Abstract
Background/Aims: A child presented with hepatic veno-occlusive disease after having been administered a short course of treatment with a traditional herbal remedy. The child subsequently died. Postmortem liver histology confirmed the diagnosis. This study aimed to investigate the hypothesis that the herbal remedy was the cause of veno-occlusive disease.
Methods: Extracts of the traditional remedy were analysed by colorimetry and gas chromatography/mass spectrometry. Cultured hepatocytes were treated with an extract of the plant material and examined for morphological changes.
Results: The screening analyses indicated the presence of toxic pyrrolizidine alkaloids, which were later confirmed by gas chromatography/mass spectrometry. The cell studies indicated dose related toxicity, with necrosis at high concentrations and apoptosis and abnormalities of the cytoskeleton at lower concentrations.
Conclusions: The simple screening techniques used allowed rapid confirmation of the presence of toxic pyrrolizidines in the remedy. The in vitro method confirmed the toxicity of herbal extracts to hepatocytes.
Keywords: traditional remedy, poisoning, hepatotoxicity, pyrrolizidine alkaloids
In South Africa, the use of herbal remedies containing plant and other materials are an integral part of traditional culture, with 60–80% of the population relying on such remedies.1 The potential toxicity of plants containing herbal remedies is well documented and especially so for the pyrrolizidine alkaloids. In humans, chronic exposure causes veno-occlusive disease (VOD) of the liver.2–5 The diagnosis of VOD is made on clinical evidence and confirmed on liver histology, but methods for confirmation of the presence of pyrrolizidines are not readily available to clinicians. Screening tests have been used in veterinary practice for the analysis of plants that are thought to be implicated in livestock poisoning. They have also been used forensically in the study of contaminated flour in several incidences of mass poisoning in humans. There are no reports of the use of such screening tests in clinical practice, although in a small number of cases pyrrolizidine alkaloids have been detected retrospectively in herbal teas that have caused VOD.6–8
“The potential toxicity of plants containing herbal remedies is well documented and especially so for the pyrrolizidine alkaloids”
We looked for toxic pyrrolizidine alkaloids in a complex herbal mixture administered to an infant who presented with VOD from which she subsequently died. We used non-specific colorimetry to detect alkaloids and confirmed these as toxic pyrrolizidines by a more specific method and by gas chromatography/mass spectrometry (GC/MS). The hepatoxicity of the herbs was confirmed by treating HuH-7 cells in culture with an extract of the plant material and observing the morphological changes that occurred.
MATERIALS AND METHODS
Ethical clearance was obtained from the University of the Witwatersrand.
Case study
A 3.5 month old female infant was admitted to an outlying hospital with a two day history of diarrhoea and vomiting requiring intravenous rehydration. She had been given an infusion of a herbal medicine daily for one week before admission. The reason for administration and the amount given could not be ascertained. Seven days after admission she developed massive ascites and was referred to tertiary care for management. Clinical examination showed an alert non-jaundiced infant with a weight of 6 kg, massive ascites and hepatomegaly, but without concomitant splenomegaly. She was in moderate respiratory distress. From our considerable experience with such cases, a diagnosis of probable VOD was made. Electrolytes and glucose were unremarkable. Liver function tests showed: total bilirubin, 43 μmol/litre (normal, < 21); alkaline phosphatase, 200 U/litre (normal range, 25–280), γ glutamyl transferase, 121 U/litre (normal, < 35); alanine aminotransferase, 48 U/litre (normal range, 5–40); aspartate aminotransferase, 101 U/litre (normal, < 40); protein, 33 g/litre (normal range, 60–85); albumin, 20 g/litre (normal range, 35–55); international normalised ratio, 2.52. Aspirated ascitic fluid at first grew Enterococcus faecalis but after appropriate antibacterial treatment both blood and ascitic fluid cultures proved negative. Ascites was treated using our repeated protocol of high volume therapeutic paracentesis, albumin administration, and diuretics (spironolactone and hydrochlorothiazide). Ultrasonography confirmed the clinical findings of ascites and hepatomegaly (9 cm), in addition to progressive splenomegaly. These clinical findings are classic in the cases of VOD that we deal with on a regular basis. The clinical course was one of gradual deterioration with loss of weight, development of jaundice, and intractable and persistent hyponatraemia. She developed Streptococcus pneumoniae peritonitis and septicaemia and died three months after admission. A postmortem liver biopsy revealed mild fibrosis, hepatocellular necrosis, and the collapse of reticulin structure in zone 3, all of which are consistent with a diagnosis of VOD. The absence of the typical perivenular congestion was thought to result from the delay in biopsy, which was performed only three months after presentation.
