Abstract
Aims: It has been shown previously (by immunohistochemistry) that gastric adenocarcinomas harbouring Epstein-Barr virus (EBV) frequently lose p16 protein. This study aimed to examine the mechanisms of inactivation of the CDKN2A gene and correlate the results with clinicopathological features.
Methods: Methylation specific polymerase chain reaction was used to detect CDKN2A promoter methylation in gastric adenocarcinomas from American patients. In addition, immunohistochemistry was used to detect the loss of the p16 protein and in situ hybridisation was used to detect the presence of EBV. The tumours were also analysed for the presence of microsatellite instability.
Results: Eleven (10%) of 107 tumours harboured EBV in the malignant cells. In gastric cancers without EBV, 32% exhibited CDKN2A promoter methylation and 26% had p16 protein loss. In contrast, 91% of the tumours containing EBV had CDKN2A promoter methylation (p = 0.0003) and 90% showed p16 protein loss (p = 0.0001). The presence of EBV was also associated with male sex (p = 0.03) and was more common in tumours from Texas Hispanics than from non-Hispanic whites or African–Americans (p = 0.01). EBV was not associated with microsatellite instability, histological subtype, stage, or grade of the tumour, or age or survival time of the patient.
Conclusions: The presence of EBV in gastric adenocarcinomas is strongly associated with CDKN2A inactivation by promoter methylation. In addition, these findings suggest that there are ethnic differences in tumour virology and pathogenesis.
Keywords: CDK2A, Epstein-Barr virus, methylation
Gastric cancer is distinctive in its association with two different infectious agents that may contribute to the development of the disease. The first of these associations to be identified was that with Helicobacter pylori, a bacterium that colonises the stomach and serves as a risk factor for gastric carcinoma of the intestinal subtype.1–3 The second infectious agent found to be associated with a subset of gastric cancers is the Epstein-Barr virus (EBV). This virus was initially recognised for its association with Burkitt’s lymphoma, Hodgkin’s disease, and nasopharyngeal carcinoma. More recently, it was found to be present in gastric adenocarcinomas, especially lymphoepithelioma-like (LEL) variants, which morphologically resemble nasopharyngeal carcinomas, but also in gastric cancers not morphologically distinguishable from non-EBV containing gastric adenocarcinomas.4–10 The virus is present in 3–16% of gastric adenocarcinomas, in 67–100% of LEL carcinomas, and in 29–35% of gastric remnant adenocarcinomas7,11–17 (for a review, see Takada18). Monoclonality in the size of the fused termini of the viral genome indicates that the virus was present in the original cell from which the tumour arose, and identification of EBV in premalignant lesions further implicates the virus as an early acting contributor to the process of carcinogenesis.11,12,19–22 When gastric epithelial cells were infected with EBV containing a selectable marker, clones arose that had higher proliferation rates and a higher saturation density than control cells, and which formed colonies in soft agar.23 Clearly, EBV is able to drive gastric epithelial cells in vitro towards a malignant phenotype, but the mechanisms by which the virus accomplishes this remain obscure.
The CDKN2A gene encodes p16, or cyclin dependent kinase inhibitor 2a, also known as INK4a, for inhibitor of cyclin dependent kinase 4a. Binding of p16 inhibits kinase activity of cyclin dependent kinase 4 or 6, so that the retinoblastoma protein is hypophosphorylated and consequently binds E2F transcription factors, thus inhibiting cell cycle progression from G1 to S.24,25 The CDKN2A gene is inactivated in a wide variety of human cancers, by mutation, by homozygous mutation, or by promoter methylation,26–32 as reviewed in Liggett and Sidransky32 and Ruas and Peters.33 Homozygous deletions of CDKN2A are reported to occur in approximately 10% of gastric cancers.34 Chen et al reported the presence of aberrant CDKN2A transcripts, including intragenic deletions in five of 11 diffuse-type gastric cancers and in three of 10 intestinal-type gastric cancers.35 Others have found p16 mutations to occur in 10% or less of gastric cancers.34,36–38 CDKN2A promoter methylation has been found in 42% of gastric cancers.39
“Epstein-Barr virus is able to drive gastric epithelial cells in vitro towards a malignant phenotype, but the mechanisms by which the virus accomplishes this remain obscure”
In a previous report,40 we found that EBV associated gastric adenocarcinomas showed p16 loss, as measured by immunohistochemistry (IHC), more frequently than did non-EBV associated tumours. To gain insight into the mechanisms of this loss, we performed methylation specific polymerase chain reaction (MS-PCR) and microsatellite analysis on a larger set of gastric cancers. Here, we report the striking association of EBV with promoter methylation of the CDKN2A gene in gastric cancers.
METHODS
Specimens
Formalin fixed, paraffin wax embedded gastrectomy specimens (n = 122) from patients with gastric adenocarcinoma were obtained from hospitals in San Antonio, Texas (n = 67); New Orleans, Louisiana (n = 50); and Rochester, Minnesota (n = 5). Specimens were serially acquired, but cases with small amounts of tumour in the section were omitted from the study, as were cases with finely dispersed tumour cells. Methylation analysis data were obtained for 118 of the 122 cases, and EBV analysis data were obtained for 107 cases. The ethnicity, age, and sex of the patient and anatomical site of the tumour were obtained from medical records or tumour registries. The ethnic groups represented were white (persons of European ancestry; n = 48, 36 men, 12 women), Texas Hispanics (persons of predominantly Mexican ancestry; n = 47, 35 men, 12 women), and African–Americans (n = 25, 16 men, nine women); the ethnic origins of two patients were unknown. Staging was performed using standard TNM criteria.41 Tumours were graded and classified as intestinal, diffuse, or mixed subtype by an experienced surgical pathologist (JCB) according to the criteria of Laurén.42 Survival data were available for 100 patients from Texas and Louisiana. All specimens and patient information were obtained with the approval of the institutional review boards of the Louisiana State University Health Sciences Center and/or the University of Texas Health Sciences Center at San Antonio. This set of cases overlaps but is not identical to that used in our previous study of EBV in gastric cancer.22
Harvesting of DNA from formalin fixed, paraffin wax embedded gastric tumour samples
Paraffin wax embedded sections of gastric adenocarcinomas were prepared for microdissection as described previously.43 Tumour cells and benign cells were harvested by laser capture microdissection (LCM) with the PixCell I instrument (Arcturus Engineering, Mountain View, California, USA) using a 30 μm laser spot with 50 to 60 mW power and a 50 to 100 msec pulse width. Cell preparations of 2000 to 3000 pulses were harvested and digested in 40 μl of proteinase K (1 mg/ml in 50mM Tris/HCl buffer, pH 8.0, with 1mM EDTA and 0.45% Tween 20) at 52°C overnight. Digested material was heated at 95°C for 15 minutes to denature the proteinase K and was used as template for PCR without additional purification.
