Abstract
Aims: The matrix metalloproteinases (MMPs) are a family of proteolytic enzymes collectively capable of degrading all extracellular matrix components, in particular fibrillar collagen. The importance of this group of proteins in the processes of tumour invasion and metastasis is now widely acknowledged. MMP-13 (collagenase 3) has a central role in the MMP activation cascade. The purpose of this study was to investigate the presence and activity of MMP-13 in colorectal cancer and relate these to clinicopathological features.
Methods: Immunohistochemistry for MMP-13 was performed on formalin fixed, paraffin wax embedded sections of a large series of colorectal cancers (n = 249), all of which had uniform clinical and pathological information available. Immunoreactivity to MMP-13 was detected with a monoclonal antibody to MMP-13 using a Dako TechMate™500 automated immunostaining system. The presence and cellular localisation of MMP-13 was assessed using a semiquantitative scoring system. Gelatin zymography was used to detect and measure MMP-13 activity. The zymography was performed on a subset of the cases studied by immunohistochemistry using two groups of 10 paired Dukes’s C tumours and normal samples, selected by either having “good” or “poor” survival.
Results: Immunoreactivity to MMP-13 was identified in 91% of cases and immunoreactivity was localised to the cytoplasm of tumour cells. A high MMP-13 staining score showed a trend towards poorer survival. Tumours had significantly greater MMP-13 activity compared with normal colonic mucosa (p < 0.001). Furthermore, the tumour to normal tissue ratio was significantly higher in the poor survival group (p = 0.02).
Conclusions: These results show that MMP-13 is frequently present and active in colorectal cancer and suggest that the activity of MMP-13 is associated with poorer survival in colorectal cancer.
Keywords: colorectal cancer, matrix metalloproteinase, tumour invasion
Colorectal cancer is the second most common cause of death from cancer in the UK. The prognosis of colorectal cancer depends on the extent of local and metastatic tumour spread, and the degradation of the extracellular matrix (ECM) surrounding tumour cells is a crucial step in tumour invasion.1 The main group of enzymes involved in matrix degradation is the matrix metalloproteinases (MMPs).2,3 The MMPs are a family of proteolytic enzymes collectively capable of degrading all major components of the ECM and they are the only group of enzymes that can degrade fibrillar collagen. It is now widely accepted that the MMPs have a central role in facilitating tumour invasion and metastasis.
“Matrix metalloproteinase 13 (MMP-13) has a central position in the MMP activation cascade, both activating and being activated by several MMPs”
Several MMPs have been investigated previously in colorectal cancer. MMP-1 immunostaining is associated with a poor prognosis in colorectal cancer.4 A high tumour to normal MMP-9 RNA ratio identified by northern blotting predicts shorter disease free and overall survival,5 and MMP-7 secretion correlates with metastatic disease.6 MT1-MMP immunostaining is more frequent in cases with vascular invasion,7 and also correlates with increasing tumour stage.8
MMP-13 (collagenase 3) was first identified in human breast cancer in 1994,9 and is active against a wide variety of ECM components.10 MMP-13 is synthesised as a latent proenzyme and undergoes activation by cleavage of the N-terminal peptide.10 MMP-13 has a central position in the MMP activation cascade, both activating and being activated by several MMPs. MMP-13 is activated by MMP-2, MMP-3, and MT1-MMP and can activate MMP-2 and MMP-9.10–13 It has been found in several types of cancer,14–18 but there have been no previous investigations of this MMP in colorectal cancer. In view of the central role of MMP-13 in the MMP activation cascade the aim of our study was to investigate the presence of MMP-13 immunoreactivity and activity in colorectal cancer.
MATERIALS AND METHODS
Tissue samples
Immunohistochemical analysis of MMP-13 was performed on formalin fixed, paraffin wax embedded sections from 249 consecutive colorectal cancer resections collected over the period 1994–8 at the Aberdeen Royal Infirmary, with approval from the local research ethics committee. Table 1 summarises the clinical and pathological characteristics. One tumour section for each case was analysed by immunohistochemistry.
