Abstract
Aims: To determine important factors influencing recurrence after local excision of duct carcinoma in situ (DCIS) of the breast.
Materials and methods: The extent (size) in millimetres, classification (by cytonuclear grade (NHSBSP system), by extent of necrosis, and by the Van Nuys system), and excision margins of 115 cases of screen detected DCIS treated by local excision were measured. A prognostic index was calculated by the addition of the Van Nuys classification (low grade, 1; moderate grade, 2; high grade, 3), margin score (≥ 10 mm, 1; 1–9 mm, 2; < 1 mm, 3), and size score (≤ 15 mm, 1; 16–40 mm, 2; and ≥ 41 mm, 3), giving a total score of 3–9.
Results: Classification using cytonuclear grade, extent of necrosis, or the Van Nuys system did not correlate significantly with recurrence. The excision margin (in millimetres) was associated with recurrence (p = 0.027) and if excision margin status was simplified using the scoring system (≥ 10 mm, 1; 1–9 mm, 2; < 1 mm, 3), the margin score was significantly associated with recurrence (p = 0.03). A prognostic index based on the Van Nuys score, margin status, and size was significantly associated with recurrence (p = 0.003).
Conclusion: The results support the hypothesis that the margin of excision is the most important factor predicting the recurrence of DCIS after local excision.
Keywords: duct carcinoma of breast, duct carcinoma in situ, breast
The introduction of breast screening programmes has led to an increase in the detection of ductal carcinoma in situ (DCIS) of the breast, with up to 20% of breast neoplasia being in situ. Although mastectomy in these cases is almost always curative for localised DCIS this seems to be over treatment. Selected patients may be offered breast conserving surgery with wide local excision (WLE) of the DCIS without axillary node dissection in the first instance. If the DCIS is completely excised on pathological examination and no invasion is found then patients are followed up. If excision is incomplete (DCIS at cut margin or less than 1 mm clearance in some centres) further local excision or mastectomy may be offered. The use of tamoxifen and/or radiotherapy to the residual breast tissue to prevent recurrence is controversial and is the subject of clinical trials. There has been considerable clinical interest in developing methods to identify those patients at high risk of recurrence as DCIS or invasive carcinoma in the remaining breast. Development of a classification system for DCIS that could predict recurrence is seen as a potential advance. Several classifications based on architectural features, cytonuclear features, cell polarity, necrosis, or combinations of these features have been proposed,1–4 but none has been widely accepted.5 There is consensus that observation and recording of these features is important in histopathological reporting of DCIS6 and evidence that acceptable interobserver agreement can be attained by non-specialist pathologists.7 The classification of the DCIS, the extent of the disease (size), and the margins of excision have been proposed as potential predictors of local recurrence. The classification, size, and excision margin of the DCIS have been combined as a prognostic index for DCIS, which has been shown to correlate with recurrence.8 Subsequent work has shown that margin of excision is the most important predictor of recurrent disease.9, 10 The aim of our present study was to provide independent data in support of these preliminary conclusions.
“There has been considerable clinical interest in developing methods to identify those patients at high risk of recurrence as ductal carcinoma in situ or invasive carcinoma in the remaining breast”
MATERIALS AND METHODS
Case material
From 1989 to 1998 there were 1110 cases of DCIS in our hospital, and 646 of these were screen detected. Five hundred and eleven of the 1110 patients were treated by WLE, and 346 of 646 screen detected patients were treated by WLE.
Screening by mammography for women between the ages of 50 and 65 was introduced in South East Wales in 1989 as part of the National Breast Screening programme. For our study, 131 women with pure screen detected DCIS were identified. Sixteen of these women proceeded to a completion mastectomy following local excision and were excluded from the study. Nine women had re-excision but retained breast tissue and these were included in the analysis. The pathological data reported are for 115 women, among whom there were 15 recurrences.
All patients were within the age range for the National Breast Screening Programme in the UK (50–65 years) at the time of diagnosis. In the absence of evidence of benefit from clinical trials the policy of the screening unit was not to offer adjuvant treatment (radiotherapy or tamoxifen) to women after complete local excision of DCIS. Women with radiologically extensive (> 50 mm) or central disease were advised to undergo mastectomy.
