Abstract
Aim: The value of immunohistochemical staining in differentiating between malignant mesothelioma and pulmonary adenocarcinoma was re-examined using newly available commercial antibodies, with the aim of increasing the sensitivity and specificity of diagnosis, and simplifying the antibody panel required.
Methods: Forty one malignant mesotheliomas and 35 lung adenocarcinomas were studied. Commercial antibodies to calretinin, E-cadherin, N-cadherin, surfactant apoprotein A (SP-A), thyroid transcription factor 1 (TTF-1), thrombomodulin, and cytokeratin 5/6 were applied using the streptavidin–biotin–peroxidase complex procedure on formalin fixed, paraffin wax embedded tissue.
Results: E-cadherin was expressed in all adenocarcinomas and in 22% of the mesotheliomas. TTF-1 expression was detected in 69% of the adenocarcinomas and none of the mesotheliomas. Positive staining with polyclonal anticalretinin was detected in 80% of the mesotheliomas and 6% of the adenocarcinomas. N-cadherin was expressed in 78% of mesotheliomas and 26% of adenocarcinomas. Thrombomodulin was expressed in 6% of the adenocarcinomas and in 53% of the mesotheliomas. Cytokeratin 5/6 expression was detected in 6% of the adenocarcinomas and 63% of the mesotheliomas. The results were compared with the standard laboratory panel for mesothelioma diagnosis: anticarcinoembryonic antigen (anti-CEA), LeuM1, BerEP4, and HBME-1.
Conclusion: Of the antibodies used in this study, E-cadherin was 100% sensitive for pulmonary adenocarcinoma and TTF-1 was 100% specific for pulmonary adenocarcinoma. The application of these two antibodies alone was adequate for the diagnosis of 69% of adenocarcinomas and 78% of mesotheliomas. Where TTF-1 is negative and E-cadherin is positive, a secondary panel of antibodies, including BerEP4 and LeuM1 (CD15) and antibodies directed against CEA, calretinin, cytokeratin 5/6, thrombomodulin, and N-cadherin, is required for differentiation between malignant mesothelioma and pulmonary adenocarcinoma.
Keywords: mesothelioma, adenocarcinoma, immunohistochemistry, diagnosis
The diagnosis of malignant mesothelioma is dependent on an assessment of clinical and radiological findings in conjunction with pleural fluid cytopathology and pleural biopsy.1 Even when thoracoscopy is used to obtain sufficient tissue, the histological diagnosis may prove elusive for several reasons. These include distinguishing well differentiated epithelioid mesothelioma from reactive mesothelial proliferation, sarcomatoid or desmoplastic mesothelioma from reactive pleural fibrosis, and epithelioid mesothelioma from metastatic or pseudomesotheliomatous carcinoma, usually adeno-carcinoma.2–9 Immunohistochemistry has proved most useful in the last of these situations but, despite many antibodies showing potential, it is generally agreed that no one antibody shows absolute specificity or sensitivity for either tumour.10
Therefore, laboratories dealing with mesothelioma cases on a regular basis have developed panels of antibodies, whereby the probability of a tumour being a mesothelioma can be assessed.11 To refine this process further, we have used a group of newer antibodies in addition to our standard panel of antibodies and suggest a process whereby a clear diagnosis can be reached in most cases.
“Despite many antibodies showing potential, it is generally agreed that no one antibody shows absolute specificity or sensitivity for either tumour”
MATERIAL AND METHODS
Tumour specimens
The material included in our study was obtained from the archives of the department of cellular pathology at the Southampton General Hospital. The 76 cases included 41 open or thoracoscopic biopsies of malignant mesothelioma (11 epithelioid, seven sarcomatoid, and 23 mixed) and 35 sequential cases of resected primary pulmonary adenocarcinomas. The mesothelioma cases were from 1990 to 1997, whereas the adenocarcinomas were from the years 1997 and 1998. All biopsy tissues were fixed in 10% neutral buffered formalin and routinely processed to paraffin wax.
