Abstract
Background/Aims: Paediatric primary follicular lymphoma of the testis (PPFLT) is exceptional: the few reported cases seem to lack BCL-2 gene rearrangement and/or protein expression. The aim of this study was to characterise a PPFLT arising in a 4 year old boy.
Methods: This case was characterised using conventional histological analysis, immunohistochemistry, and a polymerase chain reaction based method for the detection of immunoglobulin VH chain rearrangements.
Results: The neoplasm was staged IE/A; left orchiectomy and chemotherapy were performed, producing complete remission. Histology showed a predominantly follicular lymphoid infiltrate mainly composed of centroblast-like cells. The phenotype was CD20+, CD79a+, CD10+, bcl-6+, B cell specific activating protein+, κ light chain+, CD30−/+, interferon regulating factor 4−/+, c-myc−/+, λ light chain−, CD3−, bcl-2−, p53−, cytokeratin−, and placental alkaline phosphatase−. Lymphomatous elements were found within a CD21+ follicular dendritic cell network and 70% were positive for Ki-67/MIB-1. Molecular analysis revealed monoclonal immunoglobulin heavy chain gene rearrangement and BCL-6 mutations, in the absence of BCL-2 major breakpoint and BCL-2 minor cluster region rearrangements, p53 mutations, and death associated protein kinase gene hypermethylation.
Conclusions: These findings suggest a different pathogenesis of PPTFL compared with adult follicular lymphoma and might explain its favourable course in spite of aggressive histology.
Keywords: follicular lymphoma, testis, childhood, immunohistochemistry, molecular biology
Follicular lymphoma (FL) is one of the most common lymphomas: it usually occurs in adults, involves lymph nodes, and may finally transform into a diffuse large B cell lymphoma.1,2 Three morphological grades are recognised,1,2 namely: grades I and II are indolent tumours that regularly carry the t(14;18) translocation with Bcl-2 protein overexpression; grade III presents aggressively with a greater tendency to evolve into diffuse large B cell lymphoma and can show different chromosomal aberrations, lacking detectable BCL-2 mutations and Bcl-2 protein expression in a minority of cases.1–6 Paediatric testicular tumours most frequently display germ cell derivation, whereas gonadal stromal neoplasms, rhabdomyosarcomas, leukaemias, and lymphomas are much more rare.7,8 In general, lymphomas occur in the elderly, with aggressive histology and poor prognosis.9 Thus, the occurrence of a primary testicular lymphoma in children is noteworthy per se ,10–15 and particularly unusual when of the follicular type, as shown by the few reports in the literature.13–15
We report an additional example of primary FL of the testis in a 4 year old boy.
CASE REPORT
In September 1999, a 4 year old boy was admitted to another hospital with left testis swelling without other signs and symptoms. On the incision biopsy a diagnosis of possible high grade lymphoma was made. The patient then entered the haematological oncology unit of the paediatric department of Bologna University. No history of infectious diseases was recorded, blood laboratory tests were unremarkable (including lactic dehydrogenase, α fetoprotein, and serology for Epstein-Barr virus, cytomegalovirus, hepatitis C virus, and Mycobacterium tuberculosis). Both total body axial computed tomography and nuclear magnetic resonance showed an enlarged (20 mm across) left testis with non-homogeneous structure of the upper pole and an 8 mm diameter cyst in the left kidney that remained unmodified in the follow up scans. The remaining organs and apparata were unremarkable. Bilateral bone marrow biopsies were slightly hypocellular and disease free; the cerebrospinal fluid was normal. Left orchiectomy and right testis incisional biopsy were performed. The diagnosis was made at the pathology unit of the institute of haematology and clinical oncology of Bologna University. High dose chemotherapy according to the Berlin-Frankfurt-Munster group16 was administered with complete remission. At the last follow up (January 2002), the patient was well and remained in complete remission.