Extraction of plant material
The specimen (fig 1) (“muti”) and dried Senecio latifolius were ground to a powder and the pyrrolizidines extracted using the method of van Wyk et al.9 For GC/MS the dried extracts were reconstituted in methanol. For the cell studies, 1 g powdered samples were infused with 10 ml of hot distilled water for 15 minutes. The suspensions were centrifuged and filtered through a 0.22 μm filter. Dilutions were made in culture medium. The osmolality of the plant extracts was between 125 and 160 mmol/litre.
Figure 1.
The traditional remedy (muti).
Colorimetric methods
Screening
The method of Birecka et al was used for the determination of total alkaloids.10 The specific method of Mattocks was used for the detection of toxic pyrrolizidine compounds containing a D-3 pyrroline ring. The sensitivities of these methods are 0.5 mg/litre (our data) for the non-specific method and 5 mg/litre for the specific method,11 both with respect to retrorsine.
Gas chromatography/mass spectrometry
The method used was similar to that of Hostege et al.12 A HP6890 gas chromatograph equipped with an HP 7683 auto injector and HP 5973 mass selective detector (Agilent Technologies, Palo Alto, California, USA) was used for GC/MS. Data collection and integration were performed with HP Chem Station software. Chromatographic separations were performed with a DB-1 capillary column (30 m; internal diameter, 250 μm; film thickness, 0.1 μm; J&W Scientific, Folson, California, USA).
Samples (2 μl) were injected in the pulsed splitless mode, the pulse time being 1.5 minutes and the pressure 200 kPa. The injector temperature was 250°C. The helium carrier gas flow rate was 1.0 m/minute. The column temperature programme was as follows: 0.5 minutes at 50°C, from 50°C to 200°C at 10°C/minute, then to 290°C at 30°C/minute, with a final isothermal period of 6.5 minutes. Total run time was 25 minutes. All of the mass spectra were recorded at 70 eV. The mass range was 50–650 Da. Single ion monitoring data were collected at the expected masses for senecionine, platyphylline, and retrorsine.
Cell culture studies
Aliquots of 100 μl of the filtered infusions, or dilutions, were added to 3 ml cultures of a human hepatoma cell line (HuH-7) grown on coverslips. The cells were maintained in Dulbecco's modified Eagle's medium, supplemented with 5% fetal calf serum, penicillin (50 units/ml), streptomycin (0.05 μg/litre), and glutamine (2mM). A 330 ng/ml solution of standard retrorsine served as a positive control. Morphological changes were investigated using light microscopy with haematoxylin and eosin staining following treatment for 3, 6, 18, 24, 36, 48, and 72 hours. The effects of these compounds on the cytoskeleton were studied using indirect immunofluorescence with anti-β tubulin and antinuclear antibodies.13 Staining with 0.1 mg/litre of 4,6, diamino-2-phenylindole (DAPI) was used to show the altered morphology of the nuclear chromatin.
RESULTS
Colorimetry
The alkaloid assay indicated a yield of 1.3 mg/g total pyrrolizidines in S latifolius and 1.0 mg/g in the muti. The method of Mattocks gave a yield of 1.75 for the S latifolius and 1.2 mg/g for the muti. These values compare well with the studies of Watt,14 who found 0.76–1.72% in S latifolius, depending on the season.
Gas chromatography/mass spectrometry
The S latifolius extract was found to contain retrorsine and senecionine. The traditional remedy was found to contain the pyrrolizidines retrorsine, seneciphylline, and platyphylline.
Toxicity studies
Morphological changes
Examination of haematoxylin and eosin stained cells treated with the undiluted muti extract showed necrosis with pyknotic nuclei and cytoplasmic remnants (fig 2B). Control cells are shown in fig 2A. Similar changes were found with S latifolius.
