Abstract
Aims: To investigate the distribution and roles of α smooth muscle actin (ASMA) positive stromal cells (ASMA+ cells), which belong to the myofibroblast group, within gastric carcinomas, with reference to three histological types (diffuse type, intestinal type, and solid type).
Methods: In total, 74 surgically resected gastric carcinomas (24 diffuse type, 43 intestinal type, and seven solid type) were examined. ASMA positive and high molecular weight caldesmon (HCD) negative stromal cells were regarded as ASMA+ cells. The distribution of CD34 positive stromal cells (CD34+ cells) was also analysed immunohistochemically.
Results: In the 24 diffuse-type gastric carcinomas, six of the 13 carcinomas invading the subserosa had ASMA+ cells in the tumour stroma, whereas all six diffuse-type gastric carcinomas confined to the submucosa and all five invading the muscularis propria had no ASMA+ cells in the tumour stroma. In the 43 intestinal-type gastric carcinomas, only five of the 21 carcinomas confined to the submucosa had ASMA+ cells in the tumour stroma, whereas 21 of the 22 intestinal-type gastric carcinomas invading the muscularis propria and the subserosa had ASMA+ cell bundles in the tumour stroma. The distribution of CD34+ cells in diffuse-type and intestinal-type gastric carcinomas was similar to that seen in a previously published series. All seven solid-type gastric carcinomas examined had ASMA+ cells but not CD34+ cells in the tumour stroma. No stromal cells double positive for ASMA and CD34 were detected within the diffuse-type tumours examined.
Conclusions: These results suggest that ASMA expression in stromal cells is associated with tumour stroma formation of diffuse-type gastric carcinomas invading the subserosa, intestinal-type gastric carcinomas invading the muscularis propria and subserosa, and solid-type gastric carcinomas.
Keywords: α smooth muscle actin, high molecular weight caldesmon, stromal cell, diffuse type, intestinal type, gastric carcinoma
Based on the expression of CD34, CD31, α smooth muscle actin (ASMA), and high molecular weight caldesmon (HCD), five different immunophenotypes of stromal cells (CD34 positive stromal cells,1,2 endothelial cells,1–3 ASMA positive stromal cells,4–7 smooth muscle cells,1,2,5–8 and colorectal pericryptal fibroblasts5) are found in normal and pathological conditions in the gastrointestinal tract (table 1). (Inflammatory cells and interstitial cells of Cajal are excluded.) ASMA positive stromal cells belong to the myofibroblast group,9 and are found in pathological conditions but not in normal tissue.1,4–7,9 ASMA positive stromal cells are major cellular components of desmoplastic stroma.1,4,9 In colorectal carcinomas, desmoplasia at the tumour edge is associated with an unfavourable prognosis.10 Intestinal type gastric carcinomas invading the subserosa have a large number of ASMA positive stromal cells at the tumour border facing the subserosa.7 To date, the relation between intratumoral ASMA positive stromal cell distribution and histological type has not been studied extensively in human carcinomas.
Table 1.
Immunophenotypes of the stromal cells in the gastrointestinal stromal tissue
| Immunohistochemical markers | ||||
| Cell types | ASMA | HCD | CD34 | CD31 |
| CD34+ cells1,2 | – | – | + | – |
| Endothelial cells1–3 | – | – | + | + |
| ASMA+ cells (except PCF)4–7 | + | – | – | – |
| Smooth muscle cells1,2,5–8 | + | + | – | – |
| PCF4,5 | + | + | – | – |
ASMA, α smooth muscle actin; HCD, high molecular weight caldesmon; CD34+ cells, CD34 positive stromal cells/immature fibroblastic cells; ASMA+ cells, ASMA positive stromal cells/mature fibroblastic cells; PCF, colorectal pericryptal fibroblasts; +, positive; –, negative.
