Abstract
Aims: Regulation of cell cycle progression is a fundamental control process, linked to cellular differentiation and apoptosis in normal tissues. p21WAF1 is a nuclear protein that regulates cell cycle progression. p21WAF1 can be transcriptionally upregulated by p53, but may be activated independently of p53—for example, during terminal differentiation. Loss of topological control of p21WAF1 expression is an early feature of malignancy in the colorectal system. Similar to the colonic mucosa, sebaceous glands contain cells that are constantly going through a process of cell division, differentiation, and cell death. This study investigated the expression of p53, p21WAF1, and the proliferation marker Ki67 in normal sebaceous glands, sebaceous adenoma, sebaceoma, and sebaceous carcinoma.
Methods: Serial sections were stained with monoclonal antibodies to p21WAF1, p53, and Ki67 (MIB1) using standard immunohistochemical techniques.
Results: In normal sebaceous glands, p21WAF1 positive cells were only seen within the differentiating compartment, which was spatially distinct from the cycling peripheral Ki67 positive cells. In sebaceous adenoma and sebaceoma, topological control was maintained, with the distribution of markers being similar to that seen in normal sebaceous glands. Loss of topological control of markers of cellular control was seen in sebaceous carcinoma only. This contrasts with colonic tumours, in which loss of p21 compartmentalisation is seen in adenomas at an early stage of tumour progression.
Conclusion: This work confirms the hypothesis that the dysregulation of cell cycle progression is an important process in the development of malignancy within sebaceous glands, although loss of topological control was seen only in sebaceous carcinoma.
Keywords: sebaceous tumours, Ki67, p21
Regulation of cell cycle progression is a fundamental control process that is conserved throughout evolution. It is linked to cellular differentiation and apoptosis in normal tissues. Dysregulation of cell cycle progression appears to be an important process in the development of malignancy and the investigation of proteins involved in these processes has led to a greater understanding of the aetiology and progression of tumours.
p53 is a major controlling factor of both cell growth and transformation and is frequently described as the “guardian of the genome”. p53 prevents cells that contain damaged DNA from proliferating, either temporarily by arresting the cell cycle so that DNA repair can occur, or permanently, by entering the cell into a pathway of programmed cell death (apoptosis). These effects are mediated through the transcriptional upregulation of specific genes, including p21WAF1, which mediates growth arrest, Gadd, which is involved in signal transduction pathway(s) in response to DNA damage, and the apoptosis inducing factor Bax. p21WAF1 regulates the G1–S transition and mediates G1 cell cycle arrest by forming a complex with proliferating nuclear cell antigen (PNCA), cyclins, and cyclin dependent kinases (CDKs), inhibiting kinase activity and preventing cell cycle progression. p21WAF1 may also be upregulated by p53 independent pathways, as illustrated in cells lacking functional p53.1–4 Furthermore, experiments suggest that the upregulation of p21WAF1 via p53 independent pathways is linked to cellular differentiation.1–7 Immunostaining for Ki67 (a marker for proliferation), p53, and p21WAF1 in normal colonic mucosa (a rapidly proliferating and subsequently differentiating tissue) has shown a clear demarcation between proliferating and differentiating compartments and has also shown that cell cycle inhibitors are under precise topological control.2,5,7–11 The loss of topological control of p21WAF1 expression is one of the earliest features of malignancy in the colorectal system.7
“p21WAF1 regulates the G1–S transition and mediates G1 cell cycle arrest by forming a complex with proliferating nuclear cell antigen, cyclins, and cyclin dependent kinases, inhibiting kinase activity and preventing cell cycle progression”
Sebaceous glands are holocrine glands, forming a secretion by total cellular disintegration. Similar to the colonic mucosa, they contain cells that are constantly going through a process of cell division, differentiation, and cell death. The proliferating sebocytes are situated at the periphery of the gland. As sebocytes move towards the centre of the gland they produce lipid and differentiate. Cellular disintegration occurs in the section of the gland closest to the duct. Previous work has demonstrated intense p21WAF1 nuclear immunostaining of normal differentiating sebocytes in the absence of p53 immunostaining,7 and also the presence of Ki67 expressing cells towards the periphery of the gland.12 Sebaceous glands may form both benign and malignant tumours. We investigated the expression of p53, p21WAF1, and Ki67 in normal sebaceous glands, sebaceous adenoma, sebaceoma, and sebaceous carcinoma to ascertain whether the loss of topological control of these proteins is important in the development/progression of neoplasia in this tissue.
