Abstract
Background/Aims: The gastric cardia mucosa is a narrow band of tissue between the oesophagus and the stomach. The physiological role of this tissue is unknown. This study examined the presence and characteristics of neuroendocrine cells at this site.
Methods: Biopsy samples were obtained from across normal appearing squamocolumnar junctions. The cardiac mucosa was defined as the presence of special type mucosa composed of mucous secreting glands in the immediate vicinity of oesophageal squamous epithelium. Biopsy specimens were stained with haematoxylin and eosin, alcian blue (pH 2.5) periodic acid Schiff, and modified Giemsa. The chromogranin A and Fontana-Masson stains were used to identify neuroendocrine cells, which were also stained immunohistochemically for gastrin, serotonin, glucagon, pancreatic polypeptide, somatostatin, and vasoactive intestinal peptide.
Results: Chromogranin positive cells were seen in 18 cases with adequate biopsy specimens from the gastric cardia mucosa. These cells were all serotonin positive, but stains for other peptide hormones remained negative. Serotonin positive cells were detected only at the base of foveolae at the periphery of mucous secreting cardiac glands, giving a microscopic appearance resembling that of endocrine cells at the gastric antrum. The presence and numbers of serotonin positive cells did not correlate with chronic inflammation or intestinal metaplasia of the cardiac mucosa. These cells were seen both in Helicobacter pylori positive and negative patients.
Conclusions: Serotonin positive cells appear to be the sole neuroendocrine cell type at the gastric cardia mucosa. These cells may have a role in regulating the physiology of the gastric cardia mucosa and the lower oesophageal sphincter.
Keywords: gastric cardia, immunohistochemistry, neuroendocrine cells
Neuroendocrine cells possess molecular machinery for the uptake and release of neurotransmitters and neuropeptide hormones. These cells enable autocrine communication both with adjacent (paracrine) and more distant (endocrine) cells.1 Gastric neuroendocrine cells comprise about 1% of the volume of the oxyntic mucosa, and at least six distinct cell types have been identified.2 The gastric mucosa contains enterochromaffin-like cells, G cells, and D cells, the respective secretory products of which include chromogranins and histamine, gastrin, and somatostatin.
The gastric cardia mucosa is a specialised type of mucosa on the gastric side of the squamous and columnar junction, which is composed of pits that occupy one half of the thickness of the mucosa; the glands are coiled and occasionally branched and secrete only mucous.3 Recent data indicate that the gastric cardia is a very narrow band between oesophageal squamous mucosa and gastric fundic mucosa.4,5 There is strong evidence that the cardiac mucosa is congenital and gastric in origin.6,7 Electron microscopy has shown that the gastric cardia contains numerous endocrine cells, which mainly occupy recesses within lateral surfaces of adjacent mucous or parietal cells.8 This study was published in the 1970s and endocrine cells were not identified by immunohistochemical methods.
“The gastric cardia mucosa is a specialised type of mucosa on the gastric side of the squamous and columnar junction”
Neuroendocrine cells are involved in normal physiological processes at the gastric mucosa in addition to disease states. The role of gastrin secreted from antral G cells in the production of gastric acid is established. Hypochlorhydria may result in hyperplasia of gastric enterochromaffin cells or even in the development of carcinoid tumours of the gastric mucosa. Because only sparse data are available on gastric cardia neuroendocrine cells, we wanted to investigate the presence and characteristics of neuroendocrine cells at the gastric cardia mucosa.
MATERIALS AND METHODS
Biopsy specimens were obtained from gastric antrum and body, from the normal appearing squamocolumnar junction and from the columnar lined distal oesophagus (two specimens from each site). During endoscopy, the normal squamocolumnar junction or Z line was considered to be at the level of the proximal end of the gastric folds. Biopsy forceps were targeted across the squamocolumnar junction to obtain both columnar and squamous mucosa. If columnar appearing mucosa extended orally from the gastro-oesophageal junction, biopsy specimens were obtained separately from this mucosa to detect Barrett’s oesophagus. The presence of hiatal hernia was defined as the entire gastro-oesophageal vestibule remaining intrathoracic during both inspiration and expiration.9 Endoscopic examinations were performed by a single investigator (MV).