Microsatellite instability
Gastric tumours in our study had been categorised previously for microsatellite instability (MSI)43 following recommendations of the National Cancer Institute (NCI) Workshop on Microsatellite Instability. 44 In accordance with these guidelines, cases were designated as having high frequency microsatellite instability (MSI-H), low frequency microsatellite instability (MSI-L), or as being microsatellite stable (MSS), using the five markers recommended by the NCI convention, BAT25, BAT26, D2S123, D5S346, and D17S250. We used the NCI convention nomenclature of MSI-H if amplification of DNA from the tumour produced bands with altered mobility at 30% or more of the markers, MSI-L if amplifications showed alterations in less than 30% of markers, and MSS if there were no alterations.
Bisulfite modification
Digested samples from LCM harvested cells (20 μl) were denatured with 2 μl of 3M NaOH at 75°C for 15 minutes. Bisulfite modification was initiated by adding 250 μl of freshly prepared, pH 5.0, 4.8M sodium bisulfite and 14 μl of 10mM hydroquinone and incubating for four hours at 55°C. The samples were concentrated with centrifugal filter devices (Centricon YM-30, Millipore Corporation, Bedford, Massachusetts, USA). Desulfonation was performed by adding 4.5 μl of 3M NaOH to each sample, incubating at 37°C for 15 minutes, and then adding 28 μl of 5M ammonium acetate to neutralise the solution. DNA was precipitated with ethanol using glycogen as a co-precipitant and resuspended in 25 μl of water.
Methylation specific polymerase chain reaction
The sodium bisulfite modification technique relies on the capacity of bisulfite to convert unmethylated cytosine residues, but not 5-methylcytosine, to uracil by deamination.45 Primer sequences, as described by Herman et al, discriminated methylated from unmethylated sequences of the CDKN2A promoter46 based upon the chemically induced differences induced by bisulfite modification. Forward primers were labelled with γ-32P ATP using T4 polynucleotide kinase. PCR mixtures contained 100nM of each primer, 200μM of each dinucleotide triphosphate, and 1.5mM MgCl2. Annealing temperatures for primers were 60°C for the primers specific for unmethylated DNA and 63°C for the primers specific for methylated DNA. After 95°C for three minutes, amplifications were carried out for 40 cycles consisting of 95°C for 45 seconds, 60°C/63°C for 45 seconds, and 72°C for one minute. PCR products were electrophoresed on 6% polyacrylamide gels for two hours at 45 W. Gels were dried under vacuum at 80°C and analysed by autoradiography on Kodak X-Omat AR film.
Immunohistochemistry
Unstained sections (5 μm) were cut on to capillary gap slides, and heated at 60°C for 20 minutes. The anti-p16 monoclonal antibodies used were: Ab7 (Lab Vision Corporation, Fremont, California, USA) and clone PMG175-405 (PharMingen, San Diego, California, USA). Normal mouse IgG was used as a negative control. Sections of all cases were incubated with Ab7 at 1 μg/ml at 4°C overnight, after antigen retrieval in 0.1M EDTA, pH 8.0 (20 minutes at 95–100°C).47 A subset of cases was also reacted with the PMG175-405 monoclonal antibody, as described previously.48 The Vectastain Elite ABC kit from Vector (Burlingame, California, USA) was used for detecting bound antibodies, according to the manufacturer’s recommendations. Diaminobenzidine with a haematoxylin counterstain was used for colour development. Normal colonic mucosa and a p16 positive lung cancer xenograft were used as external positive controls. In addition, non-neoplastic stromal cells served as internal positive controls for p16 in every tumour section. A mesothelioma and pancreatic adenocarcinoma, both of which were p16 negative, were used as external negative controls. Sections were examined for evidence of nuclear staining above any cytoplasmic background; cytoplasmic staining itself was disregarded.48 If there was nuclear staining in a diffuse or mosaic distribution throughout the tumour, it was considered positive for p16. If the neoplastic nuclei failed to stain whereas admixed non-neoplastic cells reacted positively, the lesion was scored as negative. In a subset of carcinomas, loss of p16 reactivity was not diffuse, but limited to well defined areas within the lesion. This staining pattern was also considered abnormal and may reflect a relatively late event in the development of the tumour.
In situ hybridisation
In situ hybridisation for EBV encoded RNA 1 (EBER1) and U6 was performed on paraffin wax embedded sections, as described previously, using digoxigenin labelled riboprobes complementary to EBER1 transcripts.22 U6 transcripts served as a control for RNA preservation because U6 is a ubiquitous cellular RNA similar in size, copy number, and nuclear localisation to EBER1. Dr R Ambinder provided probe templates for EBER1 (RA386) and U6 (RA390). Positive controls included EBV related Hodgkin’s disease cases, and negative controls included normal epithelium and other non-malignant structures in each case. Rare small lymphocytes stained with the EBER1 probe in some tumour sections, but only tumour specific EBER1 staining was considered to be a positive result.
Statistical analysis
The relation between EBV or methylation status and categorical variables such as sex, site, and grade was analysed using the χ2 test or Fisher’s exact test. The time to event was used to calculate the relative hazard of death given EBV status. A Kaplan-Meier curve was constructed, and the association with EBV status was determined using the log rank test. All analyses were done with SAS (SAS Institute Inc, Cary, North Carolina, USA).