MATERIALS AND METHODS
Tissue samples were divided into two portions, one of which was cryopreserved in liquid nitrogen and stored at −80°C, whereas the other was fixed in 10% buffered formalin for 24 hours and then routinely processed. Sections were stained with haematoxylin and eosin, Giemsa, periodic acid Schiff (with and without diastase digestion), and Gomori silver impregnation. Immunohistochemistry was performed on a TechMate 500 immunostainer using the alkaline phosphatase antialkaline phosphatase (APAAP) technique and a panel of antibodies, which included reagents against: CD3, CD20, CD21, CD30, CD45, immunoglobulin (Ig) κ and λ light chains, Bcl-2 protein, p53, wide spectrum cytokeratins, placental alkaline phosphatase (PLAP), Ki-67 proliferation associated nuclear antigen (antibodies from Dako, Glostrup, Denmark), CD79a (from Professor DY Mason, Oxford, UK), CD68, interferon regulating factor 4 (IRF4), Bcl-6 protein (from Professor B Falini, Perugia, Italy), B cell specific activating protein (BSAP) (from Santa Cruz, California, USA), c-Myc, and CD10 (from Novocastra, Newcastle, UK). Antigen retrieval was performed as reported previously.17 Positive and negative controls were always used. The positive neoplastic cells were graded semiquantitatively as: +, 75–100%; +/−, 50–75%; −/+, 25–50%; rare, 1–25%; and −, 0%. The staining intensity and pattern of positivity were recorded. In situ hybridisation (ISH) for Epstein-Barr virus (EBV) was performed with EBER 1 and 2 (EBV encoded small non-coding RNAs) probes (Dako).17
For molecular studies, DNA was extracted from fixed and frozen material according to previously reported methods and amplified by a polymerase chain reaction (PCR) based method for the detection of IgVH chain rearrangements.17 Oligonucleotides homologous to the conserved sequences of the leader, framework II and III regions, and to the joining region of the heavy chain (JH) consensus region were used as primers. A two step amplification of BCL-2/IgVH rearrangement at the major breakpoint and minor cluster regions was performed, according to previous reports.17 Mutations of the BCL-6 and p53 genes, in addition to the methylation status of the p73, DAP kinase (death associated protein kinase), and MGMT (06-methylguanine-DNA-methyltransferase) genes were analysed as described previously.18–21
PATHOLOGICAL FINDINGS
The upper pole of the left testis was replaced by a tan/grey, fleshy, poorly demarcated tissue without macroscopic infiltration of the tunica albuginea, epididymis, and spermatic cord.
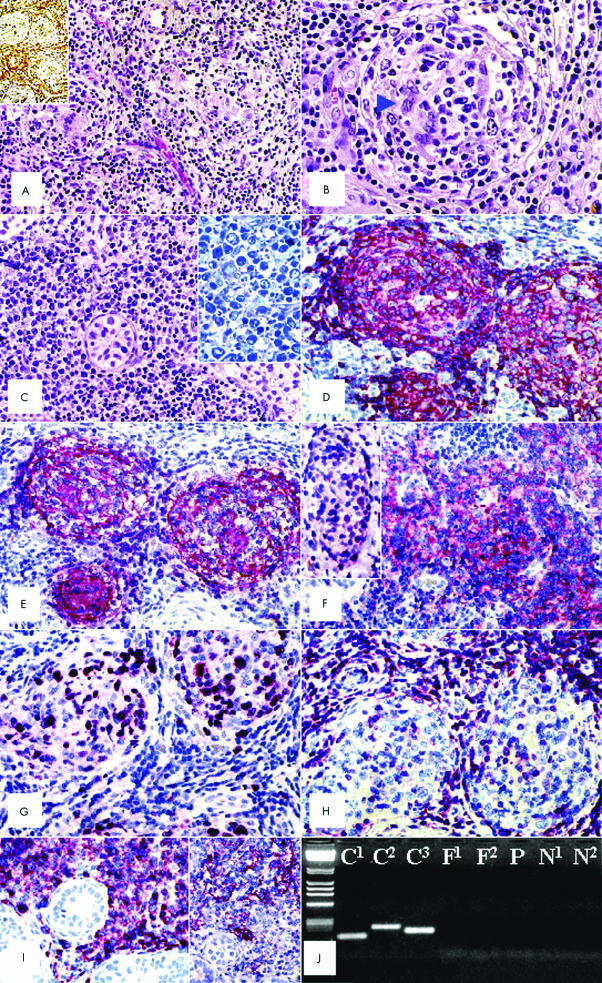
Microscopically, the tumour infiltrated the parenchyma with displacement of seminiferous tubules, showing fibrosis, and mainly growing in “back to back” follicles, without a well defined mantle zone or germinal centre polarisation (fig 1A,B,D); some germinal centres looked fibrotic with follicular dendritic cell (FDC) hyperplasia (fig 1B). Areas with diffuse growth accounted for 25% of the neoplasm (fig 1C). Most tumoral cells were large with centroblastic morphology, although multilobular or centrocytic nuclei were seen (fig 1B,C). Mitoses were numerous. The tunica albuginea, epididymis, spermatic cord, and the right incisional biopsy were negative.