Table 1.
Patient demographics
| Characteristic | Number | Per cent |
| Sex | ||
| Male | 134 | 53.8 |
| Female | 115 | 46.2 |
| Age | ||
| Mean | 68 | |
| <55 years | 37 | 14.9 |
| ≥55 years | 212 | 85.1 |
| Dukes’s stage | ||
| A | 32 | 12.9 |
| B | 123 | 49.4 |
| C | 94 | 37.8 |
| Lymph node status | ||
| Positive | 94 | 37.8 |
| Negative | 155 | 62.2 |
| Site | ||
| Proximal colon | 90 | 36.1 |
| Distal colon | 100 | 40.2 |
| Rectum | 59 | 23.7 |
| Differentiation of tumour | ||
| Well | 8 | 3.2 |
| Moderate | 205 | 82.3 |
| Poor | 36 | 14.5 |
Proximal colon tumours arose proximal to the splenic flexure (the caecum, ascending colon, or transverse colon); distal colon tumours arose distal to this point (the descending colon or sigmoid colon).
Zymography for MMP-13 activity was performed on 20 matched pairs of frozen tumour and normal colorectal tissue. All the tumours analysed by zymography were a subset of the tumours studied by immunohistochemistry. These tumours were selected from the Aberdeen colorectal cancer tissue bank on the basis of patient outcome in terms of length of survival. All these cases were of Dukes’s C colorectal cancers and consisted of 10 “good” survivors and 10 “poor” survivors. Table 2 summarises the clinical and pathological characteristics of this group of patients. There was a highly significant survival difference between the two groups (p < 0.0001; log rank test).
Table 2.
Clinicopathological characteristics of the patients analysed by gelatin zymography
| Survival group | ||
| Characteristic | Good | Poor |
| Sex | ||
| Male | 4 | 6 |
| Female | 6 | 4 |
| Age | ||
| <55 years | 3 | 4 |
| ≥55 years | 7 | 6 |
| Site | ||
| Proximal colon | 1 | 8 |
| Distal colon | 8 | 2 |
| Rectum | 1 | 0 |
| Tumour differentiation | ||
| Well | 0 | 0 |
| Moderate | 9 | 10 |
| Survival | ||
| Poor | 1 | 0 |
| Mean (months) | 53.8 | 12 |
| Range (months) | 43–60 | 2–26 |
All the tumours were Dukes’s C colorectal cancers.
Immunohistochemistry
Immunohistochemistry was performed using a mouse monoclonal antibody to human MMP-13 (clone 181–15A12, Chemicon International Ltd, Harrow, UK). This antibody, which was raised against recombinant human MMP-13, recognises both the precursor and N-terminal cleaved (active) forms of human MMP-13 and does not crossreact with human MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, or MMP-9 (supplier’s data sheet).
A Dako TechMateTM500 autostainer (Dako, Ely, Cambridgeshire, UK) was used to immunostain 4 μm thick formalin fixed, paraffin wax embedded sections with the mouse anti-MMP-13 antibody at a dilution of 1/400. Initial experiments indicated that an antigen retrieval step was not required. Biotinylated goat antimouse secondary antibody, a complex of avidin with horseradish peroxidase, and diaminobenzidine were used as the detection staining system. Stained slides were examined to identify the cellular localisation of MMP-13 immunoreactivity and scored by two observers (MFL and GIM) for both intensity (−, +, ++, and +++) and proportion (0%, 1–5%, 6–25%, 26–50%, 51–75%, and > 75%) of tumour cells stained. Integer values were assigned to the scores of intensity (0–3) and proportion of tumour cells stained (0–5). These values were multiplied together to provide a single MMP-13 score for each case.
Kaplan-Meier survival analysis was performed with a moving integer cutoff to establish the relation between “low” and “high” MMP-13 scores and survival. Cox’s multivariate analysis was also performed to determine the effects of MMP-13 score, Dukes’s stage, sex, tumour site, and age at diagnosis on survival.
