Abstract
Aims: The diagnosis of duodenal gastric metaplasia (DGM) is based on the demonstration of periodic acid Schiff (PAS) positive mucin in duodenal columnar cells. Recently, groups of duodenal columnar cells were seen to be autofluorescent in haematoxylin and eosin (H&E) stained sections from a patient with DGM.
Materials and methods: Consecutive archival gastric and duodenal H&E sections from 30 patients with chronic gastritis and DGM (CG+DGM), from 30 with chronic gastritis without DGM (control group I), from 30 with normal gastric and duodenal mucosa (control group II), and from five patients with coeliac disease (control group III) were reviewed on a fluorescent microscope.
Results: The surface epithelium of the gastric mucosa in all 95 cases had autofluorescent material. In 31 cases with DGM (including one unreported case in control group I), groups of columnar duodenal cells also had autofluorescent material. In the remaining 64 cases, duodenal columnar cells were not autofluorescent. Results were confirmed with the PAS stain.
Conclusions: The method described detected apical mucin secretion in columnar cells, both in the stomach and in the duodenum from patients with DGM. The autofluorescence was induced by eosin (which binds to neutral mucin). Observing H&E stained duodenal biopsies under a fluorescence microscope may be sufficient to confirm DGM or to detect incipient DGM. Despite long observation periods and a long exposure time while photographing, the autofluorescence did not fade away. Because re-cuttings for special staining (PAS) are no longer required when this method is used, both final diagnosing time and laboratory costs can be reduced.
Keywords: duodenum, metaplasia, gastric, autofluorescence
Duodenal gastric metaplasia (DGM) is a histologically detectable mucosal injury caused by abnormally large quantities of gastric acid reaching the duodenal cap.1 DGM leads to peptic duodenitis. The hyperproduction of gastric acid usually results from infection of the gastric mucosa with Helicobacter pylori.2 DGM can also be found in the absence of H pylori infection—for example, in Crohn's disease of the duodenum, and less frequently in coeliac disease.1,2
In the normal stomach, the columnar cells of the surface epithelium secrete mucins that can be stained with periodic acid Schiff (PAS).3 In the normal duodenum, the villi are lined with columnar cells and with goblet cells. Columnar cells have a striated absorptive border and do not secrete mucin, whereas goblet cells secrete mainly sialomucins, which are stained with PAS and more specifically with the cationic dye alcian blue (AB).3
In DGM, the surface columnar cells resemble the normal superficial columnar cells of the gastric mucosa in that they acquire the capacity to secrete mucin.1,2
“The hyperproduction of gastric acid usually results from infection of the gastric mucosa with Helicobacter pylori”
Recently, while investigating archival haematoxylin and eosin (H&E) sections from the duodenal mucosa—with the purpose of analysing the characteristics of the basement membrane using a fluorescent microscope4—we found autofluorescent material in groups of superficial columnar duodenal cells. This autofluorescent material was PAS positive and the case was diagnosed as DGM.
The purpose of our present study was to investigate, in consecutive duodenal biopsies from patients previously diagnosed with DGM, whether the pathological mucin contained in duodenal columnar cells was autofluorescent.
MATERIAL AND METHODS
Consecutive archival H&E stained sections from the duodenum of 30 patients with chronic gastritis and DGM (CG+DGM), 30 patients with normal gastric and duodenal mucosa (control group I), 30 patients with chronic gastritis without DGM (control group II), and five patients with coeliac disease and duodenal villous atrophy (DVA, control group III) were reviewed under an Axioskop routine microscope (Carl Zeiss, Wetzlar, Germany) using alternatively transmitted light and incident light fluorescence (green H filter, 546 nm wl).
Giemsa stain was available in all cases. Additional sections were stained with AB and high iron diamine to label sialomucins and sulfomucins, respectively.3,5,6 When those stains were negative, a positive PAS stain was considered to be evidence of the presence of neutral mucins.3
DGM was defined as the occurrence of foci of duodenal cells without a brush border, containing apical mucous.
The Student's unpaired t test was used to compared the significance of the difference between the group means. Significance was set at p < 0.05.
RESULTS
Patients
In the chronic gastritis and DGM group (CG+DGM), there were 18 male and 12 female patients. In control group I, there were 16 male and 14 females patients, and in control group II there were 17 male and 13 female patients. The mean (SD) age in the 30 patients with chronic gastritis and DGM (CG+DGM) was 48 (10) years, in group I it was 44 (15) years, and in group II it was 45 (12) years. No significant differences in sex and age were found between these three groups.
The mean age in the five patients with DVA was 16.6 years (range, 2–71 years).