Figure 1.
(A) The tumour shows a prevalent follicular growth pattern (haematoxylin and eosin stained; original magnification, ×100) and shows a certain degree of fibrosis, as revealed by Gomori silver impregnation for reticulin fibres (inset; original magnification, ×25). (B) At higher magnification, several centroblasts are seen within a neoplastic follicle, which lacks cell polarisation and a mantle zone, looks fibrotic, and contains hyperplastic follicular dendritic cells (one of which is arrowed) (haematoxylin and eosin stained; original magnification, ×400). (C) An area with a diffuse growth pattern; note the residual seminiferous tubule (haematoxylin and eosin stained; original magnification, ×300). The lymphomatous population mostly consists of centroblast-like cells (inset; Giemsa staining; original magnification, ×400). (D) Three neoplastic follicles laying “back to back” show a strong CD20 positivity (APAAP technique; Gill's haematoxylin nuclear counterstaining; original magnification, ×250). (E) The same follicles display a prominent CD21+ follicular dendritic cell meshwork (APAAP technique; Gill's haematoxylin nuclear counterstaining; original magnification, ×250). (F) Lymphomatous elements strongly express CD10 (APAAP technique; Gill's haematoxylin nuclear counterstaining; original magnification, ×300) and the Bcl-6 protein (inset; original magnification, ×250). (G) Most of the tumour cells are in the cell cycle, as revealed by Ki-67/Mib-1 staining (APAAP technique; Gill's haematoxylin nuclear counterstaining; original magnification, ×300). (H) Negative staining of neoplastic follicles for the Bcl-2 protein; those cells that have stained are T cells (APAAP technique; Gill's haematoxylin nuclear counterstaining; original magnification, ×300). (I) In an area with a diffuse growth pattern, lymphomatous elements strongly express the CD79a molecule and are admixed with some CD21+ follicular dendritic cells (inset) (APAAP technique; Gill's haematoxylin nuclear counterstaining; original magnification, ×200). (J) No BCl-2 gene rearrangement is seen; lane 1, molecular weight markers; C1–3, positive controls; N1–2, negative controls; P, formalin-fixed, paraffin wax embedded material; F1–2, frozen tissue.
Lymphomatous elements were strongly positive for CD20, CD79a, CD10, BSAP, and Bcl-6 protein with monotypic κ light chain Ig restriction and a MIB-1 value of 70% (fig 1D,F,G,I). Approximately 10% of neoplastic cells expressed CD30, IRF4, and c-Myc. Bcl-2, p53, cytokeratins, and PLAP were negative; the anti-CD21 antibody showed tight FDC networks and loosely dispersed FDCs in the follicular and diffuse areas, respectively (fig 1E,I). The reactive T cells admixed were positive for CD3 and Bcl-2 (fig 1H). EBV integration was not demonstrable by ISH.
Analysis of the IgVH genes revealed the presence of a single PCR product, corresponding to a monoclonally rearranged VH gene. The DP-7/21-2 of the VH1 gene family was productively rearranged with the D2-15/D2 D segment and the JH6b gene. The VH gene utilised was somatically mutated, at a relatively low frequency (4.7 × 10−2 bp). BCL-6 mutations in a heterozygous condition were detected at a frequency of 4.1 × 10−3 bp at nucleotides 1069 (A → C), 1071 (A → C), 1072 (A → G), 1075 (C → A), 1085 (C → A), and 1109 (T → C). No p53 mutations or BCL-2 rearrangements were detected. Frozen and routine samples showed identical results (fig 1J). No methylation of the MGMT, DAP kinase, or p73 genes was observed.