Gelatin zymography
Frozen sections (10 μm in thickness) of tumour and normal tissue were cut using a cryostat and 10 sections from each sample were immediately solubilised in zymogram sample buffer (Bio-Rad Laboratories, Hertfordshire, UK). One section from each case was stained with haematoxylin and eosin to confirm that tumour was present in the tumour sample. The protein concentration of each sample was measured using a Bio-Rad DC protein assay, performed according to the manufacturer’s instructions. Gelatin zymography was performed using precast 10% gelatin gels (Bio-Rad Laboratories). The samples were electrophoretically separated (100 V for two hours), renatured in zymogram renaturation buffer (Bio-Rad) for one hour, and incubated in zymogram incubation buffer (Bio-Rad) at 37°C for 19 hours. The gels were stained to excess using Coomassie blue stain (Bio-Rad) and then carefully destained using destain solution (Bio-Rad), to obtain clear bands of activity against a dark background. The completed gels were then dried and each gel digitally scanned using a flat bed scanner and saved as TIFF files for image analysis.
Measurement of gelatinase activity
The scanned images were analysed using Scion Image for Windows (http://www.scioncorp.com). The image was converted to greyscale and inverted so that subsequent analysis involved the detection of dark bands on a clear background. Beginning at the top of each lane (highest molecular size), mean optical density was measured for each horizontal band of activity. A density profile line graph was then obtained with distance down the gel (decreasing molecular weight) on the x axis and mean optical density (degree of activity) on the y axis. The area under each peak (AUC) was measured and expressed as the number of pixels. AUC measurements were divided by the control sample run in every gel, which allows comparison of samples between gels. The Wilcoxon matched pairs test was used to analyse the AUC values for tumour/normal pairs. A tumour/normal (T/N) ratio of MMP-13 activity was calculated for each case and the Mann-Whitney U test was used to examine the difference in MMP-13 activity between good and poor survival groups.
Statistical analysis
SPSS version 10 for Windows95™ (SPSS UK Ltd, Woking, Surrey, UK) was used for all statistical analyses.
RESULTS
Immunohistochemistry
