Abstract
The occurrence of pelvic endometriosis is not uncommon but hepatic endometriosis is extremely rare. Only five such cases of hepatic endometriosis have been described in the literature. This report concerns another patient with hepatic endometriosis forming a large cystic mass. The clinicopathological features and the possible pathogenesis are discussed. Endometriosis should be considered in the differential diagnosis of a cystic liver mass, particularly in patients with known endometriosis.
Keywords: endometriosis, endometrial cyst, liver
Endometriosis is a benign condition characterised by the presence and proliferation of endometrial tissue in sites outside the endometrial cavity. It is usually confined to the pelvis and reproductive organs, but has been described in several remote sites including the omentum, gastrointestinal tract, peritoneum, operative scars, lymph nodes, umbilicus, skin, lungs, pleura, bladder, kidney, and pancreas.1–9 Hepatic endometriosis is extremely rare. To our knowledge, only five such cases have been reported in the literature.10–14 In this report, we describe a patient with hepatic endometriosis forming a large cystic mass presenting eight years after hysterectomy and salpingo-oophorectomy for endometriosis. Previously reported cases are reviewed.
“Endometriosis is usually confined to the pelvis and reproductive organs, but has been described in several remote sites including the omentum, gastrointestinal tract, peritoneum, operative scars, lymph nodes, umbilicus, skin, lungs, pleura, bladder, kidney, and pancreas”
CASE REPORT
A 56 year old woman presented with intermittent epigastric pain for many years, which was dull and was not associated with menstruation. She was referred to us because of a large tumour mass found on abdominal ultrasonograms. She had a history of endometriosis involving both fallopian tubes, ovaries, cervix, and pouch of Douglas, and had undergone hysterectomy and bilateral salpingo-oophorectomy eight years previously. On physical examination, no definite abdominal mass lesion was palpable and no lymphadenopathy was noted. Complete blood count and biochemical tests were normal. The α fetoprotein value was normal at < 0.3 ng/ml and serological tests for hepatitis B surface antigen and anti-hepatitis C virus antibodies were negative. Abdominal ultrasonography and subsequent nuclear magnetic resonance and computed tomography scans (fig 1A) showed a well circumscribed cystic mass of 9.0 × 6.0 cm located in the left lobe of the liver with irregular soft tissue components. Angiography revealed a large hypovascular mass. Fine needle aspiration biopsy was then performed and yielded dark brown fluid and a few atypical columnar epithelial cells. A tentative diagnosis of “suspicious of adenocarcinoma” was made. At laparotomy, a cystic mass was found occupying segment four of the liver. No enlarged lymph nodes were noted at the porta hepatis and the mesenteric root. The mass was completely removed by extended left hepatic lobectomy. The gallbladder appeared normal and was removed simultaneously.
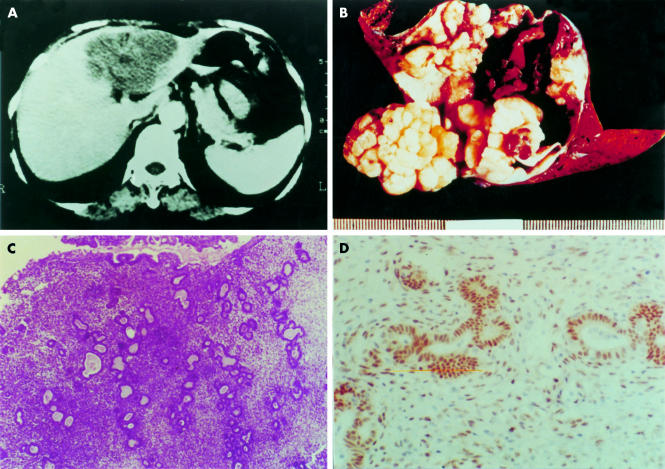
Figure 1.
(A) Computed tomography scan showing a large cystic mass in the left lobe of the liver. (B) Left liver segment opened to show a large cystic mass. After rinsing away of the chocolate coloured content, the inner surface of the cyst appears yellow/white, uneven, and nodular. (C) Photomicrography of the cyst wall shows typical endometrial tissue with both glandular and stromal components (haematoxylin and eosin stained; original magnification, ×13.2). (D) Positive nuclear immunostaining with oestrogen receptor specific antibody in both glandular and stromal cells (DAB chromogen and haematoxylin counterstain; original magnification, ×66).