METHODS
Patients and samples
Paraffin wax embedded samples (normal sebaceous glands, n = 2; sebaceous adenoma, n = 13; sebaceoma, n = 3; and sebaceous carcinoma, n = 12) were obtained from archival material in the department of pathology, Royal Victoria Infirmary, Newcastle upon Tyne. The samples of sebaceous carcinoma included five ocular tumours and seven extraocular tumours.
Immunohistochemistry
Serial sections (5 μm thick) of paraffin wax embedded tissue were dewaxed in xylene, rehydrated, and microwave enhanced antigen retrieval was performed in 0.01M sodium citrate buffer, pH 6.0, as described previously.7 Endogenous tissue peroxidase activity was blocked with 3% hydrogen peroxide in methanol, and immunohistochemistry was performed using mouse monoclonal antibodies to either p21WAF1 (1/50 dilution; Oncogene Research Products, Cambridge, Massachusetts, USA), p53 (1/50 dilution; DO7; Novocastra Laboratories, Newcastle, UK), or Ki67 (1/500 dilution; Immunotech, Marseille, France). Sections were developed with a streptavidin–biotin–peroxidase detection system (Dako K377 kit; Dako A/S, Glostrup, Denmark) and the antibody was detected with freshly prepared diaminobenzidine as the chromagen (brown). Sections were counterstained with haematoxylin and eosin. The Ki67 antibody identifies a nuclear antigen expressed in all non-G0 phases of the cell cycle.
RESULTS
Normal sebaceous glands
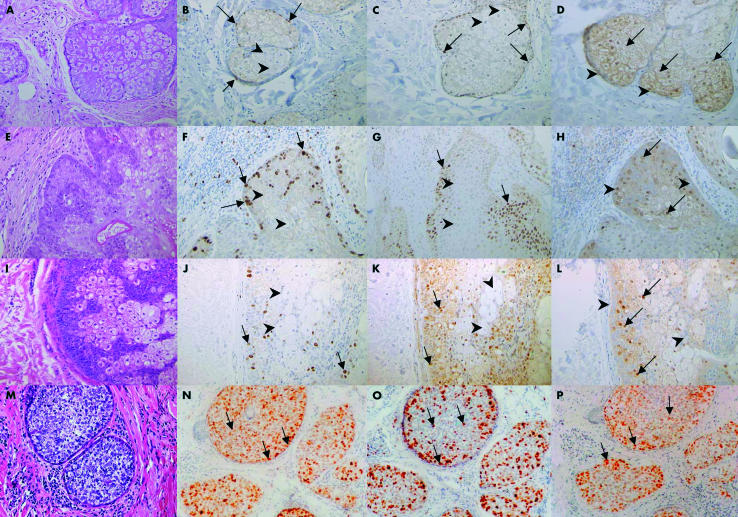
In normal human skin, Ki67 positive and p53 positive cells were confined to the nuclei of peripheral cuboidal basophilic cells of the sebaceous glands, whereas p21WAF1 positivity was confined to the nuclei of differentiating sebocytes in the centre of the gland, confirming previous work (fig 1A–D).7
Figure 1.