“α Smooth muscle actin positive stromal cells belong to the myofibroblast group, and are found in pathological conditions but not in normal tissue”
In the gastrointestinal tract, CD34 positive stromal cells are located in the submucosa, muscularis propria, subserosa, and muscularis mucosa (but not in the lamina propria).1,2 In the course of haemopoietic stem cell differentiation, the expression of CD34 decreases so that terminally differentiated cells do not express CD34.11 CD34 positive stromal cells are lacking in intestinal-type gastric carcinomas, diffuse-type early gastric carcinomas, and colorectal well and moderately differentiated adenocarcinomas, whereas CD34 positive stromal cells are detected within diffuse-type advanced gastric carcinomas.1,2 However, the relation between CD34 positive stromal cell distribution and ASMA positive stromal cell distribution has not been studied within gastric carcinoma tissues to date.
Wound healing and the generation of tumour stroma share many important properties.12 The recognised differences between tumour stroma generation and wound healing are minor.12 Desmoplasia in malignant epithelial tumours resembles wound healing, which results in hypertrophic scars and keloids.12 A recent report indicates that circulating CD34 positive fibroblast-like cells terminally differentiate into ASMA positive stromal cells and play an important role in diseased sites.13 Circulating peripheral CD34 positive fibroblastic cells are an important source of cytokines and type I collagen during both the inflammatory and the repair phase of the wound healing response.14
To elucidate the importance of ASMA positive stromal cells in tumour stroma formation and the progression of gastric carcinomas, with special reference to histological types, we examined the distribution of ASMA positive stromal cells within diffuse-type carcinomas, intestinal-type carcinomas, and one minority type of tumour (carcinoma showing a solid growth pattern). We also examined the expression of CD34 in these three types of gastric carcinoma to examine the relation between ASMA positive stromal cells and CD34 positive stromal cells. Double immunostaining for ASMA and CD34 was also performed.
MATERIALS AND METHODS
We examined 74 surgically resected invasive gastric adenocarcinomas that were confined to the gastric wall (not invading the adjacent organs) and their normal tissues from the surgical pathology files of the first department of pathology, Kochi Medical School and its affiliated hospitals from 1994 to 2001.
The definitions used for histological classification were based on the criteria of Lauren15 and the World Health Organisation’s Histological typing of oesophageal and gastric tumours (second edition, 1990)16: diffuse type (24 tumours), intestinal type (43 tumours), and solid type (seven tumours). We examined one minority tumour type, carcinoma showing a solid growth pattern,16 and it will be referred to as solid-type carcinoma. Solid-type carcinoma is carcinoma showing a solid growth pattern; the neoplastic cells are closely packed and form large tumour nests, and the tumours have well defined boundaries; these neoplastic cells may be undifferentiated or poorly differentiated (containing a few glandular structures or mucus secreting cells).16 The depth of tumour invasion was classified as submucosa (29 tumours: six diffuse type, 21 intestinal type, and two solid type), muscularis propria (14 tumours confined to the muscularis propria: five diffuse type, eight intestinal type, and one solid type), and subserosa (31 tumours: 13 diffuse type, 14 intestinal type, and four solid type). In our present study, we classified the tumours confined to the submucosa as early cancers and those invading the muscularis propria and subserosa as advanced cancers.
Immunohistochemical studies were performed by the streptavidin biotin method using the Histofine SAB-PO(M) kit (Nichirei, Tokyo, Japan). Five monoclonal antibodies against ASMA, HCD, CD34, CD31, and low molecular weight cytokeratins (CK) were used. Table 2 details the monoclonal antibodies and staining procedures used. We examined immunoreactivity for HCD in all of the tumours, to distinguish ASMA positive stromal cells from smooth muscle cells, which are positive for both ASMA and HCD.8 We also examined immunoreactivity for CD31 in all of the tumour tissues, to distinguish CD34 positive stromal cells from vascular endothelial cells, which are positive for both CD34 and CD31.3 Vascular endothelial cells were used as the internal positive control for CD34 and CD31 immunostaining. We did not apply digital subtraction.2 As in our recent studies of gastric cancer, immunostaining for CK was also performed to define the tumour border in every specimen examined.2,7
Table 2.