Immunohistochemical staining procedure
Immunohistochemical studies were performed on formalin fixed, paraffin wax embedded tissue sections using the streptavidin–biotin–peroxidase complex method. Sections were cut at 4 μm thickness and mounted on APES coated slides, dewaxed in xylene, and rehydrated in graded ethanol. The sections were treated with freshly prepared 30% hydrogen peroxide in absolute methanol for 10 minutes to inhibit endogenous peroxidase activity and washed in Tris buffered saline (TBS). Where antigen retrieval was required, the sections were pretreated with either 0.05% pronase (Dako, Ely, UK) in TBS at room temperature for 15 to 20 minutes or sections were immersed in 0.01M citrate buffer and heated by microwave or on a hot plate for 20 to 25 minutes, following by washing in TBS. To minimise non-specific background staining, sections were preincubated with normal swine serum for 20 minutes and then incubated with the primary antibodies (table 1), either for 60 minutes at room temperature or for 18 to 24 hours overnight at 4° C in a moist chamber. The secondary antibody was a 1/200 dilution of either biotinylated sheep antimouse immunoglobulin for monoclonal antibodies or biotinylated swine antirabbit immunoglobulin for polyclonal antibodies (Amersham Pharmacia Biotech, Little Chalfont, UK) for 30 minutes at room temperature. After a further rinse in TBS, the sections were incubated with streptavidin–biotin–peroxidase complexes (1/200 dilution; Dako) for 30 minutes at room temperature, followed by washing in TBS. The colour was developed with the use of 3‘,3‘-diaminobenzidine substrate solution (DAB). Sections were then washed, counterstained with Harris’s haematoxylin, dehydrated, cleared in xylene, and mounted with DPX.
Table 1.
Antibody characteristics
| Antibody/antigen | Type | Source | Dilution | Incubation | Pretreatment | Cell pattern |
| Calretinin | P | Zymed (San Francisco, USA) | 1/50 | Overnight/4° C | Hotplate | Cytoplasm |
| CEA | M | Dako (Ely, UK) | 1/20 | 30–60 min/RT | Microwave | Cytoplasm |
| Cytokeratin 5/6 | M | Dako | 1/50 | 30–60 min/RT | Microwave | Pericellular and cytoplasm |
| BerEP4 | M | Dako | 1/50 | 30–60 min/RT | Pronase | Membrane |
| E-cadherin | M | Zymed | 1/25 | Overnight/4° C | Hotplate | Membrane |
| HBME-1 | M | Dako | 1/100 | 30–60 min/RT | No pretreatment | Membrane and cytoplasm |
| LeuMI (CD15) | M | Dako | 1/40 | Overnight/4° C | Microwave | Cytoplasm |
| N-cadherin | M | Zymed | 1/25 | Overnight/4° C | Hotplate | Cytoplasm |
| SP-A | P | Chemicon (Harrow, UK) | 1/50 | Overnight/4° C | Hotplate | Membrane and cytoplasm |
| Thrombomodulin | M | Dako | 1/100 | Overnight/4° C | No pretreatment | Membrane |
| TTF-1 | M | Lab Vision (Newmarket, UK) | 1/50 | 30–60 min/RT | Microwave | Nucleus |
CEA, carcinoembryonic antigen; M, monoclonal mouse; P, polyclonal rabbit; RT, room temperature; SP-A, surfactant apoprotein A; TTF-1, thyroid transcription factor 1.
RESULTS
Adenocarcinoma
Table 2 summarises the results from the 35 cases of pulmonary adenocarcinoma. All 35 cases of adenocarcinoma were positive for E-cadherin and surfactant apoprotein A (SP-A). Immunoreactivity for E-cadherin was confined to the cell membranes of the tumour cells (fig 1A), whereas SP-A immunoreactivity appeared to be either cytoplasmic (74%) or on the cell membranes (26%). Twenty four of the 34 (69%) adenocarcinoma cases expressed thyroid transcription factor 1 (TTF-1), which was clearly located in the cell nuclei (fig 2A). The percentage positivity was similar for poorly differentiated adenocarcinomas with a solid cell pattern. Nine cases (26%) of pulmonary adenocarcinoma expressed N-cadherin, which was present mainly in the cytoplasm and less frequently on the cell membrane (fig 3A). Only two cases (6%) of adenocarcinoma showed labelling for calretinin in a cytoplasmic and nuclear pattern (fig 4A). Only two of the 35 adenocarcinomas (6%), one of which was a poorly differentiated tumour with a solid pattern, stained for thrombomodulin, expression being localised in the cell membrane. Two cases (6%) of adenocarcinoma showed staining for cytokeratin 5/6, and localisation was both pericellular at the cell membrane and cytoplasmic.
Table 2.