(A–D) Normal sebaceous gland, (E–H) sebaceous adenoma, (I–L) sebaceoma, and (M–P) sebaceous carcinoma were stained with haematoxylin and eosin (A, E, I, M) or immunostained with antibodies to Ki67 (B, F, J, N), p53 (C, G, K, O), or p21WAF1 (D, H, L, P), as indicated. Normal sebaceous gland (A–D): basaloid cells show nuclear staining for Ki67 and p53 (arrows) but are negative for p21WAF1 (arrowheads). Differentiated sebocytes show nuclear staining for p21WAF1 (arrows) but are negative for Ki67 and p53 (arrowheads). Sebaceous adenoma (E–H) and sebaceoma (I–L): basaloid cells show increased nuclear staining for Ki67 and p53 (arrows) but are negative for p21WAF1 (arrowheads). Differentiated sebocytes show p21WAF1 nuclear positivity (arrows) but are negative for Ki67 and p53 (arrowheads). Sebaceous carcinoma (M–P) shows very disordered nuclear staining for Ki67, p53, and p21WAF1 (arrows), with a lack of compartmentalisation. The staining intensity is increased compared with normal glands and adenomas.
Sebaceous adenoma and sebaceoma
Sebaceous adenoma and sebaceoma differed from normal sebaceous glands by exhibiting an expanded volume of undifferentiated basaloid cells between well organised differentiating sebocytes. Again, p21WAF1, p53, and Ki67 positive cells appeared to be compartmentalised, with p53 positive and Ki67 positive cells seen only within the expanded basaloid cells and p21WAF1 positive cells confined to the differentiating sebocytes (fig 1E–L). There were no differences in staining between sebaceous adenomas and sebaceomas.
Sebaceous carcinoma
A strikingly different pattern was seen in the sections of sebaceous carcinoma. In the lobules, there was a noticeable increase in intensity of staining for p53, p21WAF1, and Ki67, compared with normal sebaceous glands and benign sebaceous hyperplasia and adenomas. In addition, cells expressing p53, p21WAF1, and Ki67 were randomly distributed and showed no clear zones of demarcation (fig 1M–P).
DISCUSSION
Our work has confirmed previous reports demonstrating the expression of Ki67, p53, and p21WAF1 in topologically distinct compartments in normal sebaceous glands. The expression of p21WAF1 in the absence of p53 positivity in the differentiating compartment of normal sebaceous glands is consistent with previous work indicating that p53 independent upregulation of p21WAF1 is associated with cellular differentiation.1–3,5–7 p21WAF1 positivity of postmitotic cells immediately adjacent to the proliferating compartment is consistent with a role for p21WAF1 in early differentiation, perhaps initiating cell cycle arrest.
In sebaceous adenoma and sebaceoma, both benign proliferations of the sebaceous glands, topological control was maintained, with the distribution of markers being similar to that seen in normal sebaceous glands, although the proliferative compartment was expanded.
The complete loss of compartmentalisation of markers seen in the sections of sebaceous carcinoma suggests loss of topological control of specific cell cycle regulatory proteins within the tumour. The loss of compartmentalisation of p21WAF1 and Ki67 has been shown to occur as an early feature in neoplastic change in the colon, and is seen in colonic adenoma,7 a precursor of colonic neoplasia. In contrast, in sebaceous gland tumours the development of sebaceous adenomas is not associated with the loss of topographical control, in keeping with the benign nature of this tumour and the lack of evidence to suggest progression of sebaceous adenoma into sebaceous carcinoma, even in patients with Muir Torre syndrome, who have a propensity to develop sebaceous adenomas and carcinomas, in addition to bowel malignancies. Loss of topological control of markers of cellular control was seen only in sebaceous carcinoma.
The role of p53 and p21WAF1 in tumorigenesis is an area of current interest. Gonzalez et al report a G:C→T:A transversion, characteristic of mutations caused by carcinogens, which resulted in the substitution of Phe for Cys277, a residue that normally participates in hydrogen bonding to the p53 DNA binding consensus sequence in an invasive sebaceous carcinoma. They also provide evidence that p53 is not expressed in sebaceous carcinoma in situ, but is overexpressed in invasive tumours,13 suggesting that p53 mutation may be involved in tumour invasion rather than initiation. This has also been suggested in studies of premalignant and malignant lesions in breast14 and oesophagus.15 Interestingly, somatic mutations of p53 are commonly found in sporadic carcinoma of the colon, whereas germline mutation of p53 (responsible for Li Fraumeni syndrome) rarely results in cancer of the colon. Thus, p53 may play a greater role in the progression of disease rather than the initiation of tumours. However, p53 positivity on immunostaining does not necessarily correlate with the presence of p53 mutations.12 Further studies of p53 mutations in sebaceous tumours are required to clarify this issue.