Based on these findings, a diagnosis of peripheral B cell lymphoma, follicular, grade 3/3 (75%) with diffuse large B cell lymphoma (25%) was made, which was confirmed at the Workshop of the European Association for Haematopathology held in London, May 7–11, 2000.
DISCUSSION
In adulthood, testicular lymphoma is not infrequent and mostly corresponds to the diffuse large B cell variety.9 In childhood, it is rare and when it is seen it usually represents a secondary involvement by an advanced stage Burkitt's or lymphoblastic lymphoma.9 Only six reports of primary testicular lymphoma in children are quoted in the literature and in the most up to date ones the tumour was of the FL type (table 1).10–15
Table 1.
Main clinical, pathological, and biological features of the cases of primary paediatric follicular lymphoma of the testis quoted in the literature compared with the ones of the reported case
| Case (ref) | Age (years) | Testis | Tumour size | Stage | Histo | Grade | Treat | Outcome | Bcl-2 | CD10 | Bcl-6 | p53 | IgH/R | IgH/M | BCL-2/R | BCL-6/R | BCL-6/M | DAP |
| 1 (13) | 8 | Left | 2.0 cm | IE | F | 3 | OR | NED/44 mo* | – | NA | NA | NA | Clonal | NA | – | NA | NA | NA |
| 2 (14) | 3 | Left | 2.8 cm | IE | F&D | 3 | OR+CX | NED/18 mo | – | – | + | – | – | NA | – | NA | NA | NA |
| 3 (14) | 3 | Right | 2.3 cm | IE | F | 3 | OR+CX | NED/18 mo | – | – | + | – | Clonal | NA | – | + | NA | NA |
| 4 (14) | 10 | Left | 4.0 cm | IE | F | 3 | OR+CX | NED/19 mo | – | NA | + | – | Clonal | NA | – | NA | NA | NA |
| 5 (14) | 5 | Right | 4.0 cm | IE | F | 3 | OR+CX | NED/24 mo | – | NA | NA | – | NA | NA | NA | NA | NA | NA |
| 6 (15) | 6 | Right | 3.0 cm | IE | F&D | 3 | OR+CX | NED/7 mo | – | + | + | – | NA | NA | – | NA | NA | NA |
| 7 (present case) | 4 | Left | 2.0 cm | IE | F&D | 3 | OR+CX | NED/26 mo | – | + | + | – | Clonal | + | – | NA | + | – |
Bcl-2, Bcl-2 protein immunostaining; Bcl-6, Bcl–6 protein immunostaining; BCL-2/R, BCL-2 gene rearrangement; BCL-6/R, BCL-6 gene rearrangement; BCL-6/M, BCL-6 gene somatic mutations; CD10, CD10 immunostaining; CX, chemotherapy; DAP, DAP kinase gene hypermethylation; E, extranodal; F, follicular; F&D, follicular and diffuse; Histo, histology; IgH/M, IgVH gene somatic mutations; IgH/R, IgVH gene clonal rearrangement; NA, not available; NED, no evidence of disease; OR, orchiectomy; p53, p53 product immunostaining; Treat, treatment; –, negative result; +, positive result.