Percentages of tumours labelling with new antibodies and staining patterns
| Adenocarcinoma | Mesothelioma | |||||
| Antibody/antigen | Positive (%) | Negative (%) | Staining pattern | Positive (%) | Negative (%) | Staining pattern |
| Calretinin | 6 | 94 | Cytoplasm and nuclei | 80 | 20 | Cytoplasm and nuclei |
| Cytokeratin 5/6 | 6 | 94 | Pericellular and cytoplasm | 63 | 37 | 72% pericellular and cytoplasmic, 28% cytoplasm only |
| E-cadherin | 100 | 0 | Cell membrane | 22 | 78 | Cell membrane |
| N-cadherin | 26 | 74 | 22% cell membrane, 78% cytoplasm | 78 | 22 | 22% membrane, 31% cytoplasm, 47% both |
| SP-A | 100 | 0 | 26% cell membrane, 74% cytoplasm | 98 | 2 | 82% membrane, 18% cytoplasm |
| Thrombomodulin | 6 | 94 | Cell membrane | 53 | 47 | Cell membrane |
| TTF-1 | 69 | 31 | Nuclear | 0 | 100 | |
SPA-1, surfactant apoprotein A; TTF-1, thyroid transcription factor 1.
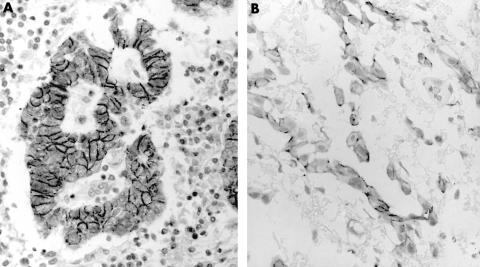
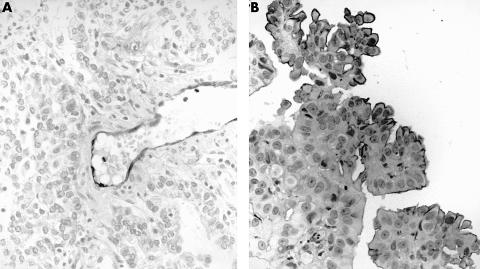
Figure 1.
Immunostaining for E-cadherin in (A) adenocarcinoma and (B) mesothelioma. The staining is localised to the cell membrane and is stronger and more consistent in adenocarcinoma.
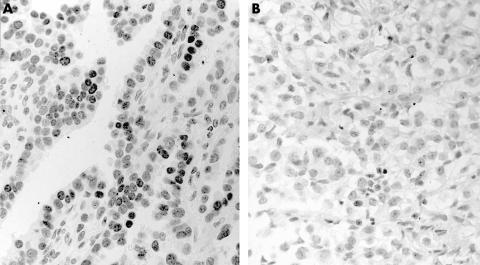
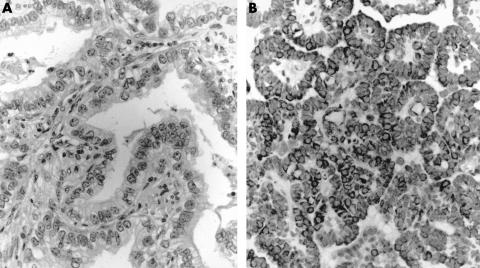
Figure 2.
Immunostaining for thyroid transcription factor 1 in (A) adenocarcinoma and (B) mesothelioma. There is strong nuclear staining in adenocarcinoma, whereas all mesotheliomas were negative.
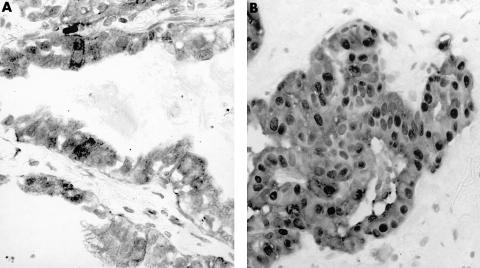
Figure 3.
Immunostaining for N-cadherin in (A) adenocarcinoma and (B) mesothelioma. Weak cytoplasmic staining was seen in adenocarcinoma, with strong staining of accompanying lymphocytes. Strong membrane staining was seen in mesothelioma.
Figure 4.
Immunostaining for calretin in (A) adenocarcinoma and (B) mesothelioma. Cytoplasmic staining was seen in occasional cells in adenocarcinoma, whereas cytoplasmic and nuclear staining was seen in mesothelioma.
Malignant mesothelioma
Tables 2 and 3 show the results of the immunohistochemical staining with the newly available antibodies in the 41 cases of mesothelioma. None of the malignant mesotheliomas showed immunoreactivity for TTF-1 or LeuM1, 2% were positive for CEA, 7% showed positivity for BerEP4, and 22% were cell membrane positive for E-cadherin (fig 1B). Of the positive markers for mesothelioma, calretinin was present in 80% of cases (fig 4B), N-cadherin was expressed in 78% of cases, and HBME-1 in 63% of cases. SP-A immunoreactivity was detected in 98% of mesotheliomas. Thrombomodulin was expressed at the cell membrane in 53% of the mesotheliomas (fig 5B). Sixty three percent of mesotheliomas showed positive staining for cytokeratin 5/6, in 72% of these staining was pericellular and cytoplasmic, whereas 24% showed cytoplasmic staining only (fig 6B).