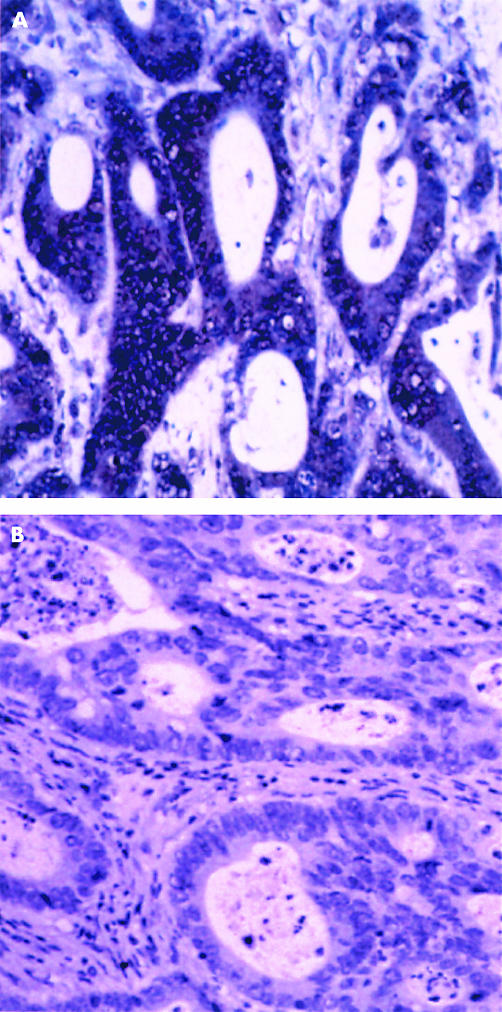
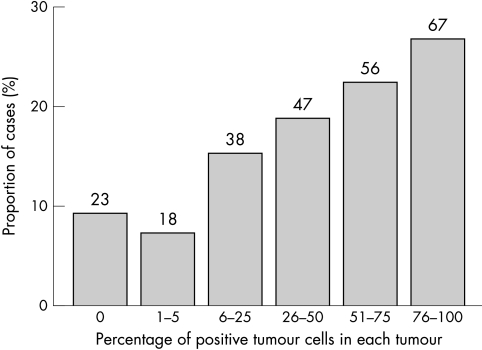
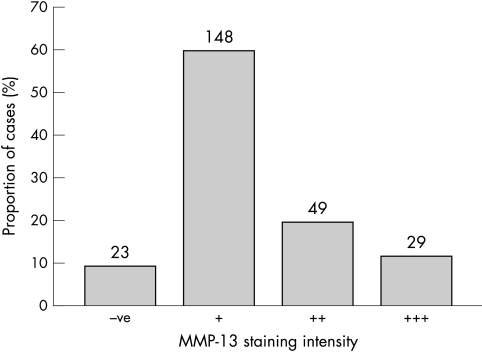
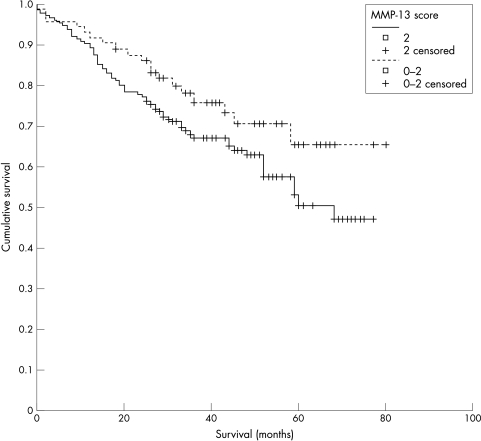
Immunoreactivity to MMP-13 staining was localised to the cytoplasm of tumour cells (fig 1), with only occasional stromal cell staining. Occasionally, nuclear staining was identified in normal epithelium adjacent to tumour cells. Two hundred and twenty six cases showed some degree of positive tumour cell staining (91%). Figure 2 shows the proportion of immunopositive tumour cells in each group, whereas figure 3 shows the intensity of immunostaining for tumours. An MMP-13 cutoff value between 2 and 3 gives 73 cases in the low staining group and 176 in the high group. The mean survivals of the low and high staining groups were 62.5 months and 53.3 months, respectively (p = 0.0875; fig 4). Cox multivariate analysis showed Dukes’s stage to be highly significant in relation to survival (p < 0.001), whereas the MMP-13 score bordered on significance (p = 0.067).
Figure 1.
Immunohistochemical localisation of matrix metalloproteinase 13 (MMP-13) in colorectal cancer. (A) MMP-13 is localised to tumour cells. (B) There is no immunoreactivity when the anti-MMP-13 antibody is omitted from the immunohistochemical protocol.
Figure 2.
The frequency distribution of the percentage of matrix metalloproteinase 13 positive tumour cells in each tumour. The number above each column represents the number of cases in that group.
Figure 3.
The frequency distribution for the intensity of matrix metalloproteinase 13 (MMP-13) immunohistochemical staining in colorectal cancer. The number above each column represents the number of cases in that group.
Figure 4.
Comparison of survival in groups with high (> 2) and low (≤ 2) matrix metalloproteinase 13 (MMP-13) staining scores. There is a trend towards a survival difference (p = 0.0875).
Gelatin zymography
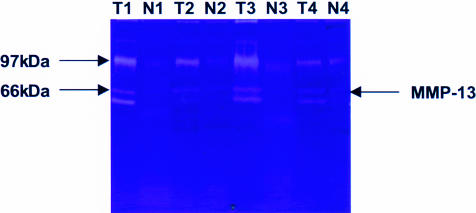
Gelatin zymography generally produced several bands of activity in the tumour samples, with much weaker activity present in the corresponding normal samples. The band of activity detected at 61 kDa is consistent with the active form of MMP-13. There was a band at 42 kDa consistent with active MMP-1 and a more intense band between 72 kDa and 92 kDa, consistent with the activity of MMP-2 and MMP-9 (fig 5).