Monoclonal antibodies used for immunohistochemical analysis
| Antibody (clone) | Specificity | Source | Working dilution | Antigen retrieval |
| 1A4 | ASMA | DAKOpatts | 1/50 | Pronase |
| h-CD | HCD | DAKOpatts | 1/50 | Microwave |
| CAM5.2 | CK | Becton-Dickinson | 1/1 (prediluted) | Pronase |
| MY10 | CD34 | Becton-Dickinson | 1/20 | None |
| JC/70A | CD31 | DAKOpatts | 1/20 | Pronase |
ASMA, α smooth muscle actin; CK, low molecular weight cytokeratins; HCD, high molecular weight caldesmon.
In the 13 diffuse-type carcinomas invading the subserosa, double immunostaining for ASMA and CD34 was performed. ASMA and CD34 were labelled with alkaline phosphatase–fast blue and 3,3`-diaminobenzidine tetrachloride (DAB), respectively.1
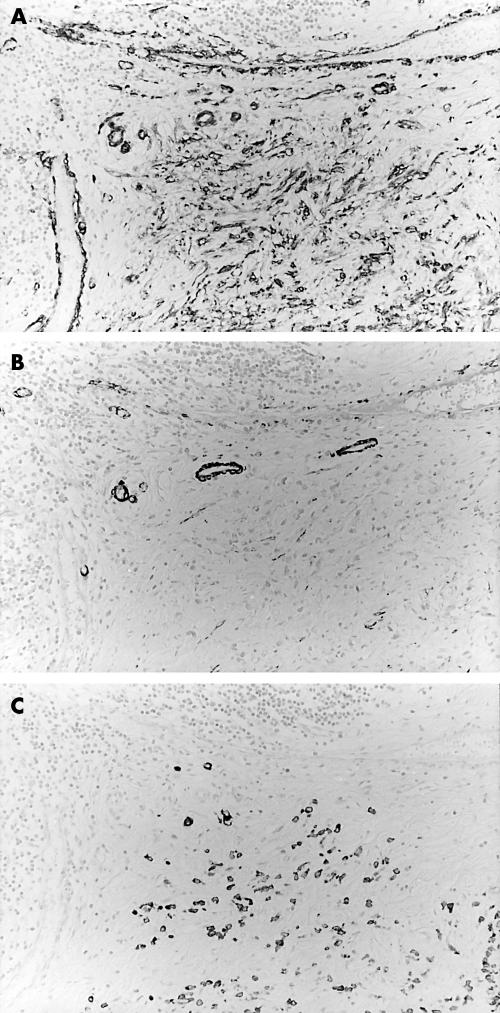
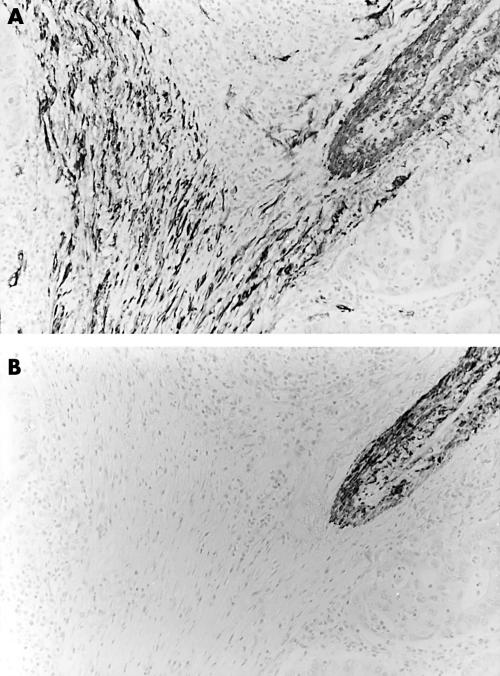
After immunostaining, we examined ASMA positive stromal cell distribution in the main body of gastric carcinoma tissues. The assessment was not based on the tumour growing edge and ulcerated surface. Tumours were classified into two types, namely: ASMA(+), tumours having ASMA positive stromal cells in the tumour stroma (fig 1A; note: fig 1B,C shows the expression of HCD and CK, respectively, in the same site as figs 1Aand 2A; note: fig 2B shows the expression of HCD in the same sites as fig 2A); and ASMA(−), tumours having no ASMA positive stromal cells within the tumour stroma. Statistical analysis was carried out using Fisher’s exact probability test. Values of p < 0.05 were considered to be significant. We did not perform statistical analysis in the solid-type carcinomas because of the low numbers of tumours analysed.