Table 3.
Comparison of conventional antibodies with new antibodies in mesothelioma
| Antibody/antigen | Positive (%) | Negative (%) |
| CEA | 2 | 98 |
| BerEP4 | 7 | 93 |
| HBME-1 | 63 | 37 |
| LeuM1 (CD15) | 0 | 100 |
| Calretinin | 80 | 20 |
| Cytokeratin 5/6 | 63 | 37 |
| E-cadherin | 22 | 78 |
| N-cadherin | 78 | 22 |
| SP-A | 98 | 2 |
| Thrombomodulin | 53 | 47 |
| TTF-1 | 0 | 100 |
CEA, carcinoembronic antigen; SPA-1, surfactant apoprotein A; TTF-1, thyroid transcription factor 1.
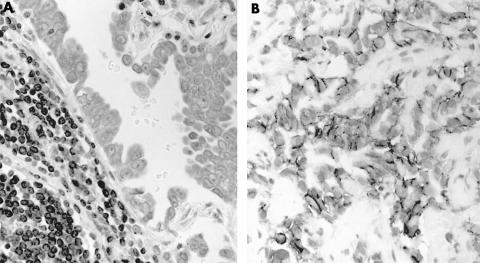
Figure 5.
Immunostaining for thrombomodulin in (A) adenocarcinoma and (B) mesothelioma. The staining is localised to the cell membrane in mesothelioma and is negative in adenocarcinoma, with positive endothelial staining.
Figure 6.
Immunostaining for cytokeratin 5/6 in (A) adenocarcinoma and (B) mesothelioma. Staining was negative in adenocarcinoma and both pericellular and cytoplasmic in mesothelioma.
Subtypes of mesothelioma
The distribution of immunoreactivity was not uniform in the subtypes of malignant mesothelioma (table 4): calretinin and E-cadherin were expressed mainly by epithelioid and mixed mesotheliomas and N-cadherin was highly expressed in all subtypes of mesothelioma. Thrombomodulin and cytokeratin 5/6 reactivity was also highest in epithelioid mesotheliomas.
Table 4.
Percentage staining of mesothelioma subtypes by different antibodies
| Mesothelioma | Percentage of subtype stained | ||||
| Antibody to | Positive (%) | Negative (%) | Epithelioid | Sarcomatoid | Mixed |
| Calretinin | 80 | 20 | 100 | 29 | 87 |
| Cytokeratin 5/6 | 63 | 37 | 92 | 14 | 62 |
| E-cadherin | 22 | 78 | 45 | 0 | 17 |
| N-cadherin | 78 | 22 | 73 | 86 | 78 |
| Thrombomodulin | 53 | 47 | 92 | 29 | 43 |
There were 11 epithelioid, 7 sarcomatoid, and 23 mixed cases.
Sensitivity and specificity
We examined the results for both the conventional laboratory panel and the new antibodies to investigate the ability of these antibodies to discriminate between mesothelioma and pulmonary adenocarcinoma. TTF-1 was 100% specific for pulmonary adenocarcinoma and detected 69% of cases. E-cadherin was 100% sensitive for adenocarcinoma but was less specific, staining 22% of mesotheliomas. The combination of these two antibodies allowed the detection of pulmonary adenocarcinoma (TTF-1 and E-cadherin positive) with 100% specificity in 69% of cases and excluded pulmonary adenocarcinoma (TTF-1 and E-cadherin negative) with 100% specificity in 78% of mesotheliomas. There were no TTF-1 positive, E-cadherin negative tumours.
The remaining cases of E-cadherin-positive, TTF-1 negative tumours required the application of the other antibodies. Each case was examined to determine the value of the individual antibodies and to determine any overlapping immunoreactivity. CEA was specific for the residual adenocarcinomas, with positivity in nine of 11 cases and no reactivity with the nine E-cadherin positive mesotheliomas. BerEP4 stained 10 of 11 TTF-1 negative adenocarcinomas, but was also positive in two of the nine mesotheliomas. LeuM1 (CD15) was negative in all the mesotheliomas and stained five of the 11 adenocarcinomas. All of the CD15 positive adenocarcinomas were also positive for BerEP4, but the BerEP4 positive mesotheliomas were CD15 negative. Calretinin was positive in one of the 11 adenocarcinomas but this case was CEA positive. Calretinin was positive in all nine E-cadherin positive mesotheliomas. N-cadherin was negative in all 11 adenocarcinomas and positive in seven of nine mesotheliomas. Thrombomodulin was negative in all 11 adenocarcinomas and was positive in six of nine mesotheliomas. Cytokeratin 5/6 was positive in one of the 11 adenocarcinomas and positive in seven of the nine mesotheliomas. HBME1 had little discriminatory value, being positive in six of 11 adenocarcinomas and positive in all the mesotheliomas.