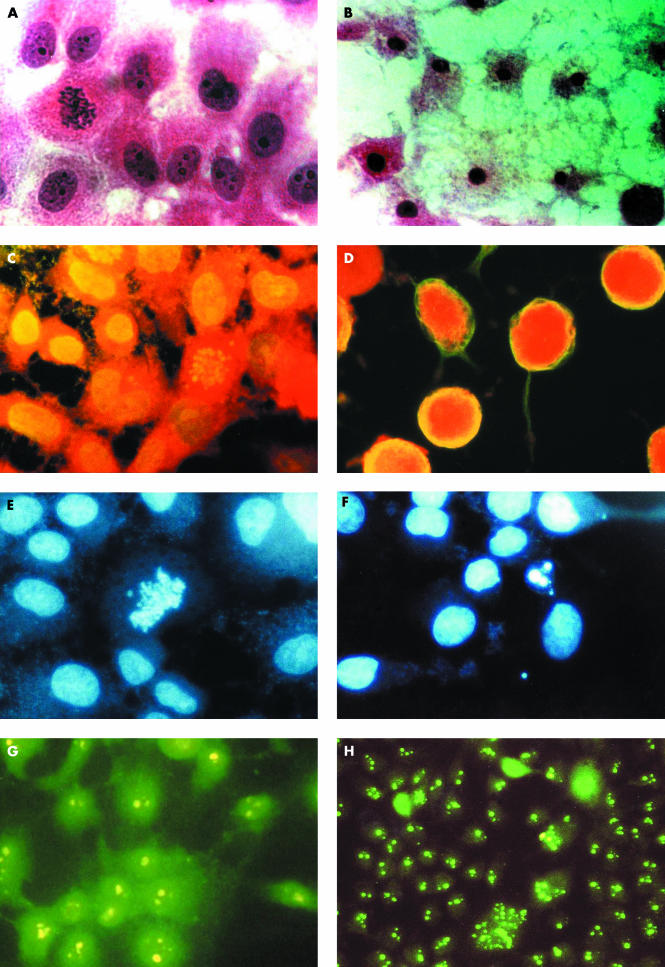
Figure 2.
Cell studies. (A) Control cells showing normal cell morphology; (B) cells treated with muti, showing necrosis; both haematoxylin and eosin stain (original magnification, ×400). (C) Control cells showing even distribution of β tubulin in the cytoplasm; (D) treated cells showing severe destruction of β tubulin with “naked nuclei”; both indirect immunofluorescence for β tubulin (original magnification, ×1000). (E) Control cells including a normal dividing cell; (F) treated cells showing apoptotic bodies; DAPI stain (original magnification, ×1000). (G) Control cells with even distribution of two or three nucleoli for each cell; (H) treated cells showing cells with multiple segregated nucleoli; indirect immunofluorescence for antinuclear antibody (original magnification, ×800).
Immunofluorescent studies
On treatment with muti extracts and S latifolius, the cytoskeleton was severely affected, with clumping of tubulin (fig 2D) compared with the control cells (fig 2C). When stained with DAPI the control cells showed a normal distribution with chromatin evenly dispersed on the equator in a dividing cell (fig 2E). Treated cells showed recognisable apoptotic body formation (fig 2F). On staining with antinuclear antibodies, control cells showed even distribution of nucleoli (fig 2G), whereas in treated cells these were segregated (fig 2H).
DISCUSSION
Pyrrolizidine alkaloids are well documented as causing both acute and chronic VOD; however, there are still those who insist that the presence of other compounds, especially antioxidants, in herbal materials, leads to amelioration of the effect of the pure substances. In our study, we demonstrated the presence of pyrrolizidines in a traditional herbal medicine that had been administered to an infant who subsequently developed VOD. We confirmed the hepatotoxicity of the remedy in vitro, where we demonstrated necrosis at high concentrations, and at lower concentrations an increased number of apoptotic and abnormal hepatocytes, destruction of β tubulin, abnormal nuclear staining, and cell division. These findings were identical to those obtained with an extract of S latifolius.
Take home messages.