Pathological material
All histological sections available were retrieved from file and reviewed.
Classification of DCIS
Cytonuclear grade
Sections were reviewed and the cytonuclear grade was determined using the method described in the NHSBSP reporting guidelines.3
High nuclear grade DCIS was composed of mitotically active cells with pleomorphic, irregularly spaced, large nuclei with coarse chromatin and pronounced variation in size. Low nuclear grade DCIS showed monomorphic, evenly spaced cells with roughly spherical, centrally placed nuclei and inconspicuous nucleoli. Intermediate nuclear grade DCIS showed moderate nuclear pleomorphism, less than that of high grade DCIS, but lacking the monotony of the low grade cells.3
Necrosis
The presence and extent of necrosis was assessed using a simple reproducible system based on the extent of necrosis,7 namely:
Extensive necrosis.
DCIS with necrosis.
DCIS without necrosis.
Cases were designated as extensive necrosis if more than 50% of the diameter of any structures present showed intraduct necrosis. Necrosis was defined as eosinophilic debris containing five or more pyknotic nuclei. DCIS without necrosis comprised those cases in which no ductular structure present showed intraduct necrosis to any degree.7
Van Nuys score4
Lesions with high grade (grade 3) nuclear features with or without necrosis were placed in the high grade group. Non-high grade DCIS was divided by the presence (group 2) or absence (group 1) of comedo-type necrosis. Necrosis was defined as eosinophilic debris containing five or more pyknotic nuclei. Occasional desquamated or individually necrotic cells were ignored and were not scored as comedo-type necrosis. No requirement for a minimum amount of comedo-type necrosis was required.7
Size
The extent (size) of the DCIS was measured on histological sections in millimetres. In cases of DCIS > 20 mm in extent (more than one histological section), contiguous sections were taken and reassembled to allow measurement across two or more slides.
Margins of excision
All wide local excision specimens were orientated with sutures by the surgeon before arrival in the pathology department. Specimen margins were inked with different coloured acrylic inks before incision of the specimens. A specimen x ray was examined to ascertain the distribution of calcification within the tissue. The sampling of the tissue was tailored to the individual specimen using a flexible iterative approach rather than a rigid protocol driven one. This approach requires careful documentation of the origin of all tissue blocks and preservation of the orientation of the specimen to allow the taking of further carefully targeted tissue blocks after initial sampling. Where necessary, the specimen was cut into 4–5 mm slices, laid out, and x rayed to identify areas of microcalcification. Blocks were taken of radiological microcalcification and usually one complete slice of the tissue was examined histologically to assess the distribution of DCIS throughout the specimen (fig 1). Random blocks were taken from the background breast and blocks were taken perpendicular to the medial and lateral margins. The first set of slides allowed an initial assessment of the distribution and size of the DCIS within the specimen. In particular, an assessment of the extent of non-calcified DCIS beyond the mammographically detected calcified area and the distribution of this non-calcified DCIS could be made. The direction in which an involved duct is running can be used to predict distal involvement and prompt further sampling. Any area in which DCIS was seen approaching a margin would prompt further adjacent blocks from serial slices to determine the closest margin. Particular note was taken of ducts containing DCIS radiating out towards a margin. These ducts were followed by levels or adjacent blocks to determine whether the margin was involved at this point (fig 1).
Figure 1.
Schematic diagram to illustrate individualised iterative approach to the examination of tissue margins. The initial slice through AB, which is blocked entirely (blocks 1–8), shows microcalcified ductal carcinoma in situ (DCIS), as expected, but also non-calcified DCIS approaching the posterior margin (blocks 4 and 2). This finding would prompt further blocks to be taken from contiguous tissue in adjacent slices 3 and 5. The presence of DCIS in block 4 would prompt further contiguous blocks to be taken from slices 2 and 3 and complete blocking of the superior margin as shown. The absence of DCIS in slices 5 and 8 (blocks 10 and 11) would indicate the absence of non-calcified DCIS extending towards the inferior margin. No further blocks would be taken from these areas.