*Follow up updated in Finn et al.14
“The tumour seems to lack protection against apoptosis and to maintain efficient DNA repair mechanisms”
Our case represents a further example of PPFLT (table 1), which confirms the peculiar clinicopathological characteristics of the tumour, provides new molecular information, and (more in general) contributes to the existing debate on the biology of FL in childhood.22 The germinal centre cell origin of the tumour was supported by its prevalent follicular aggregation, the expression of the CD10 and Bcl-6 proteins, the detection of tight FDC meshworks, and the occurrence of somatic mutations of both the IgVH and BCL-6 genes.1,2 Interestingly, CD10 positivity had previously been recorded in only one instance15 (table 1): the variable results of the search for this molecule (which in conjunction with the Bcl-6 protein is a reliable marker of normal germinal centre B cells and germinal centre cell derived lymphomas)23 might result from the usual high grade histology of PPFLT (table 1). In fact, negative or weak CD10 expression has recently been detected in 83% of grade III Fls.24 The presence of somatic mutations of the IgVH and BCL-6 genes, not analysed in previous series (table 1), provides further evidence of the germinal centre cell derivation of the tumour. In fact, these mutations characteristically occur in antigenically stimulated B cells, which enter the germinal centre, carry out Ig class switching, rearrange their Ig V region to recognise specific antigenic determinants, and undergo selection based on the affinity of the antibodies produced.25,26
In agreement with previous reports13–15 (table 1), our case was molecularly characterised by the lack of BCL-2 gene rearrangement and p53 mutations, along with negativity for the corresponding protein products. In addition, our patient did not show MGMT, DAP kinase, or p73 gene hypermethylation.19–21 These findings—possibly in conjunction with the limited extent of the disease (all patients having been staged IE)—might explain the excellent therapeutic response of PPFLT, in spite of the high grade histology and the detection of a diffuse component in three instances (table 1). In fact, the tumour seems to lack protection against apoptosis and to maintain efficient DNA repair mechanisms. Furthermore, the results suggest that PPFLT and childhood FL in general might have a different pathogenesis to FL in adults. In particular, 83–100% of grade I/II and approximately 75% of grade III adulthood FLs carry the t(14;18)(q32;q21) translocation, with consequent BCL-2 gene rearrangement, Bcl-2 protein overexpression, and protection of neoplastic cells from apoptosis.1–3,22 Because of this, Bcl-2 staining is often used for the differential diagnosis between FL (Bcl-2+) and follicular hyperplasia (Bcl-2−). In adult FL, apoptosis is also compromised by the inactivation of the proapoptotic DAP kinase gene as a result of promoter hypermethylation.20 In addition, alterations of the p53 tumour suppressor gene, and BCL-6 gene rearrangements and/or the accumulation of mutations in its 5` non-coding region seem to be involved in grade III adult FL development and/or in the transformation of FL into a diffuse large B cell tumour.1–3,5,27,28 With the exception of BCL-6 gene rearrangement, which was seen in one case by Finn et al,14 and the prognostic value of which is debatable,29 none of these molecular alterations affecting tumour resistance and progression has so far been seen in PPFLT (table 1). In particular, the lack of BCL-2 gene rearrangement and Bcl-2 protein expression might not be exclusive to PPFLT, but also be a characteristic feature of childhood FL. In fact, Lorsbach et al have recently reported their absence in 12 of 17 FLs in children, all of which occurred in patients younger than 12 years.22 Interestingly, similar to PPFLT, most of Lorsbach's patients had high grade FL, were stage I, and had an excellent outcome, with the exception of the BCL-2 positive patients, who died of their disease or required aggressive treatment with peripheral blood stem cell transplantation. Based on these findings, Lorsbach et al concluded that “in most instances FL in children has a pathogenesis that is distinct from its counterpart in the adult population”.22
Finally, PPFLT should always be differentiated from follicular hyperplasia in the course of chronic orchitis. Because the lack of Bcl-2 protein expression and BCL-2 gene rearrangement can make the diagnosis of FL less obvious, a series of parameters should always be considered, including the absence of previous infectious diseases, negativity for EBV at ISH, the lack of inflammation or granulomas in the epididymis and spared testis, back to back follicular growth pattern, non-polarised germinal centres, almost exclusive B cell antigen expression, Ig light chain restriction, and the detection of a monoclonal Ig gene rearrangement. However, it should be emphasised that the absence of monoclonal Ig gene rearrangement using conventional PCR analysis on routine material does not exclude the diagnosis of PPFLT; in fact, a negative result was recorded in one of the reported cases (table 1), which might have been produced by the limitations of amplifying DNA extracted from paraffin wax blocks30 and/or the need for a large series of primers to detect rare forms of rearrangement, such as the one found in our case.
“Furthermore, the results suggest that paediatric primary follicular lymphoma (FL) of the testis and childhood FL in general might have a different pathogenesis to FL in adults”
Take home messages.
Paediatric primary follicular lymphoma of the testis and childhood follicular lymphoma (FL) in general may have a different pathogenesis to adult FL
This might explain its favourable course despite its aggressive histology
Further studies based on microarray techniques are needed to establish firmly whether childhood FL is pathogenetically different from the adult form, irrespective of the primary site
In conclusion, further studies based on the newly developed microarray techniques31 are needed to establish firmly whether childhood FL is indeed pathogenetically different from the adult form, irrespective of the primary site.