RESULTS
To determine the mechanism of p16 inactivation in gastric cancers, we performed MS-PCR for the promoter region of the CDKN2A gene and obtained results from 118 gastric adenocarcinomas. Of these, 44 (37%) showed methylation of the CDKN2A promoter (fig 1). Details of clinicopathological associations with methylation are described elsewhere (QN Vo et al, unpublished data, 2002).
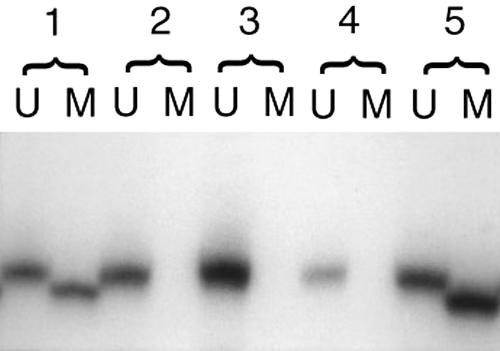
Figure 1.
Lanes marked “U” contain PCR products amplified with primers specific for unmethylated sequences, and lanes marked “M” contain PCR products amplified with primers specific for methylated sequences. Analysis of five gastric adenocarcinomas by methylation specific PCR shows that tumours 1 and 5 have bands indicating the presence of methylation in the CDKN2A promoter. Tumours 2, 3, and 4 have bands indicating only the unmethylated form of the promoter. Tumour 1 is an Epstein-Barr virus associated tumour.
For 107 of the 118 cases, EBV analysis by in situ hybridisation was also performed, and 11 (10%) cases were found to be positive. Table 1 shows the clinicopathological findings regarding the 11 EBV positive cases. None of the 11 cases was a gastric remnant tumour. All but one of the 11 EBV positive cases were also positive for methylation, showing a highly significant association (p = 0.0003). Two tumours had LEL morphology, and two more had focal LEL patterns. Table 2 shows the detailed associations of EBV infection and other clinical features of the tumours.
Table 1.
Clinicopathological findings in 11 EBV associated gastric cancers
| Case | Pattern | MS-PCR | p16 IHC | Ethnicity | Sex | Age (years) |
| 1 | Intestinal | + | – | Hispanic | Male | 80 |
| 2 | Diffuse | + | – | Hispanic | Male | 70 |
| 3 | Intestinal | + | – | Hispanic | Male | 61 |
| 4 | Intestinal | + | – | Hispanic | Male | 56 |
| 5 | LEL | – | – | Hispanic | Male | 67 |
| 6 | Intestinal | + | – | Hispanic | Male | 56 |
| 7* | Mixed | + | + | Hispanic | Male | 60 |
| 8* | Intestinal | + | – | Hispanic | Male | 63 |
| 9 | Intestinal | + | ND | Hispanic | Male | 82 |
| 10 | LEL | + | – | White | Male | 66 |
| 11 | Intestinal | + | – | White | Male | 70 |
*Had focal areas resembling LEL.
EBV, Epstein-Barr virus; IHC, immunohistochemistry; LEL, lymphoepithelioma-like morphology; MS-PCR, methylation specific polymerase chain reaction; ND, not done.
Table 2.
Clinical and biological features in association with EBV
| EBV positive | EBV negative | p Value | |
| Sex (n=108) | |||
| Male | 11 | 67 | 0.03 |
| Female | 0 | 30 | |
| Ethnicity (n=108) | |||
| White/non-Hispanic | 2 | 44 | 0.01 |
| Texas Hispanic | 9 | 34 | |
| African–American | 0 | 21 | |
| MS-PCR (n=107) | |||
| Unmethylated only | 1 | 65 | 0.0003 |
| Methylated | 10 | 31 | |
| p16 IHC (n=106) | |||
| Positive | 1 | 71 | 0.0001 |
| Negative | 9 | 25 | |
| Mean (SEM) age (n=108) | 66.5 years (2.6) | 68.3 years (1.4) | |
| Under 70 | 7 | 48 | |
| 70 or older | 4 | 49 | NS |
| Microsatellite instability (n=106) | |||
| MSI-H | 0 | 20 | NS |
| MSI-L | 1 | 9 | |
| MSS | 9 | 67 | |
| T (n=108) | |||
| 0+1 | 0 | 7 | NS |
| 2 | 6 | 57 | |
| 3 | 4 | 23 | |
| 4 | 1 | 10 | |
| N (n=106) | |||
| 0 | 1 | 14 | NS |
| 1 | 5 | 56 | |
| 2 | 5 | 25 | |
| M (n=108) | |||
| 0 | 10 | 88 | NS |
| 1 | 1 | 9 | |
| Stage (n=106) | |||
| 0–II | 5 | 53 | NS |
| III–IV | 6 | 42 | |
| Tumour grade (n=109) | |||
| Well | 1 | 19 | NS |
| Moderate | 5 | 46 | |
| Poor | 5 | 33 | |
| Histological subtype (n=109) | |||
| Intestinal | 8 | 80 | NS |
| Diffuse | 2 | 13 | |
| Mixed | 1 | 5 | |
Unless otherwise noted, values are number of patients.
EBV, Epstein-Barr virus; IHC, immunohistochemistry; MSI-L, low frequency microsatellite instability; MSI-H, high frequency microsatellite instability; MSS, microsatellite stable; MS-PCR, methylation specific polymerase chain reaction; NS, not significant.
This series of patients was larger and, coincidentally, more broadly representative of the major south Texas and Louisiana ethnic groups than the group that we examined previously for EBV22; 50 cases overlapped both studies. EBV was associated with male sex (p = 0.03) and Hispanic ethnic origin (p = 0.01). Hispanic men made up only 29% of the patients in our study, but were 82% of the patients with EBV associated tumours. EBV was associated with nine of 43 tumours from Hispanic patients, compared with two of 44 from white patients; none of the 21 African–Americans had EBV present in their tumours. In Hispanic men, 28% of gastric cancers were EBV associated, compared with 6% of tumours from white men.
The tumours were examined for p16 protein by IHC, and data for 118 cases were obtained; 36 (31%) of these showed complete or focal loss of p16 protein. Both EBV status and p16 IHC data were available in 106 cases. Of the 10 EBV positive cases for which p16 IHC data were obtained, nine were negative for p16 protein (p = 0.0001; fig 2).
