Abstract
Most post transplantation lymphoproliferative disorders (PTLDs) are Epstein-Barr virus (EBV) associated B cell proliferations. We report a case of aggressive anaplastic large cell lymphoma expressing the anaplastic lymphoma kinase (ALK) protein in a 58 year old man who had previously undergone liver transplantation. A definite diagnosis was not possible on histopathological examination. Immunostaining clearly showed a predominant population of small irregular lymphocytes, admixed with large cells strongly positive for CD30, epithelial membrane antigen, and the ALK protein. Neoplastic cells were of the T/cytotoxic phenotype. In situ hybridisation with EBV encoded early RNA probes showed only a few scattered positive non-neoplastic small lymphocytes. Polymerase chain reaction analysis of immunoglobulin and T cell receptor rearrangements was negative. The NPM–ALK fusion transcript associated with the t(2;5) translocation was detected by reverse transcription polymerase chain reaction. A review of the literature revealed 76 cases of T cell PTLD, showing a broad spectrum of morphological features and clinical behaviour. Most of these cases were EBV negative (61 of 76) and occurred after renal transplantation (48 of 76). To our knowledge, this is the first case of ALK positive lymphoma occurring in the setting of organ transplantation. This observation stresses the need for accurate immunostaining for diagnosing this rare, apparently aggressive, lymphoma in immunosuppressed patients.
Keywords: ALK+ lymphoma, immunohistochemistry, liver transplantation
Post transplantation lymphoproliferative disorders (PTLDs) are a morphologically heterogenous group of usually Epstein-Barr virus (EBV) driven B lymphoid proliferations.1,2 T cell PTLDs are much less frequent, and usually are EBV, human herpesvirus 8 (HHV-8), human T cell lymphotropic virus type 1 (HTLV-1), and HTLV-2 negative,3 with the notable exception of Japanese patients who have undergone renal transplantation and who develop tumours often associated with HTLV-1.4 Among T cell PTLDs, peripheral T cell lymphomas unspecified,4–13 T lymphoblastic lymphoma,5 large granular cell leukaemia,3,14–17 natural killer cell lymphoma,4,18,19 T γδ hepatosplenic lymphoma,20–22 cutaneous T cell lymphoma,5 and CD30+ anaplastic large cell lymphoma5,10,23,24 have been described, mostly in renal transplant recipients.
Here, we report the first case of ALK positive lymphoma occurring in the setting of solid organ transplantation and review cases of post transplant T cell lymphoma reported in the literature.
CASE REPORT
A 58 year old man underwent transplantation in July 1997 for hepatitis B cirrhosis. He had an uncomplicated course for 41 months, after which he presented with fever and axillary lymphadenopathy. After lymph node excision, the diagnosis of ALK positive anaplastic lymphoma was made. In spite of chemotherapy the patient rapidly developed lymphomatous pleural effusion and diarrhoea. Serological tests for EBV, human immunodeficiency virus, and HTLV-1 were negative. Bone marrow biopsy did not reveal lymphomatous involvement. Two months later, the patient presented with neurological problems with confusion, and a scannographic investigation suggested cerebral involvement. He died one week later. Necropsy was not performed.
PATHOLOGICAL FINDINGS
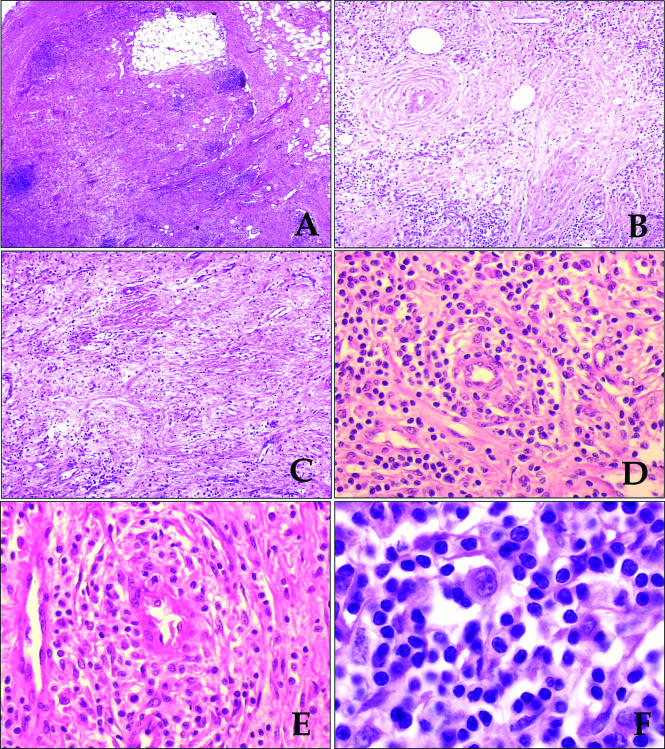
The lymph node architecture was obliterated by a lymphoid proliferation extending into the perinodal tissue. Residual follicles were seen and their dark appearance was in sharp contrast to the relatively pale staining of neoplastic sheets, which seemed to involve predominantly the paracortical area. In some areas, neoplastic cells were found in a background of oedematous, fibromyxoid stroma; collagen bands were also seen. Necrosis was absent (fig 1A–D). The morphological features of malignant cells were variable but, overall, the tumour consisted of a predominant population of small to medium sized cells with irregular, sometimes cerebriform, nuclei (fig 1E). Closer examination showed scattered large cells with the highly characteristic morphology of “hallmark cells” (fig 1F).25 Non-neoplastic cells consisted of plasma cells and histiocytes, with plasma cells predominating.
