibody
IgA tTG, IgA anti-tissue transglutaminase antibody
IIF, indirect immunofluorescence
PNPP, paranitrophenyl phosphate
ROC, receiver operating characteristic
TMB, 3,3`,5,5` tetramethylbenzidine
tTG, tissue transglutaminase
REFERENCES
- 1.Chorzelski TP, Sulej J, Tchorzewska H, et al. IgA class endomysium antibodies in dermatitis herpetiformis and coeliac disease. Ann N Y Acad Sci 1983;420:325–34. [DOI] [PubMed] [Google Scholar]
- 2.Unsworth DJ. Serological diagnosis of gluten sensitive enteropathy. J Clin Pathol 1996;49:704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collin P. New diagnostic findings in coeliac disease. Ann Med 1999;31:399–405. [DOI] [PubMed] [Google Scholar]
- 4.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 1997;3:797–801. [DOI] [PubMed] [Google Scholar]
- 5.Dieterich W, Laag E, Schöpper H, et al. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology 1998;115:1317–21. [DOI] [PubMed] [Google Scholar]
- 6.Sulkanen S, Halttunen T, Laurila K, et al. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology 1998;115:1322–8. [DOI] [PubMed] [Google Scholar]
- 7.Brusco G, Izzi L, Corazza GR. Tissue transglutaminase antibodies for coeliac disease screening. Ital J Gastroenterol Hepatol 1998;30:496–7. [PubMed] [Google Scholar]
- 8.Binder WL. New advances for serologic evaluation for celiac disease and dermatitis herpetiformis. Clinical Immunology Newsletter 1998;18:125–34. [Google Scholar]
- 9.Miller A, Elliott PR, Paspaliaris W, et al. Anti-transglutaminase antibodies and coeliac disease. Aust N Z J Med 1999;29:239–42. [DOI] [PubMed] [Google Scholar]
- 10.Lock RJ, Pitcher MCL, Unsworth DJ. IgA anti-tissue transglutaminase as a diagnostic marker of gluten sensitive enteropathy. J Clin Pathol 1999;52:274–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troncone R, Maurano F, Rossi M, et al. IgA antibodies to tissue transglutaminase: an effective diagnostic test for celiac disease. J Pediatr 1999;134:166–71. [DOI] [PubMed] [Google Scholar]
- 12.Biagi F, Ellis H, Yiannakou J, et al. Tissue transglutaminase antibodies in celiac disease. Am J Gastroenterol 1999;94:2187–92. [DOI] [PubMed] [Google Scholar]
- 13.Sárdy M, Odenthal U, Kárpáti S, et al. Recombinant human tissue transglutaminase ELISA for the diagnosis of gluten-sensitive enteropathy. Clin Chem 1999;45:2142–9. [PubMed] [Google Scholar]
- 14.Bazzigaluppi E, Lampasona V, Barera G, et al. Comparison of tissue transglutaminase-specific antibody with established antibody measurements for coeliac disease. J Autoimmun 1999;12:51–6. [DOI] [PubMed] [Google Scholar]
- 15.Amin M, Eckhardt T, Kapitza S, et al. Correlation between tissue transglutaminase antibodies and endomysium antibodies as diagnostic markers of coeliac disease. Clin Chim Acta 1999;282:219–25. [DOI] [PubMed] [Google Scholar]
- 16.Seissler J, Borns S, Wohlrab U, et al. Antibodies to human recombinant tissue transglutaminase measured by radioligand assay: evidence for high diagnostic sensitivity for celiac disease. Horm Metab Res 1999;31:375–9. [DOI] [PubMed] [Google Scholar]
- 17.Williams AJ, Annis P, Lock RJ, et al. Evaluation of a high-throughput second antibody radiobinding assay for measuring IgA antibodies to human tissue transglutaminase. J Immunol Methods 1999;228:81–5. [DOI] [PubMed] [Google Scholar]
- 18.Reeves GEM, Burns C, Hall ST, et al. The measurement of IgA and IgG transglutaminase antibodies in celiac disease: a comparison with current diagnostic methods. Pathology 2000;32:181–5. [PubMed] [Google Scholar]
- 19.Sugai E, Selvaggio G, Vazquez H, et al. Tissue transglutaminase antibodies in celiac disease: assessment of a commercial kit. Am J Gastroenterol 2000;95:2318–22. [DOI] [PubMed] [Google Scholar]
- 20.Gillett HR, Freeman HJ. Comparison of IgA endomysium antibody and IgA tissue transglutaminase antibody in celiac disease. Can J Gastroenterol 2000;14:668–71. [DOI] [PubMed] [Google Scholar]
- 21.Clemente MG, Musu MP, Frau F, et al. Immune reaction against the cytoskeleton in coeliac disease. Gut 2000;47:520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stern M. Comparative evaluation of serologic tests for celiac disease: a European initiative toward standardization. Working group on serologic screening for celiac disease. J Pediatr Gastroenterol Nutr 2000;31:513–19. [DOI] [PubMed] [Google Scholar]
- 23.Hansson T, Dahlbom I, Hall J, et al. Antibody reactivity against human and guinea pig tissue transglutaminase in children with celiac disease. J Pediatr Gastroenterol Nutr 2000;30:379–84. [DOI] [PubMed] [Google Scholar]