Figure 1.
Expression of α smooth muscle actin (ASMA) and CD34 in diffuse-type advanced gastric carcinoma tissue. Stains for (A) ASMA, (B) high molecular weight caldesmon (HCD), and (C) low molecular weight cytokeratins (CK). ASMA positive stromal cells are detected in the tumour stroma. Immunoreactivity for HCD is shown to distinguish ASMA positive stromal cells from smooth muscle cells. Immunoreactivity for CK is shown to identify diffuse-type gastric carcinoma cells.
Figure 2.
α Smooth muscle actin (ASMA) positive stromal cells in intestinal-type advanced gastric carcinoma tissue. Staining for (A) ASMA and (B) high molecular weight caldesmon (HCD). ASMA positive stromal cells—ASMA positive and HCD negative cells—are present within the tumour tissue.
RESULTS
Table 3 summarises the results. In the 74 cases examined, only six of the 24 diffuse-type gastric carcinomas had ASMA positive stromal cells in the tumour stroma, whereas 26 of the 43 intestinal-type gastric carcinomas had ASMA positive stromal cells in the tumour stroma (p = 0.00052). All of the seven solid-type carcinomas examined had ASMA positive stromal cells in the tumour stroma.
Table 3.
The relation between histological type and α smooth muscle actin (ASMA) positive stromal cells in the tumour stroma of the 74 gastric carcinomas
| ASMA positive stromal cells | |||
| Tumour depth | Number of cases | + | – |
| Diffuse type | |||
| Total | 24* | 6* | 18* |
| Early | |||
| sm | 6** | 0** | 6** |
| Advanced | |||
| mp+ss | 18*** | 6*** | 12*** |
| mp | 5** | 0** | 5** |
| ss | 13** | 6** | 7** |
| Intestinal type | |||
| Total | 43* | 26* | 17* |
| Early | |||
| sm | 21**** | 5**** | 16**** |
| Advanced | |||
| mp+ss | 22***,**** | 21***,**** | 1***,**** |
| mp | 8 | 8 | 0 |
| ss | 14 | 13 | 1 |
| Solid type | |||
| Total | 7 | 7 | 0 |
| Early | |||
| sm | 2 | 2 | 0 |
| Advanced | |||
| mp+ss | 5 | 5 | 0 |
| mp | 1 | 1 | 0 |
| ss | 4 | 4 | 0 |
*, p=0.00052; **,p=0.013; ***, p=0.000035; ****, p=0.0000011.
sm, submucosa; mp, muscularis propria; ss, subserosa; +, ASMA positive stromal cells; –, no ASMA positive stromal cells.
In the 24 diffuse type gastric carcinomas examined, all six tumours with ASMA positive stromal cells were advanced cancers invading the subserosa. Six of the 13 advanced cancers invading the subserosa were ASMA(+), whereas all of the six early cancers confined to the submucosa and all of the five advanced cancers invading the muscularis propria were ASMA(−) (p = 0.013). No stromal cells double positive for ASMA and CD34 were detected within the tumours examined.
In the 43 intestinal-type carcinomas, five of the 21 early cancers were ASMA(+), whereas 21 of the 22 advanced cancers were ASMA(+) (p = 0.0000011).
In the 45 advanced cancers (18 diffuse type, 22 intestinal type, and five solid type), six of the 18 diffuse-type carcinomas were ASMA(+), whereas 21 of the 22 intestinal-type carcinomas were ASMA(+) (p = 0.000035).
With regard to CD34 positive stromal cells, the results were almost the same as in our previous study.2 Seventeen (four invading the muscularis propria and 13 invading the subserosa) of the 18 diffuse-type advanced carcinomas had CD34 positive stromal cells in the tumour stroma, whereas the remaining seven diffuse-type carcinomas—all intestinal-type carcinomas—and all solid-type carcinomas had no CD34 positive stromal cells. No stromal cells double positive for ASMA and CD34 were detected within the diffuse-type tumours examined.