Histology
Formalin fixed biopsy specimens were embedded in paraffin wax and tissue sections were stained with haematoxylin and eosin, alcian blue (pH 2.5), periodic acid Schiff, and modified Giemsa. Only cases with typical gastric cardia mucosa in the junctional biopsy specimens were included in our analysis. The presence of incomplete-type intestinal metaplasia in the biopsy specimens from columnar lined distal oesophagus was considered the diagnostic criterion for Barrett’s oesophagus.10 Chronic cardia inflammation was defined as infiltration of cardiac mucosa by mononuclear cells.11 Gastric cardiac intestinal metaplasia was defined as the presence of goblet cells with (incomplete-type intestinal metaplasia) or without (complete-type intestinal metaplasia) acidic mucin in goblet and columnar-type cells in biopsy specimens obtained from normal appearing squamocolumnar junctions.12 Two pathologists examined the histological slides independently (MJ and PS).
Antibodies for immunohistochemical stains were supplied by Dako, Glostrup, Denmark (polyclonal antigastrin, polyclonal antiglucagon, polyclonal antipancreatic polypeptide (anti-PP), polyclonal antisomatostatin, and monoclonal antiserotonin) and by Novocastra Laboratories, Newcastle upon Tyne, UK (monoclonal antivasoactive intestinal peptide; anti-VIP). The following dilutions were used: antigastrin, 1/300; antiglucagon, 1/500; anti-PP, 1/1000; antiserotonin, 1/10; antisomatostatin, 1/750; and anti-VIP, 1/50. Before staining for somatostatin, the dewaxed sliced biopsy samples were treated with trypsin. Before staining for VIP, the dewaxed sliced biopsy samples were boiled in buffered citric acid (pH 6.0) in a microwave oven for four times five minutes. Immunohistochemical stains were performed using a Dako Techmate 500, with an incubation time of 55 minutes. A Chemnate horseradish peroxidase/diaminobenzidine kit was used to detect neuroendocrine cells. Nuclei were stained with Mayer’s haematoxylin. For each peptide hormone stain, a known positive tissue sample was used as an external control. The numbers of each type of neuroendocrine cell were classified as follows: 0, none, +, low number, ++, moderate number, +++, abundant.
Ethics
The ethics committee of the Central Hospital of Central Finland approved the study.
RESULTS
Eighteen patients with biopsy specimens from normal appearing squamocolumnar junction were included. Two of the three patients with Barrett’s oesophagus also had typical cardiac mucosa in the biopsy specimens from the gastro-oesophageal junction, bringing the number of studied patients with cardiac-type mucosa to 20 cases. These patients had been referred for endoscopy because of reflux disease symptoms (seven), dyspeptic symptoms (three), anaemia (four), coeliac disease (two), non-cardiac chest pain (one), dysphagia (one), surveillance of Barrett’s oesophagus (two), and surveillance of atrophic corpus gastritis (one). At endoscopy, hiatal hernia was detected in seven patients and signs of gastropathy (erythema and erosions) in nine, but none had erosions in the distal oesophagus. Histological examination of gastric antral and corpus biopsy specimens revealed chronic gastritis in seven patients (six Helicobacter pylori positive), whereas 13 had normal gastric histology.
Of the 20 patients with cardiac mucosa detected in biopsy specimens, four had normal non-inflamed cardiac mucosa, whereas 16 had chronic gastric cardia inflammation. In addition, six patients with “carditis” also had intestinal metaplasia at the cardiac mucosa. Chromogranin positive cells at the gastric cardia mucosa were seen in 18 of the 20 patients. In the other two patients biopsy specimens were tangentially cut and superficial; these cases were deleted from the final analysis. Of the 18 patients in the final study population, six were H pylori positive, 14 had chronic cardiac inflammation (five with intestinal metaplasia), and four had normal non-inflamed cardiac mucosa.