Histology
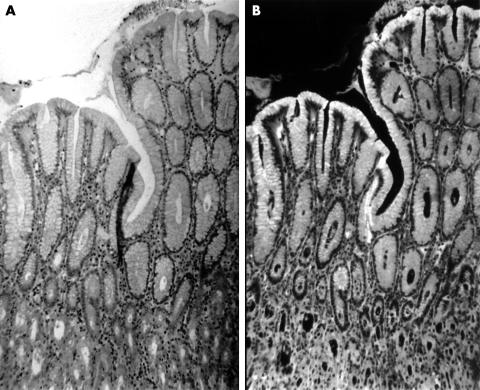
Table 1 shows that in the gastric mucosa the apical mucous of the superficial foveolar epithelium was autofluorescent in all 95 patients investigated (fig 1).
Table 1.
The number of patients showing autofluorescent material in haematoxylin and eosin stained duodenal sections excited with incident light fluorescence
| No. of patients | Superficial gastric cells | Columnar duodenal cells with DGM | |
| CG+DGM | 30 | 30/30 | 30/30 |
| Control group I | 30 | 30/30 | 0/30 |
| Control group II | 30 | 30/30 | 1/30 |
| Control group III | 5 | 5/5 | 0/5 |
| All | 95 | 95/95 | 31/95 |
CG+DGM, patients with chronic gastritis (GC) and duodenal gastric metaplasia (DGM). Control group I, patients with normal gastric and duodenal mucosa at initial diagnosis. Control group II, patients with chronic gastritis without duodenal gastric metaplasia at initial diagnosis. Control group III, patients with duodenal villous atrophy at initial diagnosis.
Figure 1.
(A) Haematoxylin and eosin (H&E) stained section from normal gastric mucosa as seen with transmitted light (original magnification, ×25). (B) The same area as (A) observed with incident light fluorescence. Note autofluorescent material in the superficial epithelium (H&E; original magnification, ×25).
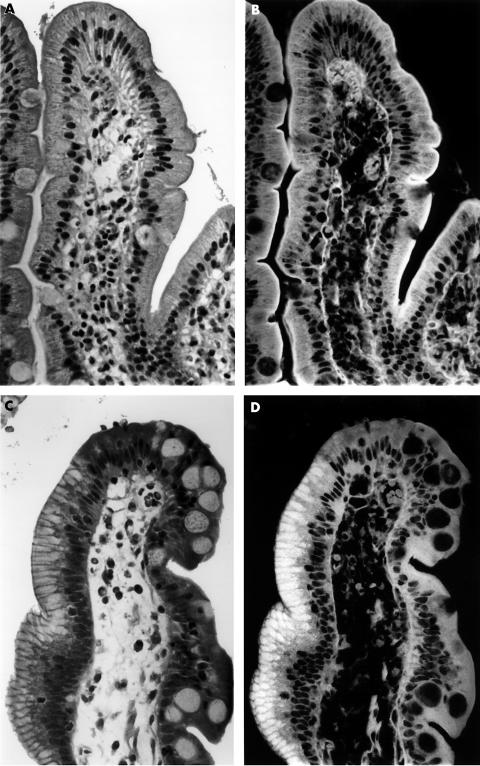
In the duodenal mucosa, the cytoplasm of the columnar and goblet cells in all 30 patients from control group I (with normal duodenal mucosa) and in 29 of the 30 patients from control group II (with chronic gastritis without DGM) were non-fluorescent (fig 2). However, the brush border of the columnar cells was autofluorescent.
Figure 2.
(A) Haematoxylin and eosin (H&E) stained section from normal duodenal mucosa as seen with transmitted light (original magnification, ×100). (B) The same area as (A) observed with incident light fluorescence. Only the brush border and the basement membrane are autofluorescent (H&E; original magnification, ×100). (C) H&E stained section from duodenal mucosa with duodenal gastric metaplasia, as seen with transmitted light (original magnification, ×100). Note apical vacuolisation of the columnar cells on the left slope of the villi. (D) The same area as (C) when observed with incident light fluorescence. Note that the material contained in the apical vacuolisation of the columnar cells shown in (C) is autofluorescent (H&E; original magnification, ×100).
At re-evaluation, the remaining case in group II (initially reported as normal on the basis of H&E stained sections alone) showed groups of columnar cells having autofluorescent material in their cytoplasm. That material was PAS positive.
Similar to the superficial epithelium of the gastric mucosa, all 30 patients with CG+DGM had groups of columnar superficial duodenal cells harbouring apical autofluorescent material (fig 2C). The cytoplasm in the remaining superficial cells of the duodenum, in addition to the goblet cells, was not fluorescent (n = 30).
Traces of sialomucins (as deduced by a weak AB stain) were detected in some cells with DGM. The HID stain was negative.
All 30 patients with CG+DGM harboured H pylori in their gastric specimens, as did 18 of 30 patients with chronic gastritis without DGM. None of the normal patients harboured H pylori.
In all five patients from control group III (with DVA), the cytoplasm of the columnar and goblet cells of the duodenal mucosa was not fluorescent.