The role of p21WAF1 in cell cycle regulation in the skin is complex. p21WAF1 is increased in differentiating suprabasal cells in psoriasis and differentiating agents including 12-tetradeconyl phorbol acetate and raised extracellular calcium induce increased p21 protein values.4 However, growth factors, including epidermal growth factor, may also result in a transient induction of p21WAF1 in mouse embryonic fibroblasts,16 and we have also observed transient increases in p21WAF1 protein concentrations in human keratinocytes in response to transforming growth factor α (A J Graham and NJ Reynolds, unpublished observations, 1999). In normal human cells, p21WAF1 exists in quaternary complexes with PCNA, cyclin, and a CDK.17,18 Evidence indicates that the stoichiometric ratio of p21WAF1 to cyclin–CDK within the complex regulates cell cycle progression. Thus, the effect of inducing p21WAF1 will also depend on whether the stimulus modulates cyclin–CDK values, so that the induction of p21WAF1 does not necessarily result in growth arrest.
Take home messages.
In normal sebaceous glands, p21WAF1 expression was only seen within the differentiating compartment, which was spatially distinct from the cycling peripheral Ki67 positive cells
This distribution of markers was similar in sebaceous adenoma and sebaceoma, both benign proliferations of the sebaceous glands, although the proliferative compartment was expanded
This work confirms the hypothesis that the dysregulation of cell cycle progression is an important process in the development of malignancy within sebaceous glands
Loss of topological control of markers of cellular control was seen in sebaceous carcinoma only
The role of p21WAF1 in carcinogenesis is not yet fully elucidated. p21WAF1 knockout mice do not develop spontaneous tumours,3 but have an enhanced susceptibility to chemically induced skin cancers19 and develop higher grade undifferentiated tumours.20 It is interesting to note that sebaceous carcinoma shows increased p21WAF1 expression in well differentiated cells, rather than a decrease or loss, as might be expected. It is not clear from our study whether this upregulation represents increased wild-type p53, which upregulates p21WAF1 in the normal situation, or mutated p53.
“Our data suggest that detecting loss of the normal compartmentalisation for p53, Ki67, and p21WAF1 through immunohistochemical studies could also help with distinguishing biopsies of sebaceomas from carcinomas”
Although early sebaceous carcinoma may be cured by surgical excision there is still considerable mortality and morbidity associated with this tumour.21 The identification of pronounced nuclear atypia, abnormal mitotic figures, and an infiltrative growth pattern usually allows the distinction of sebaceous carcinoma from adenoma and sebaceoma.22 However, although it is rarely a problem differentiating sebaceous adenoma from carcinoma, it can be difficult to distinguish biopsies of sebaceomas from carcinomas. Our data suggest that detecting loss of the normal compartmentalisation for p53, Ki67, and p21WAF1 through immunohistochemical studies could also help with the diagnosis in these circumstances.
In summary, our study has shown that the expression of p53, Ki67, and p21WAF1 occurs in topologically distinct compartments in normal sebaceous glands. The proliferative compartment in sebaceous adenoma and sebaceous hyperplasia is expanded but remains in the periphery of the gland. This contrasts greatly with sebaceous carcinoma, where there is complete loss of compartmentalisation, suggesting loss of topological control of mediators of the cell cycle.
