Abstract
A patient with chronic granulomatous disease who was being treated with steroids was diagnosed with a soft tissue Scedosporium apiospermum infection. Despite extensive treatment with antifungals progression to involve solid tissue (bone) occurred. Treatment required an HLA matched bone marrow transplant, which led to complete clearance of the fungal infection, although the patient subsequently died.
Keywords: Scedosporium apiospermum, chronic granulomatous disease, infection, bone marrow transplantation
Chronic granulomatous disease (CGD) is caused by several genetic defects in the enzymes that produce superoxide within the lysosomes of leucocytes. Most patients affected by GCD suffer from recurrent infection with catalase positive organisms. Failure to eradicate these causes inflammation and abscess formation. Antibiotic treatment may alleviate this. In addition, there is an impaired ability to eradicate certain fungi, notably aspergillus. Recurrent infection causes high morbidity and mortality in these patients and leads to a reduced life expectancy.1 Granuloma formation may occur as a result of recurrent infection and inflammation; in hollow organs such as the gut this can lead to obstructive symptoms, which are responsive to steroids.2–5 To be successful, treatment needs to eliminate both the infection and the associated inflammation. The choice of treatment needs to take into account the defective neutrophil killing. Microbiocidal agents are more likely to be effective than microbiostatic agents. The use of steroids may render the patient susceptible to other opportunistic infections. In the event of an untreatable opportunist infection bone marrow transplantation has been used successfully.6,7
Scedosporium apiospermum is a ubiquitous fungus, which is rarely pathological in the normal patient, but can cause invasive infection in neutropenic patients. It is also known to cause infection in the lungs of those who have nearly drowned in ponds.8 It is uncommon in patients with CGD. We can only speculate as to how it occurred in this patient. There was no history of the above exposure.
“The choice of treatment needs to take into account the defective neutrophil killing”
CASE HISTORY
An 18 year old male patient with known CGD was started on antibiotics and interferon γ (INF-γ) for symptomatic and documented gastric outflow obstruction, an 11 kg weight loss, and raised inflammatory markers. Before this he had been prescribed prophylactic co-trimoxazole and itraconazole; however, in the months immediately preceding this episode he had not been taking his medication regularly. Antibiotics and INF-γ did not provide relief. He was also tried on cisapride, erythromycin, and megesterone acetate. After the introduction of prednisolone (30 mg) his symptoms improved, with a 5 kg weight gain over a two month period and a reduction in inflammatory markers.
Since birth, he had suffered several episodes of urinary tract obstruction, which resolved with antibiotic treatment, and several abscesses requiring incision and antibiotics. In the past six months, shadowing at the apex of his right lung was noted; investigations, including bronchial alveolar lavage, were negative.
He began to experience a swinging fever and noted that it coincided with his three times weekly IFN-γ injections. He refused further IFN but continued on the other treatment, notably steroids. There was an increase in the shadowing at the apex of the right lung. A computed tomography scan showed the presence of an abscess (3 × 10 cm), deep to the pectoralis but anterior to the ribs. Aspiration yielded S apiospermum, which, unusually, was reported (after referral to the reference Public Health Laboratory Service (PHLS) mycology unit in Bristol, agar incorporation method) as being sensitive to amphotericin, and of intermediate resistance to other treatment. The abscess was drained surgically and he was treated with liposomal amphotericin (3 mg/kg) for four months. Steroids were discontinued and IFN-γ injections recommenced. He appeared to make a full recovery with healing of the site over nine months.