Figure 5.
A representative gel zymogram showing matrix metalloproteinase 13 (MMP-13) activity in tumour samples. There is only very weak MMP-13 activity in normal samples. N, normal tissue; T, tumour.
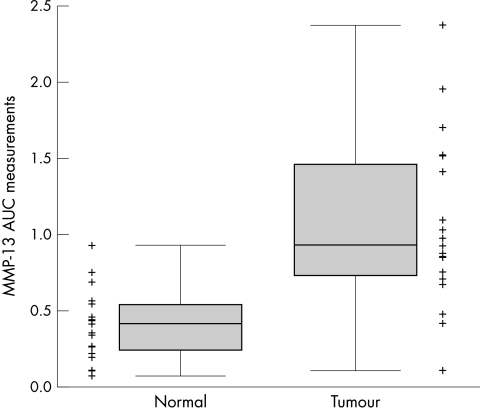
There was a highly significant difference in MMP-13 activity between tumour (T) and normal (N) samples (N mean, 0.93; T mean, 2.38; p < 0.001; fig 6). Furthermore, within both the good and poor survival groups, MMP-13 activity in tumour samples was significantly higher than that seen in normal samples; p = 0.008 and p = 0.005, respectively.
Figure 6.
Matrix metalloproteinase 13 (MMP-13) control corrected area under the curve (AUC) measurements in tumour (n = 20) and corresponding paired normal cases (n = 20). Points are the individual measurements; the box is the interquartile range, median line within; the whiskers are the minimum and maximum measurements. MMP-13 activity is significantly higher in the tumours (p < 0.001).
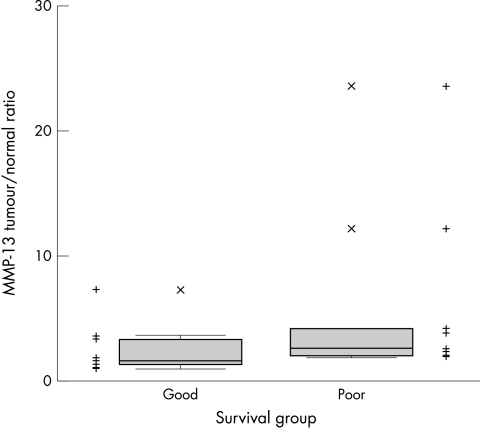
One of the normal samples from the poor survival group had no detectable MMP-13 activity and therefore was not included in the tumour/normal ratio analysis. All cases had a T/N ratio greater than 1. The good survival group had a mean T/N ratio of 1.62, the poor survival group had a mean T/N ratio of 2.49 (fig 7). The MMP-13 T/N ratio in the poor survival group was significantly higher than in the good survival group (p = 0.02; Mann-Whitney U test; fig 7).
Figure 7.
Matrix metalloproteinase 13 (MMP-13) tumour/normal ratios in good (n = 10) and poor survival groups (n = 9). Points are the ratios in individual cases; the box is the interquartile range, median line within; the whiskers are the minimum and maximum measurements. The tumour to normal ratio is significantly higher in the poor survival group (p = 0.02; Mann-Whitney U test).
DISCUSSION
Our study identified the presence of immunoreactive MMP-13 in a high proportion of colorectal cancers and found that MMP-13 is active in the subset of cases analysed for MMP-13 activity. We also found that there is an association between MMP-13 immunoreactivity and prognosis in colorectal cancer. MMP-13 staining was localised to the cytoplasm of tumour cells. This is consistent with studies of other tumours including breast cancer19 and malignant melanoma,20 which have found that MMP-13 expression is primarily present in tumour cells.