DISCUSSION
Myofibroblasts appear both at the tumour periphery and in the tumour, in response to tumour growth.9,17,18 Myofibroblasts have heterogenous cytoskeletal phenotypes with regard to their content of intermediate filaments (vimentin and desmin), ASMA, β actin, γ actin, and myosin.9 The ASMA positive stromal cells in our present study belong to the myofibroblast family.9 Our study focused on ASMA positive stromal cells, not on other types of myofibroblasts, such as the V (cells expressing vimentin) and VD (cells positive for vimentin and desmin) types. HCD is a specific marker for smooth muscle cells and is negative for myofibroblasts,8 so we recently combined immunostaining for ASMA and HCD to discriminate ASMA positive stromal cells from smooth muscle cells.
The following hypothesis regarding circulating fibroblasts is postulated13: circulating CD34 positive fibroblast precursor cells interact with activated T cells, which permits their early differentiation (towards the fibroblast phenotype), and they then migrate to the wound site.13 Within the wound site, these early differentiated fibroblasts might further interact with recruited T cells and fully differentiate and mature following exposure to transforming growth factor β.13 These fully differentiated, mature fibroblasts express increased amounts of ASMA and produce collagen and other extracellular matrix proteins that promote wound healing and contracture. ASMA positive stromal cells are fully differentiated mature fibroblastic cells.
Take home messages.
α Smooth muscle actin (ASMA) positive stromal cells and CD34 positive stromal cells appear to be associated with tumour stroma formation in diffuse-type advanced gastric carcinomas invading the subserosa
The presence of ASMA positive stromal cells and the lack of CD34 positive stromal cells is associated with tumour stroma formation of intestinal-type and solid-type carcinomas
Further molecular morphological and biological investigations will be necessary to elucidate the pathobiological relevance of these immunohistochemical findings
Accordingly, in view of the distribution of ASMA positive stromal cells,13 the stroma of diffuse-type early gastric carcinomas and diffuse-type advanced gastric carcinomas invading the muscularis propria may be immature, whereas that of intestinal-type advanced gastric carcinomas invading the muscularis propria and subserosa may be mature. In addition, because of the distribution of CD34 positive stromal cells, the stroma of diffuse-type advanced gastric carcinomas may be also immature, whereas that of intestinal-type gastric carcinomas may be mature.2 There is a possibility that transforming growth factor β produced by gastric carcinoma cells is associated with diffuse fibrous stroma formation, and that the loss of imprinting and overexpression of insulin-like growth factor II play an important role in the development of diffuse-type gastric carcinomas.19,20 The stromal cells in solid-type carcinomas may be mature, judging from the absence of CD34 and the presence of ASMA.
“To elucidate the pathobiological relevance of these immunohistochemical findings, further molecular morphological and biological investigations are needed”
In conclusion, six of the 13 diffuse-type advanced carcinomas invading the subserosa, five of the 21 intestinal-type early carcinomas, 21 of the 22 intestinal-type advanced carcinomas, and all seven solid-type carcinomas had ASMA positive stromal cells in the tumour stroma. CD34 positive stromal cells were found only in the tumour stroma of the diffuse-type advanced gastric carcinomas. These results suggest that the presence of ASMA positive stromal cells and CD34 positive stromal cells is associated with tumour stroma formation of diffuse-type advanced gastric carcinomas invading the subserosa, and that the presence of ASMA positive stromal cells and the lack of CD34 positive stromal cells is also associated with tumour stroma formation of intestinal-type and solid-type carcinomas. To elucidate the pathobiological relevance of these immunohistochemical findings, further molecular morphological and biological investigations are needed.
Acknowledgments
The authors are grateful to Ms M Yamamoto, Ms H Yamasaki, and Mr T Tokaji, First Department of Pathology, Kochi Medical School and Ms Y Yamasaki-Nabeshima and Ms M Tsutsui-Shimomura Department of Pathology, Chikamori Hospital for their excellent technical assistance. This work was partly supported by Foundation for Promotion of Cancer Research in Japan, and Grant-in-Aid for Cancer Research from the Ministry of Health and Welfare of Japan.