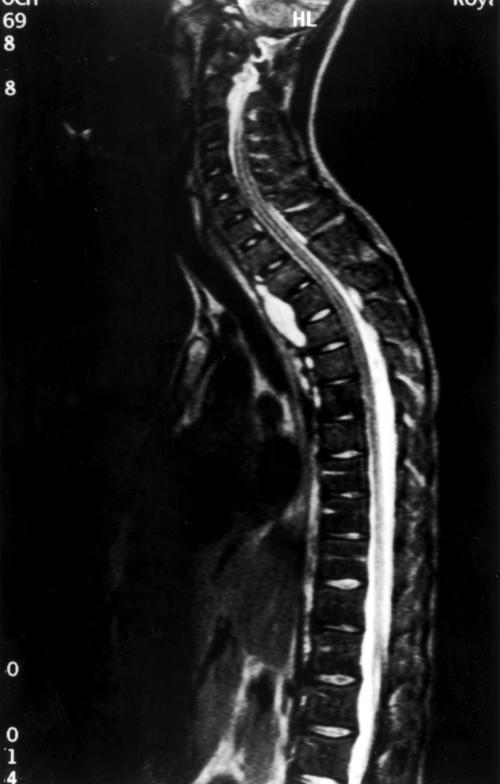
Despite feeling well, his C reactive protein (CRP) had risen to 80 mg/litre. Three months later he developed a swinging fever and pain in his cervical spine. Magnetic resonance imaging showed a paraspinal abscess with radiological evidence of bone infection (fig 1). An operative procedure obtained fluid from the abscess and culture revealed S apiospermum. This isolate was reported to be sensitive (using NCLS-M38p) only to voriconazole (Pfizer; compassionate release programme), which is not fungicidal (PHLS, Bristol). In view of the site of the lesion and the bone involvement, the patient was offered a bone marrow transplant from a human leucocyte antigen (HLA) identical sibling. Using conditioning with busulphan, melpalan, cycosporin A, and Campath 1G the patient was successfully transplanted, with clinical resolution of symptoms relating to the back pain, a normal CRP, and full neutrophil engraftment with cells that were able to produce an oxidative burst. Throughout the period of aplasia the patient was covered with voriconazole. Engraftment was complicated by several episodes of pneumonitis (not related to the original infection), which required tracheostomy. Tragically, the patient succumbed to a late complication of tracheostomy 73 days after transplantation.
Figure 1.
Magnetic resonance imaging scan of the patient.
Necropsy showed no evidence of fungal infection. The paraspinal abscess had completely resolved.
DISCUSSION
We present a rare case of infection with S apiospermum in a patient with CGD treated with steroids. The infection only resolved after treatment by bone marrow transplantation.
The ubiquitous nature of this fungus and the rarity of it as a pathogen in CGD suggest that his infection may relate uniquely to his circumstances. It is possible that the use of steroids, although necessary, could have facilitated this infection, particularly because he had discontinued his IFN-γ injections. The use of steroids, if necessary, must be carefully monitored, although they are reported to alleviate the obstructive pathology that commonly occurs in these patients as a result of the inflammatory reactions.9,10
Other cases of S apiospermum (Pseudoallescheria boydii) in CGD have been reported.11,12 In all cases there was pulmonary involvement at presentation. In three of the cases there was extension to soft tissue. Paraspinal involvement occurred in two patients11,12 after treatment with amphotericin, which has led to the suggestion by Jabado and colleagues11 that this may predispose to subsequent infection with S apiospermum as an opportunistic infection. However, our case and that of Philips et al,12 with no amphotericin before infection, indicate this is unlikely to be true. In patients with CGD, evidence of persistent infection while on adequate treatment for other pathogens, or raised inflammatory markers with chest disease and failure to isolate more common pathogens, should raise the suspicion of S apiospermum.
“It is possible that the use of steroids, although necessary, could have facilitated this infection”
Take home messages.
This unusual fungal infection with Scedosporium apiospermum in the rare condition of chronic granulomatous disease (CGD) may have been caused by further immunosuppression as a result of steroid treatment
This infection had high morbidity, was not responsive to treatment, and probably progressed to involve bone, a dangerous situation in patients with CGD
The new antifungal, voriconazole, showed promise as an agent to contain this infection while the patient was neutropenic
If an HLA matched donor is available, bone marrow transplantation may be the best option for the treatment of bone infections in this group of patients
Was this a single continuous infection, or re-infection? The sensitivity results do not help because resistance may have developed, the assay parameters were changed during the interval between the two isolates (Warnock/Brown, personal communication), and there was no typing of the isolates available at that time. The short time between ending treatment and recurrence, raised CRP, progressive chest x ray changes, and poor weight gain all led us to suspect that this was a continuous infection that failed to respond to treatment. In fact, it probably progressed to involve bone, despite the discontinuation of steroids. In our patient with impaired granulocyte killing, S apiospermum had a slow but relentless course, even when therapeutic immunosuppression was removed.
Recurrent episodes of fungal infection are a feature of CGD. Aspergillus infection carries a 30% mortality.13 In addition, other non-pathogenic fungi can cause systemic infection if there is a large exposure.13,14
Infections at superficial sites may be drained or left open to heal. In CGD, bone infection is particularly dangerous because of the lack of good fungicidal treatments that penetrate bone. The choices are neutrophil transfusions and antibiotics and/or bone marrow transplantation. The survival of an HLA matched transplant is 70% using a minitransplant technique,6 and neutrophil infusions were not shown to have a significantly better outcome.13 Therefore, both the patient and the family face a difficult choice when solid tissue fungal infection occurs.