DISCUSSION
In the normal stomach, the luminal aspect of the cytoplasm of the surface and foveolar columnar cells contains neutral mucin. In the neck region of the glands of the body of the stomach, traces of acid mucin may be present, but only in small amounts.3
In the normal duodenum, the jejunum, and the ileum at the luminal aspect the cytoplasm of the surface columnar cells does not contain neutral mucin, sialomucins,1 or sulfomucins.3 In contrast, the cytoplasm of columnar cells of the duodenum with DGM contains PAS positive mucin and traces of AB positive sialomucins. Consequently, most of the PAS positivity in DGM cells is the result of neutral mucin,3 because neutral mucin is defined by the failure to demonstrate acid groups reacting with cationic dyes (AB or HID).3
In our present study, autofluorescent material was demonstrated in H&E stained sections excited with fluorescent light. That material was present not only in the cytoplasm of the superficial foveolar columnar cells of the stomach in all three groups investigated, but also in the cytoplasm of superficial columnar duodenal cells from patients with DGM. The autofluorescent material was PAS positive. Obviously, autofluorescence demonstrated those cells able to secrete neutral mucin (as deduced by histochemistry).
The presence of neutral mucins in the cytoplasm of DGM cells seems to be the result of a cellular adaptation to the chronic insult caused by the noxious gastric juices acting upon the duodenal mucosa.3 Because neutral mucins are normally secreted by the superficial foveolar columnar cells of the gastric mucosa, it may be inferred that neutral mucin exerts a substantial buffer effect upon gastric acids, not only in the gastric milieu but also in the microenvironment surrounding DGM cells.
The reason for the absence of DGM in 29 of the 30 patients in control group II remains unclear. It is conceivable that the development of DGM may be influenced by the duration of infection with H pylori, by the presence of gastric hypochlorhydria or achlorhydria, by antacid/anti-H pylori medication, and/or by the sensitivity of the patient to the harmful HCl acting upon the duodenal mucosa.
“The autofluorescence of the eosin did not fade away despite long periods of observation under the fluorescent microscope and long periods of exposure time while photographing”
We investigated the possible cause(s) of the autofluorescence seen in the apical mucous of superficial columnar cells. For this purpose, duodenal sections from 12 patients were stained exclusively with eosin (Polysciences Incorporated, Warrington, Pennsylvania, USA), exclusively with haematoxylin (Gill's haematoxylin III; Merck, Darmstadt, Germany), or exclusively with PAS. One set of sections was not stained. Results showed that only sections stained exclusively with eosin displayed autofluorescence of the apical mucous (both in the superficial cells of the normal stomach and in the DGM cells of the duodenum). Sections that had been stained with all the other stains, in addition to the unstained sections, remained non-fluorescent. Thus, the autofluorescence reported here was exclusively elicited by the eosin of the H&E stain.
The autofluorescence of the eosin did not fade away despite long periods of observation under the fluorescent microscope and long periods of exposure time while photographing. This was very useful because it permitted repeated inspections of archival H&E stained sections under the fluorescent microscope, in the search for undiagnosed cases of DGM.
From our data, we suggest that the simple observation of H&E stained duodenal biopsies under a fluorescent microscope may be sufficient to confirm DGM. Equivocal cases of DGM (using light microscopy) may be easily confirmed or rejected by scrutinising H&E stained sections under a fluorescent microscope. Using this simple procedure, re-cuttings for special staining are no longer required, and consequently, the final diagnosing time and laboratory costs have been reduced in our department.
Take home messages.
This method can detect apical mucin secretion in columnar cells, both in the stomach and in the duodenum, in haematoxylin and eosin stained slides from patients with duodenal gastric metaplasia (DGM)
The method could be useful in the diagnosis of equivocal cases of DGM
The autofluorescence was induced by eosin (which binds to neutral mucin)
Despite long observation periods and a long exposure time while photographing, the autofluorescence did not fade away
This method could help reduce both the time to final diagnosis and laboratory costs
Abbreviations
AB, alcian blue
CG, chronic gastritis
DGM, duodenal gastric metaplasia
DVA, duodenal villous atrophy
H&E, haematoxylin and eosin
PAS, periodic acid Schiff
REFERENCES
- 1.Harris A, Gummett P, Walter J, et al. Relation between gastric output, Helicobacter pylori, and gastric metaplasia in the duodenal bulb. Gut 1996;39:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McColl K. Helicabacter pylori, gastric acid, and duodenal gastric metaplasia. Gut 1996;39:615–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filipe MI. Mucins in the human gastrointestinal tract. A review. Invest Cell Pathol 1979;2:195–216. [PubMed] [Google Scholar]
- 4.Rubio C. A simple method to evaluate the thickness of collagen in collagenous colitis. Scand J Gastroenterol 2000;35:223–4. [DOI] [PubMed] [Google Scholar]
- 5.Rubio CA, Huang C. Quantification of the sulphomucin-producing cell population of the colonic mucosa during protracted stress in rats. In Vivo 1992;6:81–4. [PubMed] [Google Scholar]
- 6.Rivera F, Rubio CA. Quantitative studies of the extension of gastric intestinal metaplasia in gastrectomy specimens from Swedish patients. Eur J Gastroenterol Hepatol 1993;5:521–5. [Google Scholar]