MMP-13 immunostaining was detected in over 90% of cases examined. This indicates that MMP-13 is commonly produced in colorectal cancer. In addition, most tumours had a high proportion of tumour cells containing MMP-13. The frequency of MMP-13 expression in colorectal cancer is similar to immunohistochemical studies of other types of tumour.17,19–21
A semiquantitative scoring system was used to categorise MMP-13 immunostaining as high and low. By varying the cutoff point between high and low scores the maximum difference in survival was found using a cutoff score of 2 to divide high and low MMP-13 expressing tumours. At that cutoff value there was a trend towards a poorer survival in the high score group (p = 0.0875).
To investigate the presence of MMP-13 activity in colorectal cancer two groups of cases at the extremes of survival duration were identified from the database. This “enrichment approach”, which we have described previously,22,23 allows the rapid examination of a small number of cases to guide further investigation. Using two groups divergent for a single characteristic (survival) increases the sensitivity by comparing cases at the extremes of a continuous variable.22,23
“By varying the cutoff point between high and low scores the maximum difference in survival was found with a cutoff score of 2 to divide high and low matrix metalloproteinase 13 expressing tumours”
The highly significant difference in MMP-13 activity between tumour and normal samples (p < 0.001) is entirely consistent with the immunohistochemistry results, where MMP-13 was expressed predominantly in tumour cells and only occasionally weakly expressed in stromal cells. The increase in MMP-13 probably represents MMP-13 upregulation, with high amounts of MMP-13 being necessary for the activation of other MMPs that have previously been identified in colorectal cancer. Furthermore, it is logical that tumours have far higher amounts of MMP-13 activity because this molecule catalyses the breakdown of ECM necessary for invasion. Similarly, normal colon cells have no requirement for MMP-13 mediated ECM breakdown, or increased activation of other MMPs.
MMP-13 tumour/normal activity ratios are representative of the degree of MMP-13 upregulation and activation in tumour cells, and are significantly higher in the poor survival group. This might indicate that MMP-13 is activating other MMPs to promote tumour invasion, or catalysing ECM breakdown, or both. Regardless of the mechanism, these findings suggest a role for active MMP-13 in colorectal cancer progression.
The results of our study show that MMP-13 is frequently present and active in colorectal cancer and suggest that the activity of MMP-13 is associated with poorer survival in colorectal cancer.
Take home messages.
Matrix metalloproteinase (MMP-13) is frequently present and active in colorectal cancer
The activity of MMP-13 appears to be associated with poorer survival in colorectal cancer
Acknowledgments
Financial support was provided to MFL by the Wolfson Foundation. The Aberdeen Colorectal Cancer Research Initiative is supported by a University of Aberdeen Development Trust Grant (steering committee: Professor J Cassidy, Dr G I Murray, Dr H L McLeod, Professor N Haites, Professor J Little, and Dr W T Melvin). The technical assistance of Mrs V Ross is gratefully acknowledged.