The patient suffered recurrent fever with IFN injections. It may be that, in the presence of infection, IFN increases the inflammatory response and the morbidity felt by the patient.
In summary, we report a rare fungal infection with S apiospermum in a rare condition, CGD, possibly caused by further immunosuppression resulting from steroid treatment. The infection had high morbidity, was not responsive to current treatment, and indirectly resulted in the death of the patient. The new antifungal, voriconazole, showed promise as an agent to contain this infection while the patient was neutropenic. In this group of patients, bone marrow transplantation may be the only option if an HLA matched donor is available.
Acknowledgments
Thanks to the family, the mycology unit, PHLS, Bristol, and other clinical staff involved in patient care.
Abbreviations
CGD, chronic granulomatous disease
CRP, C reactive protein
IFN-γ, interferon γ
HLA, human leucocyte antigen
PHLS, Public Health Laboratory Service
REFERENCES
- 1.Dinauer MC, Ezekowitz RA. Interferon-gamma and chronic granulomatous disease [review]. Current Opin Immunol 1991;3:61–4. [DOI] [PubMed] [Google Scholar]
- 2.al-Tawil YS, Abramson SL, Gilger MA, et al. Steroid-responsive esophageal obstruction in a child with chronic granulomatous disease (CGD). J Pediatr Gastroenterol Nutr 1996;23:182–5. [DOI] [PubMed] [Google Scholar]
- 3.Korman SH, Lebensart P, Shvil Y. Hydronephrosis caused by ureteric obstruction in chronic granulomatous disease: successful treatment by percutaneous nephrostomy and antibiotic therapy. J Pediatr 1990;116:740–2. [DOI] [PubMed] [Google Scholar]
- 4.Casale AJ, Balcom AH, Wells RG, et al. Bilateral complete ureteral obstruction and renal insufficiency secondary to granulomatous disease. J Urol 1989;142:812–14. [DOI] [PubMed] [Google Scholar]
- 5.Dickerman JD, Colletti RB, Tampas JP. Gastric outlet obstruction in chronic granulomatous disease of childhood. Am J Dis Child 1986;140:567–70. [DOI] [PubMed] [Google Scholar]
- 6.Horwitz ME, Barrett AJ, Brown MR, et al. Treatment of chronic granulomatous disease with nonmyeloablative conditioning and a T-cell-depleted hematopoietic allograft. N Engl J Med 2001;344:881–88. [DOI] [PubMed] [Google Scholar]
- 7.Leung T, Chik K, Li C, et al. Bone marrow transplantation for chronic granulomatous disease: long-term follow-up and review of literature. Bone Marrow Transplant 1999;24:567–70. [DOI] [PubMed] [Google Scholar]
- 8.Richardson MD, Warnock DW. Scediosporium. In: Fungal infection: diagnosis and management. Blackwell scientific publications, 1997:220–2.
- 9.Danziger RN, Goren AT, Becker J, et al. Outpatient management with oral corticosteroid therapy for obstructive conditions in chronic granulomatous disease. J Pediatr 1993;122:303–5. [DOI] [PubMed] [Google Scholar]
- 10.Chin TW, Stiehm ER, Falloon J, et al. Corticosteroids in treatment of obstructive lesions of chronic granulomatous disease. J Pediatr 1987;111:349–52. [DOI] [PubMed] [Google Scholar]
- 11.Jabado N, Casanova JL, Haddad E, et al. Invasive pulmonary infection due to Scedosporium apiospermum in two children with chronic granulomatous disease. Clin Infect Dis 1998;27:1437–41. [DOI] [PubMed] [Google Scholar]
- 12.Phillips P, Forbes JC, Speert DP. Disseminated infection with Pseudallescheria boydii in a patient with chronic granulomatous disease: response to gamma-interferon plus antifungal chemotherapy. Pediatr Infect Dis J 1991;10:536–9. [DOI] [PubMed] [Google Scholar]
- 13.Cohen MS, Isturiz RE, Malech HL, et al. Fungal infection in chronic granulomatous disease. The importance of the phagocyte in defense against fungi. Am J Med 1981;71:59–66. [DOI] [PubMed] [Google Scholar]
- 14.Conrad DJ, Warnock M, Blanc P, et al. Microgranulomatous aspergillosis after shoveling wood chips: report of a fatal outcome in a patient with chronic granulomatous disease. Am J Ind Med 1992;22:411–18. [DOI] [PubMed] [Google Scholar]