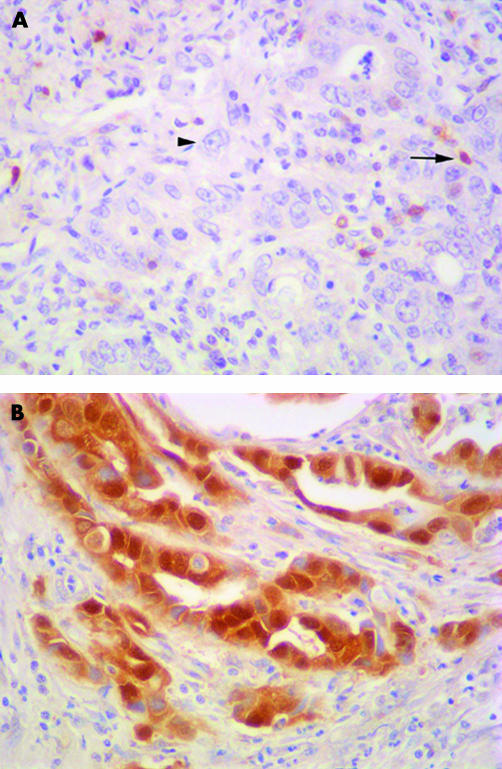
Figure 2.
(A) Immunohistochemical staining using an antibody against p16 in this Epstein-Barr virus (EBV) associated gastric cancer reveals that tumour cell nuclei (arrowhead) have no detectable p16 protein, in contrast to the p16 positive inflammatory and stromal cells adjacent to the tumour (arrow) and to the p16 positive staining tumour nuclei in the EBV negative carcinoma (B). Haematoxylin stained.
No significant associations were shown between EBV status and stage, histological subtype, or grade (table 2). Information regarding the anatomical location of the tumour was available for only three of the 11 EBV positive adenocarcinomas (two arose in the body, one in the cardia). Median survival for patients with EBV positive tumours was 207 days, compared with 329 days for patients with EBV negative tumours, but this difference was not significant; neither was there a significant difference in the ages of the patients who had EBV positive and negative tumours.
We found no significant association with EBV status and MSI. Nine of the 11 EBV positive cases were MSS, one was MSI-L (showing MSI at one of the five NIH convention markers), and one was not completely characterised using the NIH conference markers, but appeared to be MSS, based on its stability at marker BAT26 and at three dinucleotide repeat markers.
DISCUSSION
In this set of gastric adenocarcinomas, 10% were EBV related, as defined by in situ hybridisation to EBER transcripts, a method that is considered the gold standard for defining EBV associated tumours. This result was similar to the percentage reported by us (12%) in a previous study on a smaller set of mostly North American cases,22 and similar to the percentage reported in a study on Japanese Americans (10%).7 Similar percentages have also been reported by other groups18 in patients from areas with a higher incidence of gastric cancer, and somewhat higher values (16%) were reported in another set of American gastric cancers.5
The current cohort of patients with gastric cancer is more ethnically diverse than in our previous study. Our current study included non-Hispanic white, Hispanic, and African–American populations, mostly from South Texas and Louisiana. Remarkably, nine of the 11 patients with EBV positive tumours were found to be Hispanic men. In south Texas, Hispanic men have an increased incidence of gastric cancer, over twice as high as that of non-Hispanic white men.49 It is possible that EBV contributes to this increased risk in this population. However, a study of 135 consecutive gastric cancers in Mexico found only 8% of cases to be EBV related, a proportion consistent with results reported in North American and European patients.50 A population based study of south Texan ethnic groups would be useful to confirm and characterise the interesting variation in host–virus interactions that our data suggest.
Geographical variations in EBV associated tumours have long been noted. Burkitt’s lymphoma, the first tumour known to be associated with EBV, is found primarily in equatorial Africa51 and in New Guinea.52 Hodgkin’s disease is more frequently associated with EBV in Hispanic Americans.53 EBV associated nasopharyngeal carcinoma has its highest incidence in Southeast Asia.54 The reasons for such geographical or ethnic variations remain obscure because EBV infection is carried by over 90% of adults worldwide.
“The ability of the virus to control the expression of viral genes by promoter methylation provides a selective advantage that allows the virus to persist in the host for many years”
Previously, we found in gastric cancers a significant association between EBV and p16 loss, as determined by IHC.40 This association was confirmed in our current set of tumours (p = 0.0001). Furthermore, the presence of EBV was highly associated with methylation of the CDKN2A promoter (p = 0.0003). In all cases but three, tumours were both negative by immunostaining and methylation positive. In one case, IHC could not be performed, but the tumour showed methylation. In another case, a tumour with methylation was p16 positive by IHC. Such an event could occur if methylation was present in only one allele of the gene. In the third case, no methylation was present, although p16 protein was not detected by IHC. Such results could be explained by gene inactivation by mutation or homozygous deletion.
Suppression of p16 expression associated with EBV infection or EBV proteins has been observed in other systems. In mouse embryo fibroblasts, EBV latent membrane protein 1 (LMP1) acts to prevent senescence by inhibiting the induction of p16.55 Consistent with the capacity of EBV to inactivate p16, we and others have previously reported frequent p16 loss (as determined by IHC) in nasopharyngeal carcinomas.56,57
As with infection with H pylori, EBV infection persists over decades, evading attempts by the immune system to eradicate it. Normal individuals live in symbiosis with the virus by mounting an effective defence against EBV driven cell proliferation through the response of CD8 positive T cells.58–60 The T cells of individuals with EBV associated gastric cancer are able to cause regression of proliferating foci of exogenously infected autologous B cells.11 In the presence of a vigorous immune response, the virus apparently evades immune surveillance by maintaining latency, in which very few viral genes are expressed, and this control is achieved largely by promoter methylation.
EBV appears to be distinctive among large DNA viruses in showing a lower CpG content than would be expected by chance.61 This could be explained by its use of methylation in suppressing the expression of viral proteins. Because of the spontaneous deamination of methylated cytosines, CpGs tend to be reduced in the viral genome.