Pathological examination revealed a 9.0 × 9.0 × 6.0 cm, well circumscribed cystic mass containing dense chocolate coloured fluid. The inner surface was yellow/white, uneven, and nodular (fig 1B). Microscopically, the cyst wall was partially composed of endometrial glandular and stromal elements (fig 1C), characteristic of endometriosis. This was confirmed by positive immunostaining for oestrogen and progesterone receptors (fig 1D) and vimentin in both the glandular and stromal components. The adjacent liver tissue and the gallbladder were normal.
DISCUSSION
Intraparenchymal endometriosis of the liver is extremely rare. The first case was reported by Finkel et al in 1986 in a 21 year old woman who complained of epigastric pain, nausea, and vomiting.10 She was found to have an endometrial cyst measuring 13 cm in diameter located in the left lobe of the liver. Subsequently, four additional cases have been reported. The major clinical features of these previously reported five cases and the present case are summarised in table 1. They occurred in women with an age range from 21 to 62 years. They were cystic and located either in the left or the right lobe of the liver. In four cases, the tumours were solitary and large with a diameter of more than 10 cm. They most often presented with abdominal pain, which was not associated with menses. Three patients had a coexistent or past history of pelvic endometriosis. Four of them had previously undergone pelvic surgery.
Table 1.
Clinical details for six patients with hepatic endometriosis
| Author/ year (ref) | Age (years) | Gross involvement of liver | Tumour size | Location | Symptoms and signs | Coexisting endometriosis | Previous pelvic operation | Treatment |
| Grabb/1986 (11) | 21 | Solitary unilocular cystic mass | 13.5 cm | Left lobe | Chronic epigastric pain with nausea and vomiting, hepatomegaly with right subcostal mass | None | Fallopian tube cyst removed 3 years before | Deroofing, Danazol |
| Rovati/1990 (12) | 37 | Solitary multilocular cystic mass | 10.0 cm | Left lobe | Chronic epigastric pain, epigastric mass | Left ovary, peritoneum | None | Left lateral segmentectomy, Danazol |
| Verbeke/1996 (13) | 34 | Solitary mass | 12.0 cm | Right lobe | Acute abdomen | None | None | Excision |
| Verbeke/1996 (13) | 62 | Solitary cystic mass | 12.0 cm | Left lobe | Right epigastric pain | None | Abdominal operation for Meckel's diverticulum in early childhood | Excision |
| Weinfeld/1998 (14) | 60 | Two cystic masses | 3.1 cm 2.8 cm | Right lobe, falciform ligament* | Right upper abdominal tenderness | Both ovaries, pouch of Douglas | Hysterectomy and bilateral oophorectomy 23 years before, resection of endometriosis adjacent to the urinary bladder 4 years before | Excision of right lobe tumor, left hepatectomy |
| Eng/2002 (present care) | 56 | Solitary multilocular cystic mass | 9.0 cm | Left lobe | Epigastric pain, tender right upper abdominal mass | Both ovaries, uterine cervix, pouch of Douglas | Hysterectomy, bilateral oophorectomy | Left hepatectomy |
*This lesion was complicated by malignant transformation with moderately differentiated endometrioid adenosquamous carcinoma.
“Vascular/lymphatic spread of endometrial fragments offers a better explanation than tubal regurgitation for rare and distant sites of disease”
The pathogenesis of extra-abdominal endometriosis remains uncertain. Many theories have been proposed, including coelomic metaplasia, retrograde menstruation, iatrogenic injury, and haematogeous/lymphatic dissemination.15–17 Metaplasia or differentiation from celomic epithelium, triggered by many stimuli, including hormonal alternation, inflammation, and trauma, has been observed frequently. Transportation of endometrial fragments by one means or another is important to the histogenesis and is seen not infrequently after surgical procedures that involve the endometrium.6,7 Endometrial fragments have been seen in the oviduct and in the peritoneal cavity, in addition to the lymphatics and blood vessels, implicating these vessels as vehicles of endometrial tissue dissemination. Regurgitated menstrual fluid is not rare and may be responsible for the development of pelvic endometriosis; but vascular dissemination probably occurs more frequently after surgical trauma than it does spontaneously.