DISCUSSION
Some pulmonary adenocarcinomas and many metastatic adenocarcinomas produce epithelial mucin and the simplest and most reliable way to identify these is by means of periodic acid Schiff staining after diastase digestion, although incomplete glycogen digestion or, rarely, true positivity may occur focally in epithelioid mesotheliomas.12 For the remainder of the cases, a wide variety of antibodies has been used to make the distinction from malignant mesothelioma, and the use of a panel of antibodies is now accepted practice. The choice of antibodies varies and it is recognised that those chosen for our study are not comprehensive. Among those not included but of potential value are antibodies to cytokeratin 713 and specific antibodies raised against mesothelial cells.14–16
The antibody HBME-1 is widely used but its value depends on reported differences in staining patterns between mesothelioma and carcinoma, rather than the specificity of immunoreactivity. In our experience, these appearances are not easy to interpret in an individual case.17–19 Experience with the other antibodies in our established panel, monoclonal anti-CEA, LeuM1, and BerEP4, are well documented and their usefulness depends on their general lack of reactivity with mesothelioma cells. Our current study supports the findings of previous series and shows that no single antibody is entirely satisfactory in this regard.20–24 Although it seems that LeuM1 may be most specific because it did not stain mesotheliomas, it also failed to stain six of the 11 adenocarcinomas and is therefore less sensitive than the other antibodies. BerEP4 is the most sensitive of the three but at the same time is the least specific.
“Although E-cadherin shows some lack of specificity, use can be made of the fact that no example of adenocarcinoma in our series failed to stain with this antibody, the inference being that if staining is negative the tumour cannot be an adenocarcinoma”
The antibody to SP-A was disappointing in its ability to discriminate between the two types of tumour and lacked sufficient specificity to justify inclusion in any panel designed for this purpose. Regardless of slight differences in the pattern of staining for SP-A in mesothelioma, this antibody reacts with most types of tumour. Comparable findings are reported in other series.25,26 Although E-cadherin shows some lack of specificity, use can be made of the fact that no example of adenocarcinoma in our series failed to stain with this antibody, the inference being that if staining is negative the tumour cannot be an adenocarcinoma. N-cadherin is less specific27,28 but its staining of sarcomatoid mesotheliomas may be useful.
TTF-1 is a 38 kDa nuclear protein that was originally identified in thyroid epithelial cells, where it acts as a mediator of thyroid specific gene transcription and activates the transcription of thyroglobulin and thyroperoxidase. It is also present in the developing fetal lung, where it is localised to the nuclei of developing airways. In the mature lung, it is selectively expressed by type II alveolar epithelial cells and a subset of bronchiolar epithelial cells, and may act as a factor for the transcription of surfactant apoproteins. It is not expressed in mesoderm derived cells and this may account for its absence from mesothelial cells. Previous studies have shown that TTF-1 expression is retained in thyroid carcinomas and in up to 75% of pulmonary adenocarcinomas.29,30 It is not expressed in adenocarcinomas from other sites, and our results support the findings of other authors that malignant mesotheliomas are also invariably negative.31,32
Anticalretinin is one of the few antibodies that is more frequently reactive in mesotheliomas than in adenocarcinomas. Like anti-CEA, care must be taken to exclude positive staining of inflammatory cells because reactivity was seen in tumour associated macrophages. However, we found it to be useful in discriminating E-cadherin positive epithelioid mesotheliomas from pulmonary adenocarcinomas. Thrombomodulin is a protein cofactor expressed on endothelial cell surfaces that modifies the substrate specificity of thrombin, activating the protein C anticoagulant pathway.33 Thrombomodulin has been shown to be more reactive in mesotheliomas than adenocarcinomas.34–36 In our study, we found that thrombomodulin was expressed in 53% of the mesotheliomas, but the staining pattern tended to be focal. The remaining 47% may have included false negatives as a result of the focal expression of thrombomodulin. The sensitivity of this marker might be improved by staining of multiple blocks from large tumour specimens, but this is not feasible with small biopsies. Anticytokeratin 5/6 has been reported as highly sensitive for epithelioid mesotheliomas.37,38 We found that cytokeratin 5/6 was expressed by only 63% of mesotheliomas. Expression was highest in epithelioid mesothelioma, with 92% positivity, but only 14% of sarcomatoid mesotheliomas showed positivity.