Acknowledgments
This paper was supported by grants from AIRC (Milan), MURST (Rome), and Fondazione “Piera Pietro e Giovanni Ferrero” (Alba). The authors thank Mr L Chilli and Mrs F Sandri for their skilled technical assistance.
Abbreviations
APAAP, alkaline phosphatase antialkaline phosphatase
BSAP, B cell specific activating protein
DAP, death associated protein
EBV, Epstein-Barr virus
FDC, follicular dendritic cell
FL, follicular lymphoma
Ig, immunoglobulin
IRF4, interferon regulating factor 4
ISH, in situ hybridisation
MGMT, 06-methylguanine-DNA-methyltransferase
PCR, polymerase chain reaction
PLAP, placental alkaline phosphatase
PPFLT, paediatric primary follicular lymphoma of the testis
REFERENCES
- 1.Harris NL, Jaffe ES, Stein H, et al. A revised European–American classification of lymphoid neoplasms: a proposal from the international lymphoma study group. Blood 1994;84:1361–92. [PubMed] [Google Scholar]
- 2.Nathwani BN, Piris MA, Harris NL, et al. Follicular lymphoma. In: Jaffe ES, et al, eds. Tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press, International Agency for Research on Cancer, 2001:162–7.
- 3.Weisenburger DD, Gascoyne RD, Bierman PJ, et al. Clinical significance of the t(14;18) and BCL2 overexpression in follicular large cell lymphoma. Leuk Lymphoma 2000;36:513–23. [DOI] [PubMed] [Google Scholar]
- 4.Capello D, Vitolo U, Pasqualucci L, et al. Distribution and pattern of BCL-6 mutations throughout the spectrum of B-cell neoplasia. Blood 2000;95:651–9. [PubMed] [Google Scholar]
- 5.Lo Coco F, Gaidano G, Louie DC, et al. p53 mutations are associated with histologic transformation of follicular lymphoma. Blood 1993;82:2289–95. [PubMed] [Google Scholar]
- 6.Yano T, Jaffe ES, Longo DL, et al. MYC rearrangements in histologically progressed follicular lymphomas. Blood 1992;80:758–67. [PubMed] [Google Scholar]
- 7.Murphy SB, Fairclough DL, Hutchinson RE, et al. Non-Hodgkin lymphoma of childhood: an analysis of the histology, staging, and response to treatment of 338 cases at a single institution. J Clin Oncol 1989;7:186–93. [DOI] [PubMed] [Google Scholar]
- 8.Key R. Prepubertal testicular tumor registry. J Urol 1993;150:671–4. [DOI] [PubMed] [Google Scholar]
- 9.Zucca E, Conconi A, Mughal TI, et al. Patterns of survival in primary diffuse large B-cell lymphoma (DLCL) of the testis: an international survey of 373 patients. 42nd annual meeting of the American Society of Hematology, December 1–5, 2000, San Francisco[abstract]. Blood 2000;96:A1443. [Google Scholar]
- 10.Turley HK, Moore TD. Malignant lymphoma primary manifested as a testicular tumor. J Urol 1952;68:744–6. [DOI] [PubMed] [Google Scholar]
- 11.Weitzner S, Gropp A. A primary reticulum sarcoma of testis in a 12-year-old boy. Cancer 1976;37:935–8. [DOI] [PubMed] [Google Scholar]
- 12.Rao SP, Miller ST, Menll J, et al. Localized non-Hodgkin's lymphoma of testis in a child. Am J Pediatric Hematol Oncol 1993;15:443. [PubMed] [Google Scholar]
- 13.Moertel CL, Watterson J, McCormick SR, et al. Follicular large cell lymphoma of testis in a child. Cancer 1995;75:1182–6. [DOI] [PubMed] [Google Scholar]
- 14.Finn LS, Viswanatha DS, Belasco JB, et al. Primary follicular lymphoma of the testis in childhood. Cancer 1999;85:1626–35. [DOI] [PubMed] [Google Scholar]
- 15.Lu D, Medeiros J, Eskenazi AE, et al. Primary follicular large cell lymphoma of the testis in a child. Arch Pathol Lab Med 2001;125:551–4. [DOI] [PubMed] [Google Scholar]
