Abstract
Aim: To assess the specificity and sensitivity of the commonly used enzymatic colorimetric test for plasma cholesterol determination.
Methods: Interference with an enzymatic method for cholesterol measurement by several non-cholesterol sterols (β sitosterol, campesterol, stigmasterol, stigmastanol, desmosterol, and lathosterol) was assessed. Some of these compounds are present in plasma at higher than normal concentrations either in rare genetic disorders, such as phytosterolaemia, or after the consumption of phytosterol enriched foods.
Results: The non-cholesterol sterols were detected by the assay in a linear manner. There was no competitive interference in the presence of cholesterol.
Conclusions: This crossreactivity may affect the diagnosis and treatment of non-cholesterol dyslipidaemias, including phytosterolaemia and cerebrotendinous xanthomatosis. Similarly, changes in plasma lipid compositions after the consumption of phytosterol enriched foods cannot be specifically determined by this enzymatic assay. Until a more specific enzymatic assay is developed, alternative methods such as gas chromatography should be used to differentiate between cholesterol and non-cholesterol sterols.
Keywords: cholesterol, non-cholesterol sterols, enzymatic assay
Increased plasma cholesterol concentrations are a causal factor in the development of coronary heart disease (CHD). Both American and Canadian guidelines recommend annual plasma cholesterol/lipid measurements in patients with more than two major risk factors for CHD.1,2 Patients with fewer risk factors may only need to check their plasma cholesterol values every five years or less.1,2 In addition, CHD develops in rare non-hypercholesterolaemic recessive genetic disorders, such as phytosterolaemia3 and cerebrotendinous xanthomatosis (CTX)4; in particular, CHD related deaths are frequently reported in young men with phytosterolaemia.5 Higher absorption and lower excretion of plant sterols play a causal role in the development of phytosterolaemia.5 The accumulation of cholestanol in the plasma and tissues of patients with CTX may account for multiple coronary artery aneurysms and myocardial ischaemia.4
Patients with hypercholesterolaemia respond to treatment with cholesterol lowering agents such as statins, fibrates, or bile acid resins. However, some of these agents are not of value in phytosterolaemia and CTX. In fact, bile acid resins, a treatment of choice for sitosterolaemia, are contraindicated in CTX. Another strategy for reducing plasma cholesterol concentrations in individuals with hypercholesterolaemia is the consumption of phytosterol enriched foods.3 These may cause an increase in plasma concentrations of plant sterols, particularly β sitosterol and campesterol.6,7 Similarly, both human and animal studies showed that the consumption of plant sterols is associated with increases or the appearance of desmosterol and lathosterol in plasma.8–10 Although the longterm importance of this small increase in the concentrations of non-cholesterol sterols in human plasma is unknown, both patients and physicians should be able to detect such changes.
“Higher absorption and lower excretion of plant sterols play a causal role in the development of phytosterolaemia”
The commonly used enzymatic assay for cholesterol measurement lacks the specificity to discriminate plasma cholesterol from non-cholesterol sterols. The aim of our present study was to determine the extent of specificity of the enzymatic cholesterol assay for several phytosterols and other non-cholesterol sterols that may appear in plasma as a result of changes in cholesterol metabolism during the management of hypercholesterolaemia, or in certain rare genetic disorders of lipoprotein metabolism.
MATERIALS AND METHODS
All chemicals including cholesterol (catalogue number, C 8667; purity, > 99%), β sitosterol (catalogue number, S 9889; purity, > 97%), campesterol (catalogue number, C 5157; purity, ∼ 65%), stigmasterol (catalogue number, S 2424; purity,∼ 95%), stigmastanol (catalogue number, S 4297; purity, > 95%), desmosterol (catalogue number, D 6513; purity, > 85%), and lathosterol (catalogue number, C 3652) were purchased from Sigma (Oakville, Ontario, Canada). The stock solutions were prepared by dissolving the chemicals in cholesterol diluent (Wako Chemicals GmbH, Neuss, Germany) using a hot plate and stirring. Five different concentrations were made from each stock solution. The concentrations of the chemicals were determined by the enzymatic colorimetric test for cholesterol determination currently used in the clinical laboratory, St Paul’s Hospital, Vancouver, Canada. Briefly, Hitachi analyser 911 (Boehringer Mannheim, Indianapolis, Indiana, USA) and cholesterol reagent (catalogue number, 1489232; Boehringer Mannheim) were used. This method is based on the determination of Δ4 cholestenone after enzymatic cleavage of the cholesterol ester by cholesterol esterase, conversion of cholesterol by cholesterol oxidase, and the consequent formation of a red dyestuff after the reaction of 4-aminophenazone with phenol under the catalytic action of peroxidase.11 The colour intensity is directly proportional to the concentration of cholesterol and is determined photometrically. The “false positive” results (cholesterol reading) were plotted against the real concentrations of each specific compound. A free cholesterol standard solution (catalogue number, 274–47109; Wako Chemicals GmbH) containing 2.6 mmol/litre cholesterol was also used to determine the extent of competitive interference in the presence of native cholesterol. There was no need to obtain ethics approval for our study because of the lack of use of animals, humans, or their tissues.