Abbreviations
ASMA, α smooth muscle actin
HCD, high molecular weight caldesmon
REFERENCES
- 1.Nakayama H, Enzan H, Miyazaki E, et al. Differential expression of CD34 in colorectal normal tissue, peritumoral inflammatory tissue, and tumour stroma. J Clin Pathol 2000;53;626–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakayama H, Enzan H, Miyazaki E, et al. CD34-positive stromal cells in gastric adenocarcinomas. J Clin Pathol 2001;54:846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miettinen M, Lindenmayer AE, Chaubal A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens: evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factor. Mod Pathol 1994;7:82–90. [PubMed] [Google Scholar]
- 4.Sappino AP, Dietrich PY, Skalli O, et al. Colonic pericryptal fibroblasts. Differentiation pattern in embryogenesis and phenotypic modulation in epithelial proliferative lesions. Virchows Arch A Pathol Anat Histopathol 1989;415:551–7. [DOI] [PubMed] [Google Scholar]
- 5.Nakayama H, Miyazaki E, Enzan H. Differential expression of high molecular weight caldesmon in colorectal pericryptal fibroblasts and tumor stroma. J Clin Pathol 1999;52;785–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakayama H, Enzan H, Miyazaki E, et al. The role of myofibroblasts at the tumor border of invasive colorectal adenocarcinomas. Jpn J Clin Oncol 1998;28:615–20. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama H, Enzan H, Miyazaki E, et al. Myofibroblasts at the tumor border of invasive gastric adenocarcinomas: with reference to tumor depth and histological type. Oncol Rep 2000;7:1011–15. [DOI] [PubMed] [Google Scholar]
- 8.Lazard D, Sastre X, Frid MG, et al. Expression of smooth muscle-specific proteins in myoepithelium and stromal myofibroblasts of normal and malignant human breast tissue. Proc Natl Acad Sci U S A 1993;90:999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schurich W, Seemayer TA, Gabbiani G. The myofibroblast; a quarter century after its discovery. Am J Surg Pathol 1998;22:141–7. [DOI] [PubMed] [Google Scholar]
- 10.Halvorsen TB, Semin E. Association between invasiveness, inflammatory reaction, desmoplasia and survival in colorectal cancer. J Clin Pathol 1989;42:162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Civin CI, Strauss LC, Brovall C, et al. Antigenic analysis of hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol 1984;133:157–65. [PubMed] [Google Scholar]
- 12.Dvorak HF. Tumor: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986;315:1650–9. [DOI] [PubMed] [Google Scholar]
- 13.Abe R, Donnelly SC, Peng T, et al. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001;166:7556–62. [DOI] [PubMed] [Google Scholar]
- 14.Chesney J, Metz C, Stavitsky AB, et al. Regulated production of type I collagen inflammatory cytokines by peripheral blood fibrocytes. J Immunol 1998;160:419–25. [PubMed] [Google Scholar]
- 15.Lauren P. The two main types of gastric carcinoma. Diffuse and so-called intestinal type carcinomas. Acta Pathol Microbiol Scand 1965;64:31–49. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe H, Jass JR, Sobin H, in collaboration with pathologists in eight countries. Histological typing of oesophageal and gastric tumors, 2nd ed, edited by the World Health Organization (WHO), Berlin, Heidelberg, Germany: Springer-Verlag, 1990.
- 17.Seemayer TA, Lagace R, Schurch W, et al. Myofibroblasts in the stroma of invasive and metastatic carcinoma. A possible host response to neoplasia. Am J Surg Pathol 1979;3:525–33. [DOI] [PubMed] [Google Scholar]
- 18.Hewitt RE, Powe DG, Carter GI, et al. Desmoplasia and its relevance to colorectal tumor invasion. Int J Cancer 1993;53:62–9. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida K, Yokozaki H, Niimoto M, et al. Expression of TGF-beta and procollagen type I and type III in human gastric carcinomas. Int J Cancer 1989;44:394–8. [DOI] [PubMed] [Google Scholar]
- 20.Wu MS, Wang HP, Lin CC, et al. Loss of imprinting and overexpression of IGF-2 gene in gastric adenocarcinoma. Cancer Lett 1997;120:9–14. [DOI] [PubMed] [Google Scholar]