In nasopharyngeal carcinomas, lymphomas, and normal B cells, methylation in the viral CpG rich BamH1 C and W promoters controls expression of the EBV encoded nuclear antigen (EBNA) proteins.62–67 In vitro studies of Burkitt’s lymphoma cell lines68 and nasopharyngeal carcinoma cells69 have shown that the expression of LMP1 is associated with hypomethylation of the LMP1 regulatory sequences (for a review, see Robertson70). It seems that the ability of the virus to control the expression of viral genes by promoter methylation provides a selective advantage that allows the virus to persist in the host for many years.
Extrapolations regarding patterns of methylation in other EBV associated diseases must be performed with caution because the mechanism by which EBV contributes to gastric cancer may be distinct from the mechanisms occurring in other EBV related systems. Under in vitro conditions, the expression of LMP1 alone can transform fibroblasts.71–73 However, in gastric epithelial cells, we and others have found weak22,74 or no expression11,21,75,76 of LMP1 in EBV associated gastric cancers. Imai et al showed that in EBV associated gastric cancers inactivation of LMP1, in addition to EBNA1, EBNA2, EBNA3A, EBNA3B, EBNA3C, and Lp, was accomplished by the methylation of LMP1 regulatory sequences and the C and W promoter regions.11
Sugiura et al have described the latency pattern of EBV in gastric cancer as more closely resembling the latency I pattern in Burkitt’s lymphoma, rather than the latency II pattern of most nasopharyngeal carcinomas.75 That study reported the finding in gastric cancers of transcripts for EBNA1 (initiated from the Q promoter), LMP2A, and the BamHI-A regions of the viral genome, but no transcripts of LMP1, LMP2B, or EBNA2. Similarly, zur Hausen et al have described a transcription pattern that includes BARF1, BARF0, LMP2A, and Q/K driven EBNA1 but not LMP1, EBNA2, or ZEBRA transcripts in EBV associated gastric cancers.76 Those authors described a distinctive pattern of expression for gastric cancer that resembles that of Burkitt’s lymphoma except for the additional transcription of BARF1 and LMP2A in gastric cancer.
zur Hausen et al have proposed that BARF1 may substitute for LMP1 in its oncogenic potential in gastric cancer.76 BARF1 can transform fibroblasts77 and immortalise kidney epithelial cells in culture.78 The overexpression of BARF1 can activate the c-myc proto-oncogene in Louckes B cells.79 BARF1 has a region of homology to c-fms, a tyrosine kinase proto-oncogene, which encodes the colony stimulating factor 1 receptor.80 BARF1 may help the virus to evade the immune system by acting as an antagonist to colony stimulating factor 1, which is a macrophage/monocyte growth and differentiation factor (reviewed in Hamilton81).
“Neither of the promoters of the tumour suppressor genes hMLH1 and hMSH2 appeared to be methylated in our set of tumours”
It is possible that CDKN2A promoter methylation in EBV associated gastric cancers is a cellular change secondary to the acceleration of cell proliferation caused by the presence of a viral oncogene such as BARF1. An alternative possibility is that CDKN2A promoter methylation is more directly related to the activities of the virus, and that the ability of the virus to achieve methylation of its own genes for evasion of immune surveillance may be accompanied by the ability to influence the methylation of cellular tumour suppressor genes. If this last hypothesis is true, it is noteworthy that there is apparently some specificity of the cellular tumour suppressor genes that become methylated because neither of the promoters of the tumour suppressor genes hMLH1 and hMSH2 appeared to be methylated in our set of tumours. This we infer from the fact that no EBV positive cases were MSI-H, a defect that results from inactivation of the DNA mismatch repair system, usually caused, in gastric cancers, by hMLH1 or hMSH2 promoter methylation.82,83 A leukaemia model demonstrates how an oncogene can cause methylation of a promoter of a specific tumour suppressor gene. In this model system, a leukaemia promoting chimaeric oncogene, PML-RAR, is able to recruit DNA methyltransferases to the promoter of the tumour suppressor gene RARβ2, thus reducing its expression by hypermethylation of the promoter.84 Whether the effect of EBV upon CDKN2A promoter methylation is direct or indirect, the increased proportion of promoter methylation detected in EBV associated tumours compared with non-EBV associated tumours is thought provoking. In summary, we have identified a distinct pattern of epigenetic alteration in EBV associated gastric cancers. Such distinctiveness might become relevant to treatment in the event that treatments based on the reversal of aberrant methylation become available.
Take home messages.
The presence of Epstein-Barr virus (EBV) in gastric adenocarcinomas was strongly associated with CDKN2A inactivation by promoter methylation
This might be useful if treatments based on the reversal of aberrant methylation become available
The presence of EBV was associated with male sex and was more common in tumours from Texas Hispanics than from non-Hispanic whites or African–Americans
EBV was not associated with microsatellite instability, histological subtype, stage, or grade of the tumour, or age or survival time of the patient
Acknowledgments
We thank Drs D Bradburn, D Bostwick, W Hinchey, and E Averyt for help in obtaining specimens, and P Eagan for skillful assistance with in situ hybridisation. We thank Dr R Ambinder for the EBER1 and U6 probes and templates. We wish to acknowledge the assistance of Ms J Johnson and the staff of tumour registries of Touro Infirmary, St Luke’s Baptist Hospital, and the Audie Murphy Memorial Veterans Administration Hospital, especially M Roberts, A Burt, and B Kirk. This work was supported by the Health Excellence Fund of the Board of Regents of the State of Louisiana. Q Vo was supported in part by the Scott Graduate Scholars Program.