RESULTS
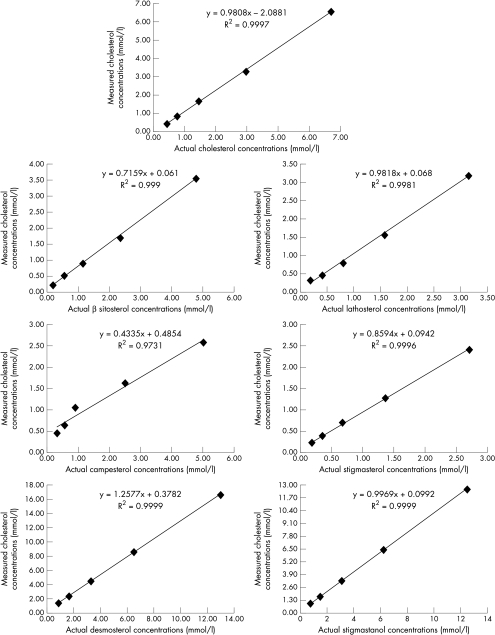
Figure 1 shows values for non-cholesterol sterols as determined by the routinely used enzymatic assay for cholesterol. All compounds tested were detected by the cholesterol assay in a linear manner. The assay was sensitive for all these compounds; the lowest concentration of sterols tested and detected was > 0.2 mmol/litre. The assay crossreacted with β sitosterol and campesterol (two plant sterols abundant in nature with relatively high absorption rates and plasma concentrations). For example, the assay read equivalent to 3.5 and 2.6 mmol/litre cholesterol for 5.2 mmol/litre of β sitosterol and campesterol, respectively, whereas it read 1.7 mmol/litre for 2.6 mmol/litre of both compounds. The reading was comparable to the actual concentrations at lower values (< 2.6 mmol/litre) for both compounds. In contrast, the assay consistently showed higher (by 30–70%) concentrations of desmosterol. This was more pronounced at lower actual concentrations (1.4 mmol/litre for 0.8 mmol/litre, a 70% increase in reading) of desmosterol as compared with higher actual concentrations (16.7 mmol/litre for 13 mmol/litre, a 28% increase in reading). The results were comparable to the actual concentrations for other compounds including cholesterol, lathosterol, stigmastanol, and stigmasterol. When various known concentrations of non-cholesterol sterol were added to a cholesterol containing solution (2.6 mmol/litre) the assay read the concentrations of both sterols (cholesterol and non-cholesterol) as cholesterol in an additive manner. This indicates a lack of competitive interference in the presence of native cholesterol.
Figure 1.
False determination of cholesterol by various non-cholesterol sterols including β sitosterol, campesterol, stigmasterol, stigmastanol, desmosterol, and lathosterol using a common enzymatic assay.
DISCUSSION
Plant sterols have chemical structures similar to that of cholesterol. Therefore, they interfere with cholesterol absorption and thus can be used for dietary management of mild hypercholesterolaemia. For plant sterols, normally only up to 5% of the dose ingested is absorbed, whereas the absorption rate for cholesterol is up to 50%. The consumption of plant sterol enriched foods results in higher plasma concentrations of some phytosterols, such as β sitosterol and campesterol, and endogenous sterols such as desmosterol and lathosterol.6–9 For example, the ratio of plasma campesterol to cholesterol concentrations increased more than 50% (from 3.39 to 5.18) in men with hypercholesterolaemia during a 30 day period of consumption of a phytosterol enriched diet.7 This reflects significant decreases in the plasma cholesterol concentration and increases in plasma campesterol concentrations. An intake of 2 g of plant sterols each day may double plasma campesterol and sitosterol values.