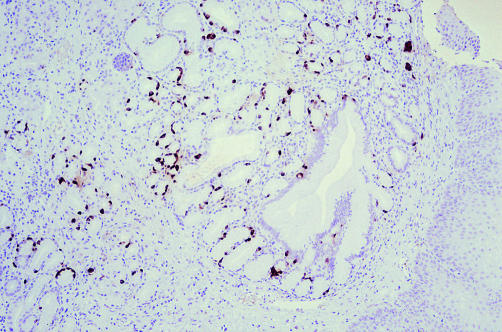
All the chromogranin positive cells at the gastric cardia were serotonin positive when stained immunohistochemically: the numbers of serotonin positive cells were classified as low (+) in 12 and moderate (++) in six cases (fig 1). The mean age of the patients with serotonin positive cells at the cardiac mucosa was 52 years (9 to 74 years) and the male to female ratio was 10:8.
Figure 1.
Moderate immunohistochemical staining for serotonin in neuroendocrine cells at the gastric cardia.
In contrast, the cardiac mucosa was negative for gastrin, glucagon, PP, somatostatin, and VIP. Of the three patients with Barrett’s oesophagus, only one sample with metaplastic oesophageal epithelium contained chromogranin positive cells that were serotonin positive. All other immunohistochemical stains for Barrett’s mucosa were negative. Instead, biopsy specimens of gastric antral mucosa contained numerous gastrin positive cells, whereas specimens of oesophageal squamous epithelium (four) contained neither chromogranin nor serotonin positive cells. Gastric antral and corpus mucosa also contained endocrine cells that were positive for VIP and somatostatin, but negative for glucagon and PP.
DISCUSSION
Our results show that serotonin is the sole secretory product of the neuroendocrine cells at the gastric cardia mucosa. In this respect cardiac mucosa differs sharply from gastric mucosa, which contains several types of neuroendocrine cells with a large variety of secretory products.2 There are conflicting opinions over whether cardiac mucosa is a normal or a metaplastic phenomen resulting from—for example, pathological gastro-oesophageal reflux.4,5,13 Our present study revealed that serotonin positive cells occur in normal and inflamed cardiac mucosa with or without intestinal metaplasia. Our results are concordant with those of Stachura et al, who showed that gastric cardiac endocrine cells were negative for gastrin, glucagon, somatostatin, VIP, secretin, motilin, PP, insulin, and substance P1.14
Although we attempted to quantify the number of neuroendocrine cells, the results must be interpreted with caution. In our study, neuroendocrine cells were present only in the base of foveolae and at the periphery of mucous secreting glands. Endoscopic biopsy specimens are often cut tangentially, and the number of neuroendocrine cells in such specimens may be low as a result of inadequate tissue sampling. The failure to detect serotonin positive cells in two cases probably resulted from problems in biopsy sample collecting and orientation.
The physiological role of gastric cardia mucosa is unknown. Based on our present study with its very limited number of cases only hypotheses can be presented. The lower oesophageal sphincter system—the main mechanism for regulating the passage of food—is controlled mainly by cholinergic innervation.15 Selective reduction of cholinergic muscle contraction was recently reported in Barrett’s oesophagus, a severe gastro-oesophageal reflux disease.16 Serotonin may stimulate cholinergic neurones to release acetylcholine, which in turn results in smooth muscle contraction.17 Furthermore, gastric mucosal functions such as secretion, growth, and repair after mucosal damage require complex interactions between neuronal and paracrine messengers, growth factors, hormones, and cytokines.2,18 We speculate that serotonin produced by neuroendocrine cells in the gastric cardia mucosa may contribute to the regulation of functions in both the gastric cardia mucosa and the lower oesophageal sphincter.
“Our present study revealed that serotonin positive cells occur in normal and inflamed cardiac mucosa with or without intestinal metaplasia”
Take home messages.