Abbreviations
CDK, cyclin dependent kinase
PCNA, proliferating cell nuclear antigen
REFERENCES
- 1.Macleod KF, Sherry N, Hannnon G, et al. p53-dependent and independent expression of p21 during cell growth, differentiation and DNA damage. Genes Dev 1995;9:935–44. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RA, Hoffman B, Iro A, et al. Induction of p21(WAF-1/CIP1) during differentiation. Oncogene 1994;9:3389–96. [PubMed] [Google Scholar]
- 3.Deng C, Zhang P, Harper JW, et al. Mice lacking p21 CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995;82:675–84. [DOI] [PubMed] [Google Scholar]
- 4.Todd C, Reynolds NJ. Up-regulation of p21WAF1 by phorbol ester and calcium in human keratinocytes through a protein kinase C-dependent pathway. Am J Pathol 1998;153:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang H, Lin J, Su ZZ, et al. Induction of differentiation in human promyelocytic HL-60 leukemia cells activates p21, WAF1/CIP1 expression in the absence of p53. Oncogene 1994;9:3397–406. [PubMed] [Google Scholar]
- 6.Skapek SX, Rhee J, Spicer DB, et al. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science 1995;267:1022–4. [DOI] [PubMed] [Google Scholar]
- 7.El-Deiry WS, Tokino T, Waldman T, et al. Topological control of p21waf1/cip1 expression in normal and neoplastic tissues. Cancer Res 1995;55:2910–19. [PubMed] [Google Scholar]
- 8.Li R, Waga S, Hannnon G, et al. Differential effects by the p21 CDK inhibitor on PCNA-dependent DNA replication and repair. Nature 1994;371:534–7. [DOI] [PubMed] [Google Scholar]
- 9.Michieli P, Chedid M, Lin D, et al. Induction of WAF1/CIP1 via a p53 independent pathway. Cancer Res 1994;54:3391–5. [PubMed] [Google Scholar]
- 10.El-Deiry WS, Tokino T, Velculescu VE, et al. WAF1 a potential mediator of p53 tumor suppression. Cell 1993;75:817–25. [DOI] [PubMed] [Google Scholar]
- 11.El-Deiry WS, Harper JW, O’Connor PM, et al. WAF1/CIP1 is induced in p53 mediated G1 arrest and apoptosis. Cancer Res 1994;54:1169–74. [PubMed] [Google Scholar]
- 12.Wu YY, Takata M, Rehman I, et al. The temporal and spatial distribution of p21WAF expression in skin appendages. Br J Dermatol 2000;142:694–701. [DOI] [PubMed] [Google Scholar]
- 13.Gonzales-Fernandez F, Kaltreider SA, Patnaik BD, et al. Tumor progression through mutational inactivation of p53. Ophthalmology 1998;105:497–506. [DOI] [PubMed] [Google Scholar]
- 14.Allred DC, O’Connell P, Fuqua SA, et al. Immunohistochemical studies of early breast cancer evolution. Breast Cancer Res Treat 1994;32:13–18. [DOI] [PubMed] [Google Scholar]
- 15.Gao H, Wang LD, Zhou Q. p53 tumor suppresser gene mutation in early esophageal precancerous lesions and carcinoma among high risk populations in Henan, China. Cancer Res 1994;54:4342–6. [PubMed] [Google Scholar]
- 16.Michieli P, Chedid M, Lin D, et al. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res 1994;54:3391–5. [PubMed] [Google Scholar]
- 17.Xiong Y, Zhang H, Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 1992;71:505–14. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Jenkins C, Nichols M, Xiong Y. Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene 1994;9:2261–8. [PubMed] [Google Scholar]
- 19.Topley G, Okuyama R, Gonzales JG, et al. p21WAF1/Cip1 functions as a suppresser of malignant skin tumor formation and a determinant of keratinocyte stem-cell potential. Proc Natl Acad Sci U S A 1999;96:9089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philipp J, Vo K, Gurley KE, et al. Tumor suppression by p27Kip1 and p21Cip1 during chemically induced skin carcinogenesis. Oncogene 1999;18:4689–98. [DOI] [PubMed] [Google Scholar]
- 21.Doxanas MT, Green WR. Sebaceous gland carcinoma. Review of 40 cases. Arch Ophthalmol 1984;102:245–9. [DOI] [PubMed] [Google Scholar]
- 22.Elder D, Elenitsas R, Ragsdale BD. Tumours of the epidermal appendages. In: Elder D, ed. Lever’s histopathology of the skin, 8th ed. Philadelphia and New York: Lippincott-Raven, 1997:747–803.