Using simple screening techniques (colorimetry and gas chromatography/mass spectrometry) we confirmed the presence of toxic pyrrolizidines in a mixed herbal remedy that is believed to have given rise to fatal hepatic veno-occlusive disease in a child
An in vitro cell culture method confirmed the toxicity of herbal extracts to hepatocytes
The application of such methods may assist clinicians in establishing early diagnoses and instituting the appropriate treatment
In two published cases of human VOD secondary to comfrey poisoning there was only circumstantial evidence to link the chronic consumption of pyrrolizidines to the diagnosis.15,16 In another case, McGee et al reported only unidentified pyrrolizidines.17 In two other cases, toxic pyrrolizidine alkaloids were identified retrospectively in the herbal teas that had been ingested.7,18 In all of these cases, the analyses were carried out after a considerable lapse of time and were not of use in patient care.
“There is a need for ongoing research into the role of apoptosis in herbal medicine induced liver disease to identify target molecules and biochemical pathways that may be modified by pharmacological treatment”
Both of the colorimetric methods described here are useful for the detection of pyrrolizidine alkaloids in plant material. We have shown that the method of Birecka and colleagues10 has the sensitivity to confirm the presence of alkaloids in the urine of poisoned patients up to 72 hours after ingestion,19 but this method does not identify the alkaloids as pyrrolizidines or differentiate between toxic and non-toxic compounds. The method of Mattocks11 confirms the presence of toxic pyrrolizidines in plant material; however, we have found that because of its lack of sensitivity it is not applicable to urine samples from small children. When the clinical picture of hepatic VOD suggests the ingestion of a toxic compound, and the muti is available, the non-specific colorimetric method is applicable to the immediate clinical situation. The alkaloids may be identified retrospectively using GC/MS. Unfortunately, we were not able to analyse a urine specimen from this infant because of the delayed admission.
The effects of pure standard pyrrolizidine alkaloids on hepatocytes in vitro have been documented and include megalocytosis, increased numbers of abnormal mitoses, cytoplasmic vacuolisation, and inhibition of nucleic acid and protein synthesis.20–22 In our study, using a crude extract of the administered material and an extract of S latifolius, we show similar changes in cell morphology; however, the additional finding of apoptosis, which we have described in detail elsewhere,23 suggests that there is a need for ongoing research into the role of apoptosis in herbal medicine induced liver disease to identify target molecules and biochemical pathways that may be modified by pharmacological treatment. Current lines of research in the treatment of pyrrolizidine poisoning are the use of anti-tumour necrosis factor24 and ursodeoxycholic acid.25
In conclusion, we have described the analysis and in vitro toxic effects of a mixed herbal remedy that is believed to have given rise to VOD in a child and have furthered our confidence in the usefulness of simple analytical methods for demonstrating the presence of toxic substances in plants in the clinical situation. The application of such methods may assist clinicians in establishing early diagnoses and instituting the appropriate treatment.
Acknowledgments
Thanks to T Snyman for colorimetric analyses, L Bekker for the GC/MS analyses, and Professor C Seegers for advice on the interpretation of morphological changes in cells. These studies were supported by grants from the South African Institute for Medical Research, the University of the Witwatersrand, and the Richard Ward research fund.
Abbreviations
DAPI, 4,6, diamino-2-phenylindole
GC/MS, gas chromatography/mass spectrometry
VOD, veno-occlusive disease
REFERENCES
- 1.Hutchings A, Terblanche S. Observations on the use of some known and suspected toxic Liliiflore in Zulu and Xhosa medicine. S Afr Med J 1989;75:62–9. [PubMed] [Google Scholar]
- 2.Willmot FC, Robertson GW. Senecio disease or cirrhosis of the liver due to senecio poisoning. Lancet 1920;2:848–9. [Google Scholar]