Abbreviations
AUC, area under the curve
ECM, extracellular matrix
MMP, matrix metalloproteinase
T/N ratio, tumour to normal tissue ratio
REFERENCES
- 1.McLeod HL, Murray GI. Tumour markers of prognosis in colorectal cancer. Br J Cancer 1999;79:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curran S, Murray GI. Matrix metalloproteinases in tumour invasion and metastasis. J Pathol 1999;189:300–8. [DOI] [PubMed] [Google Scholar]
- 3.Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer 2000;36:1621–30. [DOI] [PubMed] [Google Scholar]
- 4.Murray GI, Duncan ME, O’Neil P, et al. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med 1996;2:461–2. [DOI] [PubMed] [Google Scholar]
- 5.Zeng ZS, Huang Y, Cohen AM, et al. Prediction of colorectal cancer relapse and survival via tissue RNA levels of matrix metalloproteinase-9. J Clin Oncol 1996;14:3133–40. [DOI] [PubMed] [Google Scholar]
- 6.Adachi Y, Yamamoto H, Itoh F, et al. Contribution of matrilysin (MMP-7) to the metastatic pathway of human colorectal cancers. Gut 1999;45:252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kikuchi R, Noguchi T, Takeno S, et al. Immunohistochemical detection of membrane-type-1-matrix metalloproteinase in colorectal carcinoma. Br J Cancer 2000;83:215–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sardinha TC, Nogueras JJ, Xiong H, et al. Membrane-type 1 matrix metalloproteinase mRNA expression in colorectal cancer. Dis Colon Rectum 2000;43:389–95. [DOI] [PubMed] [Google Scholar]
- 9.Freije JMP, Diez-Itza I, Balbín M, et al. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem 1994;269:16766–73. [PubMed] [Google Scholar]
- 10.Knäuper V, López-Otín C, Smith B, et al. Biochemical characterisation of human collagenase-3. J Biol Chem 1996;271:1544–50. [DOI] [PubMed] [Google Scholar]
- 11.Knäuper V, Will H, López-Otín C, et al. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem 1996;271:17124–31. [DOI] [PubMed] [Google Scholar]
- 12.Knäuper V, Smith B, López-Otín C, et al. Activation of progelatinase b (proMMP-9) by active collagenase-3 (MMP-13). Eur J Biochem 1997;248:369–73. [DOI] [PubMed] [Google Scholar]
- 13.Cowell S, Knäuper V, Stewart ML, et al. Induction of matrix metalloproteinase activation cascades based on membrane-type 1 matrix metalloproteinase: associated activation of gelatinase a, gelatinase b and collagenase 3. Biochem J 1998;331:453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson N, Airola K, Grénman R, et al. Expression of collagenase-3 (matrix metalloproteinase13) in squamous cell carcinomas of the head and neck. Am J Pathol 1997;151:499–508. [PMC free article] [PubMed] [Google Scholar]
- 15.Boström PJ, Ravanti L, Reunanen N, et al. Expression of collagenase-3 (matrix metalloproteinase-13) in transitional-cell carcinoma of the urinary bladder. Int J Cancer 2000;88:417–23. [PubMed] [Google Scholar]
- 16.Cazorla M, Hernández L, Nadal A, et al. Collagenase-3 expression is associated with advanced local invasion in human squamous cell carcinomas of the larynx. J Pathol 1998;186:144–50. [DOI] [PubMed] [Google Scholar]
- 17.Etoh T, Inoue H, Yoshikawa Y, et al. Increased expression of collagenase-3 (MMP-13) and MT1-MMP in oesophageal cancer is related to cancer aggressiveness. Gut 2000;47:50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson N, Vaalamo M, Grénman S, et al. Collagenase-3 (MMP-13) is expressed by tumor cells in invasive vulvar squamous cell carcinomas. Am J Pathol 1999;154:469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heppner KJ, Matrisian LM, Jensen RA, et al. Expression of most matrix metalloproteinase family members in breast cancer represents a tumour-induced host response. Am J Pathol 1996;149:273–82. [PMC free article] [PubMed] [Google Scholar]
- 20.Airola K, Karonen T, Vaalamo M, et al. Expression of collagenases-1 and -3 and their inhibitors TIMP-1 and -3 correlates with the level of invasion in malignant melanomas. Br J Cancer 1999;80:733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uria J, Balbin M, López JM, et al. Collagenase-3 (MMP-13) expression in chondrosarcoma cells and its regulation by basic fibroblast growth factor. Am J Pathol 1998;153:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLeod HL, Murray GI, Mollison J, et al. Selection of markers to predict tumour response or survival: description of a novel approach. Eur J Cancer 1999;15:1650–2. [DOI] [PubMed] [Google Scholar]
- 23.McKay JA, Lloret C, Murray GI, et al. Application of the enrichment approach to identify putative markers of response to 5-fluorouracil therapy in advanced colorectal carcinomas. Int J Oncol 2000;17:153–8. [DOI] [PubMed] [Google Scholar]