The closest margin of excision was measured where possible in millimetres on the glass slides. In cases where an open biopsy established the diagnosis of DCIS a subsequent therapeutic WLE was performed. If there was DCIS in the treatment specimen the margin of excision was taken from that specimen. If no residual DCIS was demonstrated in the treatment specimen then the margin of excision was recorded as 10 mm. Similarly, where excision margins of a treatment WLE were positive for DCIS and re-excision was performed and no DCIS was found in the re-excision specimen the margin was arbitrarily recorded as 10 mm.
Prognostic index
The prognostic index for the DCIS was calculated as follows.8
The pathology score (Van Nuys classification):
Low grade, score 1.
Moderate grade, score 2
High grade, score 3.
For margins of excision:
Margin > 10 mm, score 1.
Margins 1–9 mm, score 2.
Margin < 1 mm, score 3.
For size:
< 15 mm score 1.
16–40 mm, score 2.
> 41 mm, score 3.
The prognostic index was calculated by adding together the score for pathological classification, the score for size, and the score for margins, giving a total prognostic index of 3–9.8
Statistical analysis
The data sets for non-recurrent cases were compared with data for recurrent cases using a Mann Whitney test.
The probability of recurrence was estimated using binary logistic regression with nuclear grade, necrosis, distance to margin, and size as the independent variables. The logistic model highlights which of these variables contributes most to an estimation of the probability of recurrence. The log likelihood ratio test was used to assess the relative importance of each variant in the logistic model.
Differences in grade and prognostic index in DCIS and invasive recurrences were compared using the χ2 test. Differences in extent (size in millimetres), disease free interval, and excision margins in millimetres in DCIS and invasive recurrences were compared using the Student’s t test.
RESULTS
Table 1 shows the results of the comparison of parameters observed in non-recurrent and recurrent DCIS. Classification of the DCIS using the NHSBSP system, based on the extent of necrosis or using the Van Nuys system, was not significantly correlated with recurrence.
Table 1.
Comparison of pathological features in non-recurrent and recurrent cases of ductal carcinoma in situ
| Pathological feature | p Value (Mann-Whitney) | Significance |
| Classification | ||
| Nuclear grade (NHSBSP) | 0.132 | NS |
| Necrosis | 0.286 | NS |
| Van Nuys | 0.087 | NS |
| Extent | ||
| Size score | 0.340 | NS |
| Histological size | 0.149 | NS |
| Margin | ||
| Margin distance (mm) | 0.027 | Significant |
| Margin score | 0.0315 | Significant |
| Prognostic index | 0.0028 | Significant |
NS, not significant.
The excision margin measured in millimetres was significantly associated with recurrence (p = 0.027). If the excision margins were simplified using the scoring system then the margin score was significantly associated with recurrence (p = 0.0315).
The size score and the histological size in millimetres were both associated with recurrence but failed to reach significance. The prognostic index was shown to be significantly associated with recurrence (p = 0.0028).
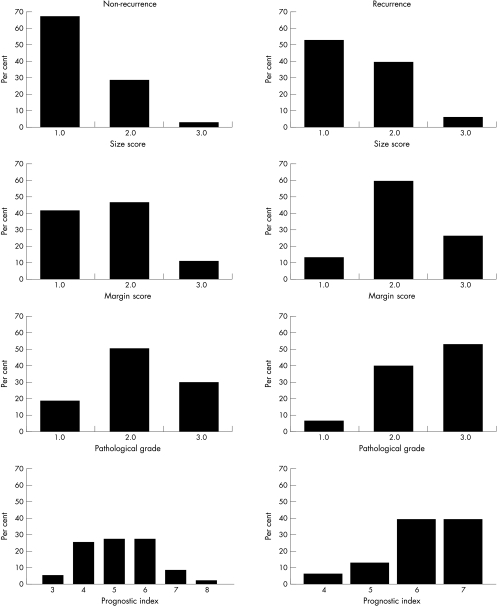
Figure 2 shows the proportion of recurrent cases in relation to the variables examined, classification using the Van Nuys system, extent of necrosis margin of excision (simplified score), and the prognostic index. Patients in whom DCIS reached the margins or had extensive disease (> 50 mm) were not offered conservation but were advised to undergo mastectomy. Sixteen patients in whom the excision margins were close (less than 1 mm) or involved chose to undergo mastectomy and were excluded. For these reasons there are few patients in this series with a prognostic index of 8 or 9. In the non-recurrent group, three patients had a prognostic index of 8 and none had a prognostic index of 9. All three patients had a margin score of 3 (two with a margin of 0.9 mm and one margin involved), one had a grade score of 2, and two had a size score of 2 (19 and 20 mm). No patients with recurrence had a prognostic index of 8 or 9 but 12 of the recurrent cases had a score of 6 or 7.