Abbreviations
CDKN2A, cyclin dependent kinase inhibitor 2A
EBER1, Epstein-Barr virus encoded RNA 1
EBNA, Epstein-Barr virus encoded nuclear antigen
EBV, Epstein-Barr virus
IHC, immunohistochemistry
LCM, laser capture microdissection
LEL, lymphoepithelioma-like
LMP1, latent membrane protein 1
MSI, microsatellite instability
MSI-L, low frequency microsatellite instability
MSI-H, high frequency microsatellite instability
MS-PCR, methylation specific polymerase chain reaction
MSS, microsatellite stable
NCI, National Cancer Institute
REFERENCES
- 1.Parsonnet J, Freidman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 1991;325:1127–31. [DOI] [PubMed] [Google Scholar]
- 2.Parsonnet J, Vandersteen D, Goates J, et al. Helicobacter infection in intestinal- and diffuse-type gastric adenocarcinomas. J Natl Cancer Inst 1991;83:640–3. [DOI] [PubMed] [Google Scholar]
- 3.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—first American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res 1992;52:6735–40. [PubMed] [Google Scholar]
- 4.Shibata D, Tokunaga M, Uemura Y, et al. Association of Epstein-Barr virus with undifferentiated gastric carcinomas with intense lymphoid infiltration. Lymphoepithelioma-like carcinoma. Am J Pathol 1991;139:469–74. [PMC free article] [PubMed] [Google Scholar]
- 5.Shibata D, Weiss LM. Epstein-Barr Virus-associated gastric adenocarcinoma. Am J Pathol 1992;140:769–74. [PMC free article] [PubMed] [Google Scholar]
- 6.Niedobitek G, Herbst H, Young LS, et al. Epstein-Barr virus and carcinomas. Expression of the viral genome in an undifferentiated gastric carcinoma. Diagn Mol Pathol 1992;1:103–8. [PubMed] [Google Scholar]
- 7.Shibata D, Hawes D, Stemmermann GN, et al. Epstein-Barr virus-associated gastric adenocarcinoma among Japanese Americans in Hawaii. Cancer Epidemiol Biomarkers Prev 1993;2:213–17. [PubMed] [Google Scholar]
- 8.Tokunaga M, Land CE, Uemura Y, et al. Epstein-Barr virus in gastric carcinoma. Am J Pathol 1993;143:1250–4. [PMC free article] [PubMed] [Google Scholar]
- 9.Leoncini L, Vindigni C, Megha T, et al. Epstein-Barr virus and gastric cancer: data and unanswered questions. Int J Cancer 1993;53:898–901. [DOI] [PubMed] [Google Scholar]
- 10.Rowlands DC, Ito M, Mangham DC, et al. Epstein-Barr virus and carcinomas: rare association of the virus with gastric adenocarcinomas. Br J Cancer 1993;68:1014–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai S, Koizumi S, Sugiura M, et al. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc Natl Acad Sci U S A 1994;91:9131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oda K, Tamaru J, Takenouchi T, et al. Association of Epstein-Barr virus with gastric carcinoma with lymphoid stroma. Am J Pathol 1993;143:1063–71. [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto N, Tokunaga M, Uemura Y, et al. Epstein-Barr virus and gastric remnant cancer. Cancer 1994;74:805–9. [DOI] [PubMed] [Google Scholar]
- 14.Baas IO, Van Rees BP, Musler A, et al. Helicobacter pylori and Epstein-Barr virus infection and the p53 tumour suppressor pathway in gastric stump cancer compared with carcinoma in the non-operated stomach. J Clin Pathol 1998;51:662–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang MS, Lee JH, Kim JP, et al. Microsatellite instability and Epstein-Barr virus infection in gastric remnant cancers. Pathol Int 2000;50:486–92. [DOI] [PubMed] [Google Scholar]
- 16.Chang MS, Kim WH, Kim CW, et al. Epstein-Barr virus in gastric carcinomas with lymphoid stroma. Histopathology 2000;37:309–15. [DOI] [PubMed] [Google Scholar]
- 17.Wu MS, Shun CT, Wu CC, et al. Epstein-Barr virus-associated gastric carcinomas: relation to H. pylori infection and genetic alterations. Gastroenterology 2000;118:1031–8. [DOI] [PubMed] [Google Scholar]
- 18.Takada K. Epstein-Barr virus and gastric carcinoma. J Clin Pathol 2000;53:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pittaluga S, Loke SL, So KC, et al. Clonal Epstein-Barr virus in lymphoepithelioma-like carcinoma of the stomach. Mod Pathol 1992;5:661–4. [PubMed] [Google Scholar]
- 20.Ott G, Kirchner T, Müller-Hermelink HK. Monoclonal Epstein-Barr virus genomes but lack of EBV-related protein expression in different types of gastric carcinoma. Histopathology 1994;25:323–9. [DOI] [PubMed] [Google Scholar]
- 21.Fukayama M, Hayashi Y, Iwasaki Y, et al. Epstein-Barr virus-associated gastric carcinoma and Epstein-Barr virus infection of the stomach. Lab Invest 1994;71:73–81. [PubMed] [Google Scholar]
- 22.Gulley ML, Pulitzer DR, Eagan PA, et al. Epstein-Barr virus infection is an early event in gastric carcinogenesis and is independent of bcl-2 expression and p53 accumulation. Hum Pathol 1996;27:20–7. [DOI] [PubMed] [Google Scholar]
- 23.Nishikawa J, Imai S, Oda T, et al. Epstein-Barr virus promotes epithelial cell growth in the absence of EBNA2 and LMP1 expression. J Virol 1999;73:1286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 1993;366:704–7. [DOI] [PubMed] [Google Scholar]
- 25.Serrano M. The tumor suppressor protein p16INK4a. Exp Cell Res 1997;237:7–13. [DOI] [PubMed] [Google Scholar]
- 26.Kamb A, Gruis NA, Weaver FJ, et al. A cell-cycle regulator potentially involved in genesis of many tumor types. Science 1994;264:436–40. [DOI] [PubMed] [Google Scholar]
- 27.Nabori T, Miura K, Wu DJ, et al. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature 1994;368:753–6. [DOI] [PubMed] [Google Scholar]
- 28.Spruck CHI, Gonzalez-Zulueta M, Shibata A, et al. p16 gene in uncultured tumors. Nature 1994;370:183–4. [DOI] [PubMed] [Google Scholar]