- 24.Sblattero D, Berti I, Trevisiol C, et al. Human recombinant tissue transglutaminase ELISA: an innovative diagnostic assay for celiac disease. Am J Gastroenterol 2000;95:1253–7. [DOI] [PubMed] [Google Scholar]
- 25.Koop I, Ilchmann R, Izzi L, et al. Detection of autoantibodies against tissue transglutaminase in patients with celiac disease and dermatitis herpetiformis. Am J Gastroenterol 2000;95:2009–14. [DOI] [PubMed] [Google Scholar]
- 26.Sardy M, Karpati S, Peterfy F, et al. Comparison of a tissue transglutaminase ELISA with the endomysium antibody test in the diagnosis of gluten-sensitive enteropathy. Z Gastroenterol 2000;38:357–64. [DOI] [PubMed] [Google Scholar]
- 27.Baldas V, Tommasini A, Trevisiol C, et al. Development of a novel rapid non-invasive screening test for coeliac disease. Gut 2000;47:628–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan A, Butzner J, McKenna R, et al. Tissue transglutaminase enzyme-linked immunosorbent assay as a screening test for celiac disease in pediatric patients [abstract]. Paediatrics 2001;107:E8. [DOI] [PubMed] [Google Scholar]
- 29.Leon F, R-Pena R, Camerero C, et al. Limitations of anti-guinea pig liver transglutaminase IgA in screening of celiac disease. Gastroenterology 2001;120:586–7. [DOI] [PubMed] [Google Scholar]
- 30.Basso D, Gallo N, Guariso G, et al. Role of anti-transglutaminase (anti-tTG), anti-gliadin, and anti-endomysium serum antibodies in diagnosing celiac disease: a comparison of four different commercial kits for anti-tTG determination. J Clin Lab Anal 2001;15:112–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaskowski TD, Schroder C, Martins TB, et al. IgA antibodies against endomysium and transglutaminase: a comparison of methods. J Clin Lab Anal 2001;15:108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahele A, Kingstone K, Bode J, et al. Anti-endomysial antibody negative celiac disease: does additional serological testing help? Dig Dis Sci 2001;46:214–21. [DOI] [PubMed] [Google Scholar]
- 33.Dahele AV, Aldhous MC, Humphreys K, et al. Serum IgA tissue transglutaminase antibodies in coeliac disease and other gastrointestinal diseases. QJM 2001;94:195–205. [DOI] [PubMed] [Google Scholar]
- 34.Dickey W, McMillan SA, Hughes DF. Sensitivity of serum tissue transglutaminase antibodies for endomysial antibody positive and negative coeliac disease. Scand J Gastroenterol 2001;36:511–14. [DOI] [PubMed] [Google Scholar]
- 35.Bonamico M, Tiberti C, Picarelli A, et al. Radioimmunoassay to detect antitransglutaminase autoantibodies is the most sensitive and specific screening method for celiac disease. Am J Gastroenterol 2001;96:1536–40. [DOI] [PubMed] [Google Scholar]
- 36.Salmaso C, Ocmant A, Pesce G, et al. Comparison of ELISA for tissue transglutaminase autoantibodies with antiendomysium antibodies in pediatric and adult patients with celiac disease. Allergy 2001;56:544–7. [DOI] [PubMed] [Google Scholar]
- 37.Fabiani E, Catassi C. The serum IgA class anti-tissue transglutaminase antibodies in the diagnosis and follow up of coeliac disease. Results of an international multi-centre study. International working group on Eu-tTG. Eur J Gastroenterol Hepatol 2001;13:659–65. [DOI] [PubMed] [Google Scholar]
- 38.Leon F, Camarero C, R-Pena R, et al. Anti-transglutaminase IgA ELISA: clinical potential and drawbacks in celiac disease diagnosis. Scand J Gastroenterol 2001;36:849–53. [DOI] [PubMed] [Google Scholar]
- 39.Gentile V, Saydak M, Chiocca EA, et al. Isolation and characterization of cDNA clones to mouse macrophage and human endothelial cell tissue transglutaminases. J Biol Chem 1991;226:478–83. [PubMed] [Google Scholar]
- 40.Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology 2001;120:636–51. [DOI] [PubMed] [Google Scholar]
- 41.Maki M, Collin P. Coeliac disease. Lancet 1997;349:1755–9. [DOI] [PubMed] [Google Scholar]
- 42.Bamber D. The area above the ordinal dominance graph and the area below the receiver operating graph. Journal of Mathematical Psychology 1975;12:387–415. [Google Scholar]
- 43.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 1993;39:561–77. [PubMed] [Google Scholar]
- 44.DeLong ER, DeLong DM, Clark-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a non-parametric approach. Biometrics 1988;44:102–6. [PubMed] [Google Scholar]
- 45.Casadio R, Polverini E, Mariani P, et al. The structural basis for the regulation of tissue transglutaminase by calcium ions. Eur J Biochem 1999;262:672–9. [DOI] [PubMed] [Google Scholar]
- 46.Nakachi K, Swift G, Wilmot D, et al. Antibodies to tissue transglutaminase: comparison of ELISA and immunoprecipitation assay in the presence and in the absence of calcium ions. Clin Chim Acta 2001;304:75–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.