Figure 2.
Distribution of components (size, margin, and grade) of the ductal carcinoma in situ (DCIS) prognostic index and of the prognostic index in non-recurrent and recurrent cases of DCIS treated by local excision.
Figure 3 shows the margins of excision of all the patients plotted in ascending order. Arrows indicate patients in whom there was recurrence.
Figure 3.
Margin of excision in 115 cases of ductal carcinoma in situ (DCIS) treated by local excision in ascending order. Recurrent cases are indicated by arrows.
Stepwise multivariate binary logistic regression is used to determine whether a particular combination of variables provides the best estimate of the relative risk of recurrence. The following model was obtained:
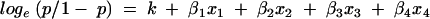 |
Where p is the probability of recurrence, k is a constant (−3.132), + βn is the regression co-efficient, and xn is a variable. Table 2 gives the results of this analysis. Each variable was excluded from the model in turn and the log likelihood test was used to determine the significance of any difference between a full model (all variables included) and the reduced model (one variable omitted). The degree of freedom for the likelihood test was determined by the difference in the number of factors between the full and reduced models. In each case, this is one, equivalent to a cut off value of χ2 of 3.841. From Table 2, only the margin variable (χ2, 4.284) is seen to contribute a significant (p = 0.05) difference when omitted from the full model. The standard errors for β for nuclear grade and necrosis are large, making the estimates of the relative contributions of these two variables less reliable than those for margin and size (table 2).
Table 2.
Analysis of logistic regression modelling
| Pathological feature contributing to estimation of probability of recurrence | β | SE β | χ2 |
| Nuclear grade (NHSBSP) | 0.2379 | 0.4580 | 0.0272 |
| Necrosis | 0.4456 | 0.5537 | 0.718 |
| Margin distance (mm) | −0.1427 | 0.0733 | 4.284 |
| Histological size | 0.0228 | 0.0225 | 0.954 |
Regression coefficients from binary logistic modelling; β is the regression coefficient, SE β is the standard error of β, χ2 values are from a log likelihood ratio test for comparing two regression equations. Comparing the full model (with all four variables) with a reduced model with margin omitted gives a χ2 value of 4.284, which is significant at p=0.05 (χ2(0.05)=3.841).
Recurrent cases
Six recurrent cases were of DCIS without invasion, eight recurred as invasive carcinoma, and one case recurred as metastatic disease. For the purposes of analysis the metastatic case was included with the invasive cases. Table 3 shows the relations of the type of recurrence to grade, extent (size in millimetres), prognostic index, disease free interval, and margins of excision. Grade 3 DCIS was more likely to recur as invasive carcinoma and invasive cases had a closer margin of excision but none of the differences were significant.
Table 3.
Characteristics of recurrent disease
| Type of recurrence | DCIS (n=6) | Invasive (n=9) |
| Van Nuys | ||
| Grade 1 | 2 | 0 |
| Grade 2 | 1 | 4 |
| Grade 3 | 3 | 5 |
| Extent (mm) | 22.1 | 16.2 |
| Prognostic index | ||
| 4 | 1 | 0 |
| 5 | 1 | 1 |
| 6 | 3 | 3 |
| 7 | 1 | 5 |
| Disease free interval (months) | 32 | 46 |
| Margin of excision (mm) | 6.0 | 2.98 |
DCIS, ductal carcinoma in situ.