The only secretory product of the neuroendocrine cells at the gastric cardia mucosa was serotonin
These serotonin secreting cells are found at the base portion of foveolae
Serotonin positive cardiac cells may play a role in regulating the functions of the gastric cardia mucosa and lower oesophageal sphincter
Serotonin may be involved in the production and perception of non-cardiac chest pain, for which gastro-oesophageal reflux disease is probably an aetiological factor.19 Sertraline, a selective serotonin reuptake inhibitor, reduced non-cardiac chest pain in a randomised placebo controlled study.20 Whether this was the result of central or peripheral action of the drug remains to be determined. Cicapride, a 5-hydroxytryptamine agonist, enhances cholinergically mediated contractions in the oesophagus, and has been used in the treatment of gastro-oesophageal reflux disease.17
Our finding that neuroendocrine cells were present in Barrett’s oesophagus agrees with previous reports. Griffin and Sweeney reported the predominant neuroendocrine cell type to be serotonin positive; they were unable to find gastrin positive cells at Barrett-type epithelium.21 Recent studies showed that Barrett’s mucosa contained chromogranin positive cells that persisted in low and high grade dysplasia but were lost during the development of oesophageal adenocarcinoma.22,23 However, neuroendocrine differentiation reportedly exists in gastric adenocarcinomas and oesophageal primary small cell carcinomas.24,25 Even a case with combined oesophageal adenocarcinoma and carcinoid in a patient with Barrett’s oesophagus has been reported.26 However, we did not detect neuroendocrine cells in the squamous epithelium. One explanation could be that Barrett’s metaplasia with neuroendocrine cells results from a multipotent stem cell, which is capable of differentiating into both squamous and columnar epithelium.27
In conclusion, our study showed that serotonin positive cells are the only neuroendocrine cell type at the gastric cardia mucosa. These cells are found at the base portion of foveolae. Serotonin positive cardiac cells may play a role in regulating the functions of the gastric cardia mucosa and lower oesophageal sphincter.
Abbreviations
PP, pancreatic polypeptide
VIP, vasoactive intestinal peptide
REFERENCES
- 1.Wiedenmann B, John M, Ahnert-Hilger G, et al. Molecular and cell biological aspects of neuroendocrine tumors of the gastroenteropancreatic system. J Mol Med 1998;76:637–47. [DOI] [PubMed] [Google Scholar]
- 2.Öberg K. Gastric neuroendocrine cells and secretory products. Yale J Biol Med 1998;71:149–54. [PMC free article] [PubMed] [Google Scholar]
- 3.Owen DA. Normal histology of the stomach. Am J Surg Pathol 1986;10:48–61. [DOI] [PubMed] [Google Scholar]
- 4.Kilgore SP, Ormsby AH, Gramlich TL, et al. The gastric cardia: fact or fiction? Am J Gastroenterol 2000;95:921–4. [DOI] [PubMed] [Google Scholar]
- 5.Chandrasoma PT, Der R, Ma Y, et al. Histology of the gastroesophageal junction: an autopsy study. Am J Surg 2000;24:402–9. [DOI] [PubMed] [Google Scholar]
- 6.Ellison E, Hassall E, Dimmick JE. Mucin histochemistry of the developing gastroesophageal junction. Pediatr Pathol Lab Med 1996;16:195–206. [PubMed] [Google Scholar]
- 7.Zhou H, Greco MA, Daum F, et al. Origin of cardiac mucosa: ontogenic consideration. Pediatric and Developmental Pathology 2001;4:358–63. [DOI] [PubMed] [Google Scholar]
- 8.Krause WJ, Ivey KJ, Baskin WN, et al. Morphological observations on the normal human cardiac glands. Anat Rec 1987;192:59–72. [DOI] [PubMed] [Google Scholar]
- 9.Savary M, Miller G, eds. The esophagus. Handbook and atlas of endoscopy. Solothurn, Switzerland: Verlag Gassmann AG, 1978.