- 16.Reiter A, Schrappe M, Parwaresh R, et al. Non-Hodgkin's lymphomas of childhood and adolescence: results of a treatment stratified for biologic subtypes and stage—a report of the Berlin-Frankfurt-Munster group. J Clin Oncol 1995;13:359–72. [DOI] [PubMed] [Google Scholar]
- 17.Pileri SA, Zinzani PL, Ascani S, et al. Diffuse large B-cell lymphoma with primary retroperitoneal presentation: clinico-pathologic study of nine cases. Ann Oncol 2001;12:1445–53. [DOI] [PubMed] [Google Scholar]
- 18.Gaidano G, Ballerini P, Gong JZ, et al. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 1991;88:5413–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corn PG, Kuerbitz SJ, van Noesel MM, et al. Transcriptional silencing of the p73 gene in acute lymphoblastic leukemia and Burkitt's lymphoma is associated with 5` CpG island methylation. Cancer Res 1999;59:3352–6. [PubMed] [Google Scholar]
- 20.Katzenellenbogen RA, Baylin SB, Herman JG. Hypermethylation of the DAP-kinase CpG island is a common alteration in B-cell malignancies. Blood 1999;93:4347–53. [PubMed] [Google Scholar]
- 21.Esteller M, Gaidano G, Goodman SN, et al. Hypermethylation of the DNA repair gene O(6)-methylguanine DNA methyltransferase and survival of patients with diffuse large B-cell lymphoma. J Natl Cancer Inst 2002;94:26–32. [DOI] [PubMed] [Google Scholar]
- 22.Lorsbach RB, Shay-Seymore D, Moore JL, et al. Clinicopathologic analysis of follicular lymphoma occurring in children. Blood 2002;99:1959–64. [DOI] [PubMed] [Google Scholar]
- 23.Ree HJ, Yang WI, Kim CW, et al. Coexpression of Bcl-6 and CD10 in diffuse large B-cell lymphomas: significance of Bcl-6 expression patterns in identifying germinal center B-cell lymphoma. Hum Pathol 2001;32:954–62. [DOI] [PubMed] [Google Scholar]
- 24.Eshoa C, Perkins S, Kampalath B, et al. Decreased CD10 expression in grade III and interfollicular infiltrates of follicular lymphoma. Am J Clin Pathol 2001;115:862–7. [DOI] [PubMed] [Google Scholar]
- 25.Harris MB, Chang CC, Berton MT, et al. Transcriptional repression of Stat6-dependent interleukin-4-induced genes by BCL-6: specific regulation of iepsilon transcription and immunoglobulin E switching. Mol Cell Biol 1999;19:7264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen HM, Peters A, Baron B, et al. Mutation of BCL-6 gene in normal B-cells by the process of somatic hypermutation of Ig genes. Science 1998;280:1750–2. [DOI] [PubMed] [Google Scholar]
- 27.Szereday Z, Csernus B, Nagy M, et al. Somatic mutation of the 5` noncoding region of the BCL-6 gene is associated with intraclonal diversity and clonal selection in histological transformation of follicular lymphoma. Am J Pathol 2000;156:1017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lossos IS, Levy R. Higher-grade transformation of follicle center lymphoma is associated with somatic mutation of the 5` noncoding regulatory region of the BCL-6 gene. Blood 2000;96:635–9. [PubMed] [Google Scholar]
- 29.Pescarmona E, De Sanctis V, Pistilli A, et al. Pathogenetic and clinical implications of BCL-6 and BCL-2 gene configuration in nodal diffuse large B-cell lymphoma. J Pathol 1997;183:281–6. [DOI] [PubMed] [Google Scholar]
- 30.Albrecht S, Bruner JM, Segall GK. Immunoglobulin heavy chain rearrangements in primary brain lymphomas. A study using PCR to amplify CDR-III. J Pathol 1993;169:297–302. [DOI] [PubMed] [Google Scholar]
- 31.Husson H, Carideo EG, Neuberg D, et al. Gene expression profiling of follicular lymphoma and normal germinal center B cells using cDNA arrays. Blood 2002;99:282–9. [DOI] [PubMed] [Google Scholar]