In our present case the lesion most probably resulted from endometrial fragments transported into the liver by lymphatic or blood vessels during surgery for pelvic endometriosis eight years previously. Although an origin from the peritoneum covering the liver cannot be ruled out completely, this possibility is less likely because the cyst was completely intraparenchymal.
Take home messages.
To our knowledge, this is only the sixth case of hepatic endometriosis to have been described in the literature
The lesion probably resulted from endometrial fragments transported into the liver by lymphatic or blood vessels during surgery for pelvic endometriosis eight years previously
Endometriosis should be considered in the differential diagnosis of a cystic liver mass, particularly in patients with known endometriosis
REFERENCES
- 1.Bergquist A. Extragenital endometriosis, a review. Eur J Sci 1992;158:7–12. [PubMed] [Google Scholar]
- 2.Bergquist A. Different types of extragenital endometriosis: a review. Gynecol Endocrinol 1993;7:207–21. [DOI] [PubMed] [Google Scholar]
- 3.Franklin RR, Navarro C. Extragenital endometriosis. Prog Clin Biol Res 1990;323:289–95. [PubMed] [Google Scholar]
- 4.Markham SM, Carpenter SE, Rock JA. Extrapelvic endometriosis. Obstet Gyncol Clin N Am 1989;16:193–219. [PubMed] [Google Scholar]
- 5.Goodman JD, Macchia R, Macasaet H, et al. Endometriosis of the urinary bladder: sonographic findings. Am J Roentgenol 1980;135:625–6. [DOI] [PubMed] [Google Scholar]
- 6.Miller WB, Melson GL. Abdominal wall endometrioma. Am J Roentgenol 1970;132:467–68. [DOI] [PubMed] [Google Scholar]
- 7.Steck WD, Helwig EB. Cutaneous endometriosis. JAMA 1965;191:167–70. [DOI] [PubMed] [Google Scholar]
- 8.Goswami AK, Sharma SK, Tandon SP, et al. Pancreatic endometriosis presenting as a hypovascular renal mass. J Urol 1986;135:112–13. [DOI] [PubMed] [Google Scholar]
- 9.Marchevsky AM, Zimmerman MJ, Aufses AH, Jr, et al. Endometrial cyst of the pancreas. Gastroenterology 1984;86:1589–91. [PubMed] [Google Scholar]
- 10.Finkel L, Marchevsky A, Cohen B. Endometrial cyst of the liver. Am J Gastroenterol 1986;81:576–68. [PubMed] [Google Scholar]
- 11.Grabb A, Carr L, Goodman JD, et al. Hepatic endometrioma. J Clin Ultrasound 1986;14:478–80. [DOI] [PubMed] [Google Scholar]
- 12.Rovati V, Faleschini E, Vercellini P, et al. Endometrioma of the liver. Am J Obstet Gynecol 1990;163:1490–2. [DOI] [PubMed] [Google Scholar]
- 13.Verbeke C, Harle M, Sturm J. Cystic endometriosis of the upper abdominal organs. Report on three cases and review of the literature. Pathol Res Pract 1996;192:300–4. [DOI] [PubMed] [Google Scholar]
- 14.Weinfeld RM, Johnson SC, Lucas CE, et al. CT diagnosis of perihepatic endometriosis complicated by malignant transformation. Abdom Imaging 1998;23:183–4. [DOI] [PubMed] [Google Scholar]
- 15.Marik JJ. Endometriosis etiology. J Reprod Med 1997;19:301–2. [Google Scholar]
- 16.Malick JE. The etiology of endometriosis. J Am Osteopath Assoc 1982;81:407. [PubMed] [Google Scholar]
- 17.Ridley JH. The histogenesis of endometriosis. Obstet Gynecol Surv 1968;23:1. [Google Scholar]