In patients with sitosterolaemia (phytosterolaemia), a rare genetic disorder, plant sterols accumulate in both the plasma and the tissues. Plasma plant sterols are increased from approximately 0.05 mmol/litre in healthy individuals to 1.2 mmol/litre in patients with sitosterolaemia.3 Although plasma cholesterol concentrations may be in the normal range or raised, the clinical symptoms of the disease resemble those of hypercholesterolaemia, including coronary artery disease and xanthomatosis.3,5
Our data provide evidence for the possibility of a low detection rate for sitosterolaemia world wide. In general, such patients may be misdiagnosed as having hypercholesterolaemia based on the results of plasma cholesterol determination. Thus, their treatment may not be appropriate. Moreover, the use of phytosterol enriched food products as one of the current strategies in the management of mild hypercholesterolaemia1 may result in the accumulation of plant sterols6,7 and other endogenous non-cholesterol sterols (desmosterol and lathosterol)8,9 in plasma, which interfere with plasma cholesterol determination.
“Our data provide evidence for the possibility of a low detection rate for sitosterolaemia world wide”
It is well documented that unlike cholesterol, relatively small increases in plasma concentrations of non-cholesterol sterols, such as plant sterols (in phytosterolaemia) or cholestanol (in CTX), can result in clinical events, including myocardial infarction. Therefore, it is important to develop an enzymatic assay with a higher specificity for cholesterol. In the meantime, the use of other methods, including gas chromatography, is recommended in patients who show no observable cholesterol lowering in response to hypocholesterolaemic agents, those with symptoms of CHD in the absence of high plasma cholesterol concentrations, subjects with a family history of sitosterolaemia or CTX, or people who are consuming phytosterol enriched diets for a prolonged period.
Take home messages.
Non-cholesterol sterols were detected by the commonly used enzymatic colorimetric test assay in a linear manner and there was no competitive interference in the presence of cholesterol
This crossreactivity may affect the diagnosis and treatment of non-cholesterol dyslipidaemias, including phytosterolaemia and cerebrotendinous xanthomatosis
In addition, changes in plasma lipid compositions after the consumption of phytosterol enriched foods cannot be specifically determined by this enzymatic assay
Until a more specific enzymatic assay is developed, alternative methods such as gas chromatography should be used to differentiate between cholesterol and non-cholesterol sterols
Abbreviations
CHD, coronary heart disease
CTX, cerebrotendinous xanthomatosis
REFERENCES
- 1.Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001;285:2486–91. [DOI] [PubMed] [Google Scholar]
- 2.Fodor JG, Frohlich JJ, Genest JJ, Jr, et al. Recommendations for the management and treatment of dyslipidaemia. Report of the working group on hypercholesterolaemia and other dyslipidaemias. Can Med Assoc J 2000;162:1441–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Moghadasian MH, Frohlich JJ. Effects of dietary phytosterols on cholesterol metabolism and atherosclerosis: clinical and experimental evidence. Am J Med 1999;107:588–94. [DOI] [PubMed] [Google Scholar]
- 4.Potkin BN, Hoeg JM, Connor WE, et al. Aneurysmal coronary artery disease in cerebrotendinous xanthomatosis. Am J Cardiol 1988;61:1150–2. [DOI] [PubMed] [Google Scholar]
- 5.Salen G, Shefer S, Nguyen L, et al. Sitosterolaemia. J Lipid Res 1992;33:945–55. [PubMed] [Google Scholar]
- 6.Weststrate JA, Meijer GW. Plant sterol-enriched margarines and reduction of plasma total- and LDL-cholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur J Clin Nutr 1998;52:334–43. [DOI] [PubMed] [Google Scholar]
- 7.Jones PJH, Ntanious FY, Raeini-Sarjaz M, et al. Cholesterol-lowering efficacy of a sitostanol-containing phytosterol mixture with a prudent diet in hyperlipidaemic men. Am J Clin Nutr 1999;69:1144–50. [DOI] [PubMed] [Google Scholar]
- 8.Gyllying H, Puska P, Vartiainen E, et al. Serum sterols during stanol ester feeding in a mildly hypercholesterolaemic population. J Lipid Res 1999;40:593–600. [PubMed] [Google Scholar]
- 9.Vanhanen HT, Kajander J, Lehtovirta H, et al. Serum levels, absorption efficacy, faecal elimination and synthesis of cholesterol during increasing doses of dietary sitostanol esters in hypercholesterolaemic subjects. Clin Sci 1994;87:61–7. [DOI] [PubMed] [Google Scholar]
- 10.Moghadasian MH, Nguyen LB, Shefer S, et al. Hepatic cholesterol and bile acid synthesis, low density lipoprotein receptor function, and plasma and fecal sterol levels in mice: effects of apolipoprotein E deficiency and probucol or phytosterol treatment. Metabolism 2001;50:708–14. [DOI] [PubMed] [Google Scholar]
- 11.Allain CC, Poon LS, Chan CS, et al. Enzymatic determination of total serum cholesterol. Clin Chem 1974;20:470–5. [PubMed] [Google Scholar]