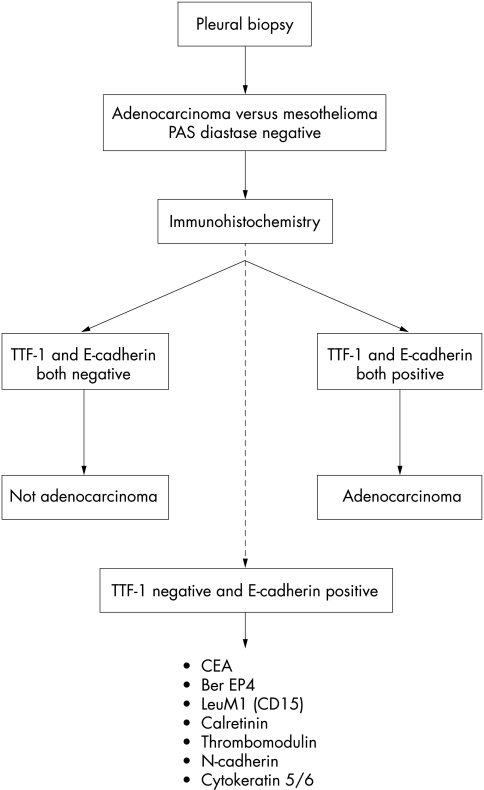
Our results suggest that the first line antibodies should be E-cadherin and TTF-1 (fig 7). Tumours that show reactivity for both of these antibodies are unequivocally not mesotheliomas and, in the case of primary tumours, are pulmonary adenocarcinomas. Individually, both antibodies have deficiencies; anti-E-cadherin is not specific and anti-TTF-1 has limited sensitivity. Nevertheless, 69% of the adenocarcinomas in our series could be confidently classified at this stage, irrespective of the degree of differentiation. Tumours that lack reactivity for both antibodies are not adenocarcinomas and this accounts for 78% of mesotheliomas.
Figure 7.
Protocol for the immunohistochemical distinction between adenocarcinoma and malignant mesothelioma. CEA, carcinoembryonic antigen; TTF-1, thyroid transcription factor 1.
No tumours were TTF-1 positive and E-cadherin negative. However, those tumours staining for E-cadherin but not for TTF-1 will include 31% of adenocarcinomas and 22% of mesotheliomas. Within this group, most tumours reacting with calretinin will be mesotheliomas. At this stage, however, a very small number of adenocarcinomas will also react and there is therefore an element of uncertainty. The use of the standard panel of antibodies (anti-CEA, CD15, and BerEP4) with the inclusion of antibodies to calretinin, N-cadherin, thrombomodulin, and cytokeratin 5/6 should enable most of these cases to be classified. This approach reduces the panel of antibodies required for most cases and allows for reporting with a greater degree of certainty as to whether a pleural tumour represents malignant mesothelioma or pulmonary adenocarcinoma.
Take home messages.
E-cadherin was expressed in all adenocarcinomas and in 22% of the mesotheliomas, whereas thyroid transcription factor 1 (TTF-1) was expressed in 69% of the adenocarcinomas and none of the mesotheliomas
Thus, E-cadherin was 100% sensitive for pulmonary adenocarcinoma and TTF-1 was 100% specific for pulmonary adenocarcinoma
The combination of these two antibodies alone was adequate for the diagnosis of 69% of adenocarcinomas and 78% of mesotheliomas
Tumours that show reactivity for both of these antibodies are unequivocally not mesotheliomas and, in the case of primary tumours, are pulmonary adenocarcinomas
Where TTF-1 is negative and E-cadherin is positive, a secondary panel of antibodies, including BerEP4 and LeuM1 (CD15) and antibodies directed against carcinoembryonic antigen, calretinin, cytokeratin 5/6, thrombomodulin, and N-cadherin, is required for differentiation between malignant mesothelioma and pulmonary adenocarcinoma.