- 3.Selzer G, Parker R. Senecio poisoning exhibiting as Chiari's syndrome: a report of twelve cases. Am J Pathol 1951;27:885–907. [PMC free article] [PubMed] [Google Scholar]
- 4.Freiman I, Schmaman A, Zamit R, et al. Veno-occlusive disease of the liver—some new aspects. S Afr Med J 1968;42:126–9. [PubMed] [Google Scholar]
- 5.Grobler A, Koen M, Brouwers F. Planttoksiene en lewersiektes by kinders in Pretoria en omgewing. Tijdschr Kindergeneeskd 1997;65:99–104. [Google Scholar]
- 6.Ridker PM, Ohkuma S, McDermott WV, et al. Hepatic veno-occlusive disease associated with the consumption of pyrrolizidine-containing dietary supplements. Gastroenterology 1985;88:1050–4. [DOI] [PubMed] [Google Scholar]
- 7.Roulet M, Laurini R, Rivier L, et al. Hepatic veno-occlusive disease in the newborn infant of a woman drinking herbal tea. J Paediatr 1988;112:43–6. [DOI] [PubMed] [Google Scholar]
- 8.Sperl W, Stuppner H, Gassner I, et al. Reversible hepatic veno-occlusive disease in an infant after consumption of pyrrolizidine-containing herbal tea. Eur J Pediatr 1995;154:112–16. [DOI] [PubMed] [Google Scholar]
- 9.van Wyk B-E, Verdoorn G, Schutte A. Distribution and taxonomic significance of major alkaloids in the genus Podalyria. Biochem Syst Ecol 1992;20:163–72. [Google Scholar]
- 10.Birecka H, Catalfamo J, Eisen R. A sensitive method for the detection and quantitative determination of pyrrolizidine alkaloids. Phytochemistry 1981;20:343–4. [Google Scholar]
- 11.Mattocks AR. Spectrophotometric determination of unsaturated pyrrolizidine alkaloids. Anal Chem 1967;39:443–7. [DOI] [PubMed] [Google Scholar]
- 12.Hostege D, Seiber J, Galey F. Rapid multi-residue screen for alkaloids in plant material and biological samples. J Agric Food Chem 1995;43:691–9. [Google Scholar]
- 13.Seegers J, Aveling M-L, van Aswegen C, et al. The cytotoxic effects of estradiol-17β, catecholestradiols and methoxyestradiols on dividing MCF-7 and HeLa cells. J Steroid Biochem 1989;32:797–809. [DOI] [PubMed] [Google Scholar]
- 14.Watt H. The alkaloids of Senecio latifolius. Journal of the Chemical Society of the Transvaal 1909;95:466–77. [Google Scholar]
- 15.Bach N, Thung SN, Schaffner F. Comfrey herb tea-induced hepatic veno-occlusive disease. Am J Med 1989;87:97–9. [DOI] [PubMed] [Google Scholar]
- 16.Weston CFM, Cooper BT, Davies JD, et al. Veno-occlusive disease of the liver secondary to the ingestion of comfrey. BMJ 1987;295:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGee JOD, Patrick R, Wood C, et al. A case of veno-occlusive disease of the liver associated with herbal tea consumption. J Clin Pathol 1976;29:788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sperl W, Stuppner H, Gassner I, et al. Reversible hepatic veno-occlusive disease in an infant after consumption of pyrrolizidine-containing herbal tea. Eur J Pediatr 1995;154:112–6. [DOI] [PubMed] [Google Scholar]
- 19.Steenkamp V, Stewart M, Zuckerman M. Clinical and analytical aspects of pyrrolizidine poisoning caused by South African traditional medicines. Ther Drug Monit 2000;22:302–6. [DOI] [PubMed] [Google Scholar]
- 20.Hirchinson V, Hill KR. The effects of senecio alkaloid (monocrotaline) on human embryo liver in tissue culture. Br J Cancer 1960;14:637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullman S, Zuckerman A. The effect of heliotrine, a pyrrolizidine alkaloid on human liver cells in culture. Br J Exp Pathol 1969;50:361–70. [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong S, Zuckerman A. The effects of lasiocarpine, retrorsine and retronecine pyrrole on human embryo lung and liver cells in culture. Br J Exp Pathol 1971;53:138–44. [PMC free article] [PubMed] [Google Scholar]
- 23.Steenkamp V, Stewart M, van der Merwe S, et al. The effect of Senecio latifolius a plant used as a South African traditional medicine, on a human hepatoma cell line. J Ethnopharmacol [In press.] [DOI] [PubMed]
- 24.Miyata M, Ueno Y, Sekine H, et al. Protective effect of beraprost sodium, a stable prostacyclin analogue, in development of monocrotaline-induced pulmonary hypertension. J Cardiovasc Pharmacol 1996;27:20–6. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues C, Ma X, Linehan-Stieers C, et al. Ursodeoxycholic acid prevents cytochrome c release in apoptosis by inhibiting mitochondrial membrane depolarization and channel formation. Cell Death Differ 1999;6:842–54. [DOI] [PubMed] [Google Scholar]