DISCUSSION
With the increased use of breast conservation surgery in the treatment of DCIS, there has been much interest in developing methods to predict the risk of local recurrence. Several classification systems for DCIS based on cytonuclear features, cell polarity, and the presence of necrosis have been proposed. None of these systems has gained widespread acceptance, although the Van Nuys system does have some advantages and is associated with a low inter observer variation.5, 7 In our study, one observer (eliminating interobserver variation) applied the classification systems used and none is predictive of recurrent disease. Previous larger studies of non-screen detected cases with a wider age range have demonstrated a significant association between high grade DCIS with comedo necrosis and recurrent disease.11–13 Other possible characteristics of the DCIS that may be associated with recurrence are the size and margin of excision. The extent of DCIS in an excision specimen may be difficult to determine histologically because of sampling error. A radiological estimate of extent may be available by mammography using the size of malignant intralumenal microcalcifications. This measurement can only give a minimum size of DCIS because there may be non-calcified DCIS invisible mammographically beyond the confines of the necrotic calcified component. Linear assessment of histological size is facilitated by the use of large sections of the whole specimen on appropriately large sheets of glass. This method requires considerable technical expertise and the large sections are not easy to store. Although this large section technique is probably optimal it is not available in many centres. The alternative is to select a series of contiguous blocks across the specimen, which can then be reassembled and the extent of DCIS measured across several glass slides. Because DCIS is a discontinuous process in two dimensions, these methods are subject to sampling error. Other investigators have argued that tumour volume is a superior measurement and that this can be assessed by the number of slides containing DCIS.10
Similar comments apply to the pathological assessment of the margin of excision. The biggest problem is that of sampling. There are two main approaches, the one used in our study being to take sections perpendicular to the margin of excision medially, laterally, superiorly, and inferiorly and measure the distance from DCIS to margin in the blocks sampled. To sample the whole surface of a local excision using this method is impracticable, requiring a large number of histological sections. In our study, an individualised iterative approach taking further appropriate targeted histology was used to define the closest margin of excision as described (fig 1). Another approach is to take tissue parallel to the surface of the local excision and if tumour is present to score the margin as positive. This approach has the advantage of sampling the whole of the surface, but the disadvantage that the real distance of tumour to the margin cannot be obtained. All that can be stated is that there is no tumour within 3–4 mm (that is within the thickness of one block) of the margin. It is clear from the above discussion that the accurate measurement of the extent of DCIS and the margins of excision is difficult, and inevitably involves compromise and an acknowledgement of the problems of sampling. Goldstein et al emphasise the importance of the excision margins in predicting recurrence but argue that the number of terminal duct lobular units or cancerisation of lobules—volume of disease adjacent to the margin—is also important for the prediction of recurrence.10
“Our study shows that the margin of excision, either expressed as a distance in millimetres or simplified as a scoring system, is significantly correlated with recurrent disease”
The combination of the three characteristics classification, extent, and margin is confirmed as significantly associated with recurrence in our study. Twelve of the recurrent cases had prognostic index scores of 6 or 7. No cases had scores of 8 or 9 because these patients were deemed unsuitable for conservation (owing to close margin of excision: < 1 mm, and/or extensive disease) and were advised to undergo mastectomy. Cases recurring as invasive carcinoma were of higher grade (Van Nuys), had a higher prognostic index, and had smaller mean resection margins than cases recurrent as DCIS, but none of these differences reached significance because the numbers are small (table 3).
Despite the difficulties associated with the estimation of extent and excision margins our study shows that the margin of excision, either expressed as a distance in millimetres or simplified as a scoring system 1–3, is significantly correlated with recurrent disease.