This approach reduces the panel of antibodies required for most cases and improves the degree of certainty as to whether a pleural tumour represents malignant mesothelioma or pulmonary adenocarcinoma
Abbreviations
CEA, carcinoembryonic antigen
SP-A, surfactant apoprotein A
TBS, Tris buffered saline
TTF-1, thyroid transcription factor 1
REFERENCES
- 1.Suzuki Y. Diagnostic criteria for human diffuse malignant mesothelioma. Acta Pathol Jpn 1992;42:767–86. [DOI] [PubMed] [Google Scholar]
- 2.Marshall RJ, Herbert A, Brave SG, et al. Use of antibodies to carcinoembryonic antigen and human milk fat globule to distinguish carcinoma, mesothelioma and reactive mesothelium. J Clin Pathol 1984;37:1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Saffar N, Hasleton PS. Vimentin, carcinoembryonic antigen, and keratin in the diagnosis of mesothelioma, adenocarcinoma and reactive pleural lesion. Eur Respir J 1990;3:997–1001. [PubMed] [Google Scholar]
- 4.Gnosh AK, Gatter KC, Dunnill MS, et al. Immunohistological staining of reactive mesothelium, mesothelioma, and lung carcinoma with a panel of monoclonal antibodies. J Clin Pathol 1987;40:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheibani K, Esteban JM, Bailey A, et al. Immunopathologic and molecular studies as an aid to the diagnosis of malignant mesothelioma. Hum Pathol 1992;23:107–16. [DOI] [PubMed] [Google Scholar]
- 6.Dejmek A. Methods to improve the diagnostic accuracy of malignant mesothelioma. Respir Med 1996;90:191–9. [DOI] [PubMed] [Google Scholar]
- 7.Wick MR, Loy T, Mills SE, et al. Malignant epithelioid pleural mesothelioma versus peripheral pulmonary adenocarcinoma: a histochemical, immunohistochemical and ultrastructural study of 103 cases. Hum Pathol 1990;21:759–66. [DOI] [PubMed] [Google Scholar]
- 8.Grove A, Paulsen SM. The value of immunohistochemistry of pleural biopsy specimens in the differential diagnosis between malignant mesothelioma and metastatic carcinoma. Pathol Res Pract 1994;190:1044–55. [DOI] [PubMed] [Google Scholar]
- 9.Kortsik CS, Werner P, Freudenberg N, et al. Immunocytochemical characterisation of malignant mesothelioma and carcinoma metastatic to the pleura: IOB3—a new tumor marker. Lung 1995;173:79–87. [DOI] [PubMed] [Google Scholar]
- 10.King JE, Hasleton PS. Immunohistochemistry and diagnosis of malignant mesothelioma. Histopathology 2001;38:471–6. [DOI] [PubMed] [Google Scholar]
- 11.Angelo P, Tos D, Doglioni C. Calretinin: a novel tool for diagnostic immunohistochemistry. Adv Anat Pathol 1998;5:61–6. [PubMed] [Google Scholar]
- 12.Hammar SP, Bolen JB. Pleural neoplasms. In: Dail DH, Hammar SP, ed. Pulmonary pathology. New York: Springer, 1988:973.
- 13.Blobel GA, Moll R, Franke WW, et al. The intermediate filament cytoskeleton of malignant mesothelioma and its diagnostic significance. Am J Pathol 1985;121:235–47. [PMC free article] [PubMed] [Google Scholar]
- 14.Brown RW, Clark GM, Tanton AK, et al. Multiple-marker immunohistochemical phenotypes distinguishing malignant pleural mesothelioma from pulmonary adenocarcinoma. Hum Pathol 1993;24:347–54. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy AD, King G, Kerr KM. HBME-1 and anti-thrombomodulin in the differential diagnosis of malignant mesothelioma of pleura. J Clin Pathol 1997;50:859–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins CL, Ordonez NG, Schaefer R, et al. Thrombomodulin expression in malignant pleural mesothelioma and pulmonary adenocarcinoma. Am J Pathol 1992;141:827–33. [PMC free article] [PubMed] [Google Scholar]
- 17.Miettinen M, Kovatich AJ. HBME-I A monoclonal antibodies useful in the differential diagnosis of mesothelioma, adenocarcinoma, and soft-tissue and bone tumors. Appl Immunohistochem 1995;3:115–22. [Google Scholar]
- 18.Ascoli V, Carnovale-Scalzo C, Taccogna S, et al. Utility of HBME-1 immunostaining in serous effusions. Cytopathology 1997;8:328–35. [DOI] [PubMed] [Google Scholar]