- 29.Herman JG, Merlo A, Mao L, et al. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res 1995;55:4525–30. [PubMed] [Google Scholar]
- 30.Cairns P, Polascik TJ, Eby Y, et al. Frequency of homozygous deletion of p16/CDKN2 in primary human tumours. Nat Genet 1995;11:210–12. [DOI] [PubMed] [Google Scholar]
- 31.Hall M, Peters G. Genetic alterations of cyclins, cyclin-dependent kinases, and CDK inhibitors in human cancer. Adv Cancer Res 1996;68:67–105. [DOI] [PubMed] [Google Scholar]
- 32.Liggett WH, Jr, Sidransky D. Role of the p16 tumor suppressor gene in cancer. J Clin Oncol 1998;16:1197–206. [DOI] [PubMed] [Google Scholar]
- 33.Ruas M, Peters G. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim Biophys Acta 1998;1378:F115–77. [DOI] [PubMed] [Google Scholar]
- 34.Wu MS, Shun CT, Sheu JC, et al. Overexpression of mutant p53 and c-erbB-2 proteins and mutations of the p15 and p16 genes in human gastric carcinoma: with respect to histological subtypes and stages. J Gastroenterol Hepatol 1998;13:305–10. [DOI] [PubMed] [Google Scholar]
- 35.Chen YJ, Chang JG, Shih LS, et al. Frequent detection of aberrant RNA transcripts of the CDKN2 gene in human gastric adenocarcinoma. Int J Cancer 1997;71:350–4. [DOI] [PubMed] [Google Scholar]
- 36.Igaki H, Sasaki H, Tachimori Y, et al. Mutation frequency of the p16/CDKN2 gene in primary cancers of the upper digestive tract. Cancer Res 1995;55:3421–3. [PubMed] [Google Scholar]
- 37.Sakata K, Tamura G, Maesawa C, et al. Loss of heterozygosity on the short arm of chromosome 9 without p16 gene mutation in gastric carcinomas. Jpn J Cancer Res 1995;86:333–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YY, Kang SH, Seo JY, et al. Alterations of p16INK4A and p15INK4B genes in gastric carcinomas. Cancer 1997;80:1889–96. [DOI] [PubMed] [Google Scholar]
- 39.Shim YH, Kang GH, Ro JY. Correlation of p16 hypermethylation with p16 protein loss in sporadic gastric carcinomas. Lab Invest 2000;80:689–95. [DOI] [PubMed] [Google Scholar]
- 40.Schneider BG, Gulley ML, Eagan P, et al. Loss of p16/CDKN2A tumor suppressor protein in gastric adenocarcinoma is associated with Epstein-Barr virus and anatomic location in the body of the stomach. Hum Pathol 2000;31:45–50. [DOI] [PubMed] [Google Scholar]
- 41.Fleming ID, Cooper JS, Henson DE, et al. AJCC cancer staging handbook. Philadelphia: Lippincott-Ravin, 1998.
- 42.Laurén P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand 1965;64:31–49. [DOI] [PubMed] [Google Scholar]
- 43.Schneider BG, Bravo JC, Roa JC, et al. Microsatellite instability, prognosis and metastasis in gastric cancers from a low-risk population. Int J Cancer 2000;89:444–52. [DOI] [PubMed] [Google Scholar]
- 44.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248–57. [PubMed] [Google Scholar]
- 45.Wang RY-H, Gehrke CW, Ehrlich M. Comparison of bisulfite modification of 5-methyldeoxycytidine and deoxycytidine residues. Nucleic Acids Res 1980;8:4777–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herman JG, Graff JR, Myöhänen S, et al. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A 1996;93:9821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geradts J, Kratzke RA Niehans GA, et al. Immunohistochemical detection of the cyclin-dependent kinase inhibitor 2/multiple tumor suppressor gene 1 (CDKN2/MTS1) product p16INK4A in archival human solid tumors: correlation with retinoblastoma protein. Cancer Res 1995;55:6006–11. [PubMed] [Google Scholar]
- 48.Geradts J, Fong KM, Zimmerman PV, et al. Loss of Fhit expression in non-small-cell lung cancer: correlation with molecular genetic abnormalities and clinicopathological features. Br J Cancer 2000;82:1191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Texas Department of Health. Cancer in Texas 1997. Texas: Department of Health, 2001.
- 50.Herrera-Goepfert R, Reyes E, Hernandez-Avila M, et al. Epstein-Barr virus-associated gastric carcinoma in Mexico: analysis of 135 consecutive gastrectomies in two hospitals. Mod Pathol 1999;12:873–8. [PubMed] [Google Scholar]
- 51.Burkitt D. A children’s cancer dependent on climatic factors. Nature 1962;194:232–4. [DOI] [PubMed] [Google Scholar]
- 52.Pope JH, Achong BG, Epstein MA. Burkitt lymphoma in New Guinea: establishment of a line of lymphoblasts in vitro and description of their fine structure. J Natl Cancer Inst 1967;39:933–45. [PubMed] [Google Scholar]
- 53.Gulley ML, Eagan PA, Quintanilla-Martinez L, et al. Epstein-Barr virus DNA is abundant and monoclonal in the Reed-Sternberg cells of Hodgkin’s disease: association with mixed cellularity subtype and Hispanic American ethnicity. Blood 1994;83:1595–602. [PubMed] [Google Scholar]
- 54.Nicholls JM, Agathanggelou A, Fung K, et al. The association of squamous cell carcinomas of the nasopharynx with Epstein-Barr virus shows geographical variation reminiscent of Burkitt’s lymphoma. J Pathol 1997;183:164–8. [DOI] [PubMed] [Google Scholar]
- 55.Yang XH, He ZM, Xin BZ, et al. LMP1 of Epstein-Barr virus suppresses cellular senescence associated with the inhibition of p16INK4a expression. Oncogene 2000;19:2002–13. [DOI] [PubMed] [Google Scholar]
- 56.Gulley ML, Burton MP, Allred DC, et al. Epstein-Barr virus infection is associated with p53 accumulation in nasopharyngeal carcinoma. Hum Pathol 1998;29:252–9. [DOI] [PubMed] [Google Scholar]
- 57.Shibosawa E, Tsutsumi K, Koizuka I, et al. Absence of nuclear p16 from Epstein-Barr virus-associated undifferentiated nasopharyngeal carcinomas. Laryngoscope 2000;110:93–7. [DOI] [PubMed] [Google Scholar]