We found that the classification of DCIS alone was not predictive of recurrence, although larger series have shown a significant correlation with high grade and comedo necrosis. However, we confirmed that the combination of classification, size, and margin as a prognostic index was significantly correlated with recurrence. Analysis of the data using a logistic regression model shows that only the margin variable makes a significant difference to the calculated probability of recurrence when excluded from the full model. These findings support those published previously, indicating that the margin of excision is the most important factor predicting recurrence. In the study reported here, seven of 15 recurrent cases had an excision margin of 1 mm or less and nine of 15 had a margin of 5 mm or less (fig 3). This indicates that a margin of 1 mm is probably insufficient and that a margin of at least 5 mm should be aimed for.
Take home messages .
Classification using cytonuclear grade, extent of necrosis, or the Van Nuys system did not correlate significantly with recurrence, but the excision margin (in millimetres) was associated with recurrence of ductal carcinoma in situ (DCIS)
A prognostic index based on Van Nuys score, margin status, and size was significantly associated with recurrence
Thus, the margin of excision appears to be the most important factor predicting recurrence of DCIS after local excision
Concentration on accurate assessment of margins of excision in DCIS is worthy of further careful study.
Abbreviations
DCIS, ductal carcinoma in situ
WLE, wide local excision
REFERENCES
- 1.Bellamy CO, McDonald C, Salter DM, et al. Non-invasive ductal carcinoma of the breast: the relevance of histologic categorisation. Hum Pathol 1993;24:16–23. [DOI] [PubMed] [Google Scholar]
- 2.Holland R, Peterse JL, Millis RR, et al. Ductal carcinoma in situ: a proposal for a new classification. Semin Diagn Pathol 1994;11:167–80. [PubMed] [Google Scholar]
- 3.National Coordinating Group for Breast Screening Pathology. Pathology reporting in breast cancer screening, 2nd ed. Sheffield: NHSBSP Publication, 1995.
- 4.Silverstein MJ, Poller DN, Waisman JR, et al. Prognostic classification of breast ductal carcinoma in situ. Lancet 1995;345:1154–7. [DOI] [PubMed] [Google Scholar]
- 5.Douglas-Jones AG, Gupta SV, Attanoos RL, et al. A critical appraisal of six modern classifications of ductal carcinoma in situ of the breast: correlation with grade of associated invasive carcinoma. Histopathology 1996;29:397–410. [DOI] [PubMed] [Google Scholar]
- 6.Consensus Conference Committee. Consensus conference on the classification of ductal carcinoma in situ, April 25–28. Cancer 1997;80:1798–802. [DOI] [PubMed] [Google Scholar]
- 7.Douglas-Jones AG, Morgan JM, and 18 participating histopathologists. Consistency in the observation of features used to classify duct carcinoma in situ (DCIS) of the breast. J Clin Pathol 2000;53:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverstein MJ, Lagios MD, Craig PH, et al. A prognostic index for ductal carcinoma in situ of the breast. Cancer 1996;77:2267–74. [DOI] [PubMed] [Google Scholar]
- 9.Silverstein MJ, Lagios MD, Groshen S, et al. Influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med 1999;340:1455–61. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein NS, Kestin L, Vicini F. Intraductal carcinoma of the breast: pathologic features associated with local recurrence in patients treated with breast-conserving surgery. Am J Surg Pathol 2000;24:1058–106. [DOI] [PubMed] [Google Scholar]
- 11.Lagios NM, Margolin FR, Westdahl PR, et al. Mammographically detected duct carcinoma in situ. Frequency of local recurrence following tylectomy and prognostic effect of nuclear grade on local recurrence. Cancer 1989;63:619–24. [DOI] [PubMed] [Google Scholar]
- 12.Solin LJ, Yet I-T, Kurtz J, et al. Ductal carcinoma in situ (intraductal carcinoma) of the breast treated with breast-conserving surgery and definitive irradiation. Correlation of pathological parameters with outcome of treatment. Cancer 1993;71:2532–42. [DOI] [PubMed] [Google Scholar]
- 13.Badve S, A’Hern RP, Ward AM, et al. Prediction of local recurrence of ductal carcinoma in situ of the breast using five histological classifications: a comparative study with long follow up. Hum Pathol 1998;29:915–23. [DOI] [PubMed] [Google Scholar]