- 10.Haggitt RC. Barrett’s esophagus, dysplasia, and adenocarcinoma. Hum Pathol 1994;25:982–93. [DOI] [PubMed] [Google Scholar]
- 11.Riddell RH. The biopsy diagnosis of gastroesophageal reflux disease, “carditis,” and Barrett’s esophagus, and sequelae of therapy. Am J Surg 1996;20(suppl 1):S31–50. [DOI] [PubMed] [Google Scholar]
- 12.Jauregui HO, Davessar K, Hale JH, et al. Mucin histochemistry of intestinal metaplasia in Barrett’s esophagus. Mod Pathol 1988;1:188–92. [PubMed] [Google Scholar]
- 13.Öberg S, Peters JH, DeMeester TR, et al. Inflammation and specialized intestinal metaplasia of cardiac mucosa is a manifestation of gastroesophageal reflux disease. Ann Surg 1997;226:522–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stachura J, Krause WJ, Ivey KJ. Endocrine cells in human cardial glands. Folia Histochem Cytochem (Krakow) 1981;19:71–6. [PubMed] [Google Scholar]
- 15.Mittal RK, Balaban D. The esophagogastric junction. N Engl J Med 1997;336:924–32. [DOI] [PubMed] [Google Scholar]
- 16.Smid SD, Blackshaw LA. Neuromuscular function of the human lower oesophageal sphincter in reflux disease and Barrett’s oesophagus. Gut 2000;46:756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim D-Y, Camillieri M. Serotonin: a mediator of the brain–gut connection. Am J Gastroenterol 2000;95:2698–709. [DOI] [PubMed] [Google Scholar]
- 18.Ekblad E, Mei Q, Sundler F. Innervation of the gastric mucosa. Microsc Res Tech 2000;48:241–57. [DOI] [PubMed] [Google Scholar]
- 19.Fass R, Fennerty MB, Ofman JJ, et al. The clinical and economic value of a short course of omeprazole in patients with noncardiac chest pain. Gastroenterology 1998;115:42–9. [DOI] [PubMed] [Google Scholar]
- 20.Varia I, Logue E, O’Connor C, et al. Randomized trial of sertraline in patients with unexplained chest pain of noncardiac origin. Am Heart J 2000;140:367–72. [DOI] [PubMed] [Google Scholar]
- 21.Griffin M, Sweeney EC. The relationship of endocrine cells, dysplasia and carcinoembryonic antigen in Barrett’s mucosa to adenocarcinoma of the oesophagus. Histopathology 1987;11:53–62. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton K, Chiappori A, Olson S, et al. Prevalence and prognostic significance of neuroendocrine cells in esophageal adenocarcinoma. Mod Pathol 2000;13:475–81. [DOI] [PubMed] [Google Scholar]
- 23.Jaskiewicz K, Louw J, Anichkov N. Barrett’s oesophagus: mucin composition, neuroendocrine cells, p53 protein, cellular proliferation and differentiation. Anticancer Res 1994;14:1907–12. [PubMed] [Google Scholar]
- 24.Qvigstad G, Sandvik AK, Brenna E, et al. Detection of chromogranin A in human gastric adenocarcinomas using a sensitive immunohistochemical technique. Histochem J 2000;32:551–6. [DOI] [PubMed] [Google Scholar]
- 25.Beyer KL, Marshall JB, Diaz-Arias AA, et al. Primary small-cell carcinoma of the esophagus. J Clin Gastroenterol 1991;13:135–41. [DOI] [PubMed] [Google Scholar]
- 26.Cary NRB, Barron DJ, McGoldrick JP, et al. Combined oesophageal adenocarcinoma and carcinoid in Barrett’s oesophagitis: potential role of enterochromaffin-like cells in oesophageal malignancy. Thorax 1993;48:404–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shields HM, Zwas F, Antonioli DA, et al. Detection by scanning electron microscopy of a distinctive esophageal surface cell at the junction of squamous and Barrett’s epithelium. Digest Dis Sci 1993;38:97–108. [DOI] [PubMed] [Google Scholar]