- 19.Fetsch PA, Abati A, Hijazi YM. Utility of the antibodies CA 19-9, HBME-I, and thrombomodulin in the diagnosis of malignant mesothelioma and adenocarcinoma in cytology. Cancer 1998;84:101–8. [DOI] [PubMed] [Google Scholar]
- 20.Wirth PR, Legier J, Wright GL. Immunohistochemical evaluation of seven monoclonal antibodies for differentiation of pleural mesothelioma from lung adenocarcinoma. Cancer 1991;67:655–62. [DOI] [PubMed] [Google Scholar]
- 21.Brown RW, Clark GM, Tanton AK, et al. Multiple-marker immunohistochemical phenotypes distinguishing malignant pleural mesothelioma from pulmonary adenocarcinoma. Hum Pathol 1993;24:347–54. [DOI] [PubMed] [Google Scholar]
- 22.Weiss LM. The search for the optimal immunohistochemical panel for the diagnosis of malignant mesothelioma. Hum Pathol 1993;24:345–6. [DOI] [PubMed] [Google Scholar]
- 23.Skov BG, Lauritzen AF, Hirsch FR, et al. Differentiation of adenocarcinoma of the lung and malignant mesothelioma: predictive value and reproductivity of immunoreactive antibodies. Histopathology 1994;25:431–7. [DOI] [PubMed] [Google Scholar]
- 24.Sheibani K. Immunopathology of malignant mesothelioma. Hum Pathol 1994;25:219–20. [DOI] [PubMed] [Google Scholar]
- 25.Tsutahara S, Shijubo N, Hirasawa M, et al. Lung adenocarcinoma with type II pneumocyte characteristics. Eur Respir J 1993;6:135–7. [PubMed] [Google Scholar]
- 26.Shijubo N, Honda Y, Fujishima T, et al. Lung surfactant protein-A and carcinoembryonic antigen in pleural effusion due to lung adenocarcinoma and malignant mesothelioma. Eur Respir J 1995;8:403–6. [DOI] [PubMed] [Google Scholar]
- 27.Soler AP, Knudsen KA, Jaurand M-C, et al. The differential expression of N-cadherin and E-cadherin distinguishes pleural mesothelioma from lung adenocarcinoma. Hum Pathol 1995;26:1363–9. [DOI] [PubMed] [Google Scholar]
- 28.Soler AP, Knudsen KA, Tecson-Miguel A, et al. Expression of E-cadherin and N-cadherin in surface epithelial-stromal tumors of the ovary distinguishes mucinous from serous and endometrioid tumors. Hum Pathol 1997;28:734–9. [DOI] [PubMed] [Google Scholar]
- 29.Holzinger A, Dingle S, Bejarano PA, et al. Monoclonal antibodies to thyroid transcription factor-1: production, characterisation, and usefulness in tumor diagnosis. Hybridoma 1996;15:49–53. [DOI] [PubMed] [Google Scholar]
- 30.Harlamert HA, Mira J, Bejarano PA, et al. Thyroid transcription factor-1 and cytokeratins 7 and 20 in pulmonary and breast carcinoma. Acta Cytol 1998;42:1382–8. [DOI] [PubMed] [Google Scholar]
- 31.Bejarano PA, Baughman RP, Biddinger PW, et al. Surfactant proteins and thyroid transcription factor-1 in pulmonary and breast carcinoma. Mod Pathol 1996;9:445–52. [PubMed] [Google Scholar]
- 32.Di Loreto C, Puglisi F, Di Lauro V, et al. TTF-1 protein expression in pleural malignant mesotheliomas and adenocarcinomas of the lung. Cancer Lett 1998;124:73–7. [DOI] [PubMed] [Google Scholar]
- 33.Sadler JE. Thrombomodulin structure and function. Thromb Haemost 1997;78:392–5. [PubMed] [Google Scholar]
- 34.Yamada T, Jiping J, Endo R, et al. Molecular cloning of a cell-surface glycoprotein that can potentially discriminate mesothelium from epithelium: its identification as vascular cell adhesion molecule 1. Br J Cancer 1995;71:562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ordonez NG. Value of antibodies 44-3A6, SM3, HBME-1 and thrombomodulin in differentiating epithelial pleural mesothelioma from lung adenocarcinoma. Am J Surg Pathol 1997;21:1399–408. [DOI] [PubMed] [Google Scholar]
- 36.Ordonez NG. The immunohistochemical diagnosis of epithelial mesothelioma. Hum Pathol 1999;30:313–23. [DOI] [PubMed] [Google Scholar]
- 37.Clover A, Oates J, Edwards C. Anti-cytokeratin 5/6: a positive marker for epithelioid mesothelioma. Histopathology 1997;31:140–3. [DOI] [PubMed] [Google Scholar]
- 38.Ordonez NG. Value of cytokeratin 5/6 immunostaining in distinguishing epithelial mesothelioma of the pleura from lung adenocarcinoma. Am J Surg Pathol 1998;22:1215–21. [DOI] [PubMed] [Google Scholar]