- 58.Saiki Y, Ohtani H, Naiato Y, et al. Immunophenotypic characterization of Epstein-Barr virus-associated gastric carcinoma: massive infiltration by proliferating CD8+ T-lymphocytes. Lab Invest 1996;75:67–76. [PubMed] [Google Scholar]
- 59.Rickinson AB, Moss DJ. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu Rev Immunol 1997;15:405–31. [DOI] [PubMed] [Google Scholar]
- 60.Kuzushima K, Nakamura S, Nakamura T, et al. Increased frequency of antigen-specific CD+ cytotoxic T lymphocytes infiltrating an Epstein-Barr virus-associated gastric carcinoma. J Clin Invest 1999;104:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karlin S, Doerfler W, Cardon LR. Why is CpG suppressed in the genomes of virtually all small eukaryotic viruses but not those of large eukaryotic viruses? J Virol 1994;68:2889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ernberg I, Falk K, Minarovits J, et al. The role of methylation in the phenotype-dependent modulation of Epstein-Barr nuclear-antigen-2 and latent-membrane-protein genes in cells latently infected with Epstein-Barr virus. J Gen Virol 1989;70:2989–3002. [DOI] [PubMed] [Google Scholar]
- 63.Allday MJ, Kundu D, Finerty S, et al. CpG methylation of viral DNA in EBV-associated tumors. Int J Cancer 1990;45:1125–30. [DOI] [PubMed] [Google Scholar]
- 64.Robertson KD, Hayward SD, Ling PD, et al. Transcriptional activation of the Epstein-Barr virus latency C promoter after 5-azacytidine treatment: evidence that demethylation at a single CpG site is crucial. Mol Cell Biol 1995;15:6150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robertson KD, Manns A, Swinnen LJ, et al. CpG methylation of the major Epstein-Barr virus latency promoter in Burkitt’s lymphoma and Hodgkin’s disease. Blood 1996;88:3129–36. [PubMed] [Google Scholar]
- 66.Robertson KD, Ambinder RF. Methylation of the Epstein-Barr virus genome in normal lymphocytes. Blood 1997;90:4480–4. [PubMed] [Google Scholar]
- 67.Tao Q, Swinnen LJ, Yang J, et al. Methylation status of the Epstein-Barr virus major latent promoter C in iatrogenic B cell lymphoproliferative disease—application of PCR-based analysis. Am J Pathol 1999;155:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Minarovits J, Hu, LF, Minarovits-Kormuta S, et al. Sequence-specific methylation inhibits the activity of the Epstein-Barr virus LMP1 and BCR2 enhancer-promoter regions. Virology 1994;200:661–7. [DOI] [PubMed] [Google Scholar]
- 69.Hu LF, Minarovits J, Cao SL, et al. Variable expression of latent membrane protein in nasopharyngeal carcinomas can be related to methylation status of the Epstein-Barr virus BNLF-1 5‘ flanking region. J Virol 1991;65:1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robertson KD. The role of DNA methylation in modulating Epstein-Barr virus gene expression. Curr Top Microbiol Immunol 2000;249:21–34. [DOI] [PubMed] [Google Scholar]
- 71.Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 1985;43:831–40. [DOI] [PubMed] [Google Scholar]
- 72.Wang D, Liebowitz D, Kieff E. The truncated form of the Epstein Barr virus latent-infection membrane protein does not transform rodent fibroblasts. J Virol 1988;62:2337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moorthy RK, Thorley-Lawson DA. All three domains of the Epstein-Barr virus-coded latent membrane protein LMP-1 are required for transformation of rodent fibroblasts. J Virol 1993;67:1638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yanai H, Takada K, Shimizu N, et al. Epstein-Barr virus infection in non-carcinomatous gastric epithelium. J Pathol 1997;183:293–8. [DOI] [PubMed] [Google Scholar]
- 75.Sugiura N, Imai S, Tokunaga M, et al. Transcriptional analysis of Epstein-Barr virus gene expression in EBV-positive gastric carcinoma: unique viral latency in the tumour cells. Br J Cancer 1996;74:625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.zur Hausen A, Brink AATP, Craanen ME, et al. Unique transcription pattern of Epstein-Barr Virus (EBV) in EBV-carrying gastric adenocarcinomas: expression of the transforming BARF1 gene. Cancer Res 2000;60:2745–8. [PubMed] [Google Scholar]
- 77.Wei MX, Ooka T. A transforming function of the BARF1 gene encoded by Epstein-Barr virus. EMBO J 1989;8:2897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei MX, de Turenne-Tessier M, Decaussin G, et al. Establishment of a monkey kidney epithelial cell line with the BARF1 open reading frame from Epstein-Barr virus. Oncogene 1997;14:3073–81. [DOI] [PubMed] [Google Scholar]
- 79.Wei MX, Moulin J-C, Decaussin G, et al. Expression and tumorigenicity of the Epstein-Barr virus BARF1 gene in human Louckes B-lymphocyte cell line. Cancer Res 1994;54:1843–8. [PubMed] [Google Scholar]
- 80.Strockbine LD, Cohen JI, Farrah T, et al. The Epstein-Barr virus BARF1 gene encodes a novel, soluble colony-stimulating factor-1 receptor. J Virol 1998;72:4015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hamilton JA. CSF-1 signal transduction. J Leukoc Biol 1997;62:145–55. [DOI] [PubMed] [Google Scholar]
- 82.Halling KC, Harper J, Moskaluk CA, et al. Origin of microsatellite instability in gastric cancer. Am J Pathol 1999;155:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kang GH, Shim YH, Ro JY. Correlation of methylation of the hMLH1 promoter with lack of expression of hMLH1 in sporadic gastric carcinomas with replication error. Lab Invest 1999;79:903–9. [PubMed] [Google Scholar]
- 84.Di Croce L, Raker VA, Corsaro M, et al. Methyltransferase recruitment and DNA hypermethylation of target promoters by an oncogenic transcription factor. Science 2002;295:1079–82. [DOI] [PubMed] [Google Scholar]