Figure 1.
(A) The lymph node had a hypocellular appearance with residual small lymphoid follicles. The capsule is shown on the left side. (B,C) Lymphoid cells were loosely dispersed in an oedematous stroma, rich in venules. (D,E) Higher magnifications showed small to medium sized lymphoid cells dispersed in a oedematous stroma, with accentuation of the cellularity around the venules. (F) Rare large cells with the features of hallmark cells (eccentrically located reniform or embryo-like nucleus and abundant amphophilic cytoplasm) were present.
IMMUNOPHENOTYPE AND GENETIC FEATURES OF NEOPLASTIC CELLS
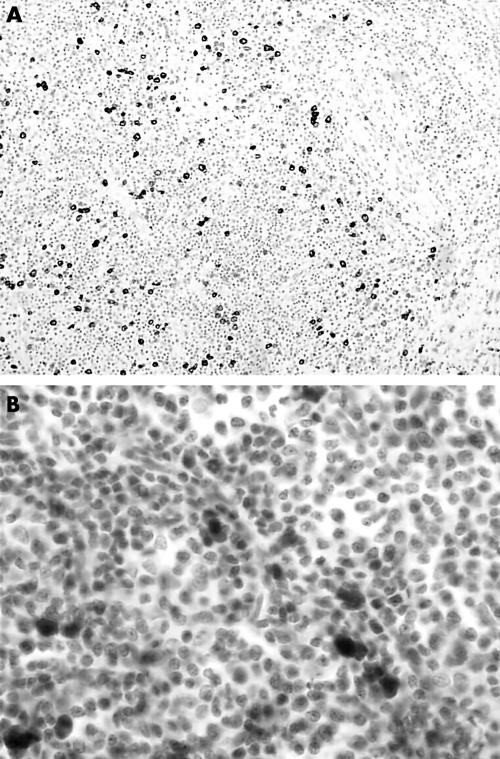
Because of the suspected diagnosis of anaplastic large cell lymphoma, immunostaining with a large panel of monoclonal antibodies was performed. The CD30/Ber-H2 antibody reacted strongly with the large cells that were often seen around blood vessels (fig 2A) and weakly with small and medium sized neoplastic cells. The most interesting pattern of staining was seen with the monoclonal anti-ALK antibody, which reacts with the tyrosine kinase domain of the ALK protein. Large neoplastic cells showed strong cytoplasmic, nuclear, and nucleolar staining, whereas in the small and medium sized neoplastic cells the staining was restricted to the nucleus (fig 2B). Antibodies directed against T cell associated antigens were difficult to interpret because of the admixture of neoplastic and reactive lymphoid cells. However, neoplastic cells were found to be strongly reactive for CD43 and weakly positive for CD3. They were also positive for TIA1, perforin, and granzyme B cytotoxic granule associated proteins. Neoplastic cells were considered to be negative for the CD2, CD4, CD5, and CD8 antigens. The CD20/L26 monoclonal antibody reacted with residual follicles and the rare immunoblasts, and the plasma cells were polyclonal. Immunostaining with an anti-latent membrane protein 1 antibody was negative and in situ hybridisation using EBV encoded early RNA probes showed very rare positive CD20 positive B cells corresponding to reservoir B cells.
Figure 2.
(A) Immunostaining for CD30 revealed a greater number of neoplastic cells than could be appreciated on routine histological sections. (B) ALK staining was observed in both the nuclei and cytoplasm of the tumour cells.
Genomic DNA was extracted from frozen lymph node sections. Polymerase chain reaction (PCR) analyses for immunoglobulin heavy chain gene rearrangement (FR1-JH, FR2-JH, and FR3-JH primers) and for T cell receptor γ and β chain gene rearrangements were negative. Total RNA was extracted from the lymph node sample and peripheral blood mononuclear cells. The detection of the nucleophosmin (NPM)–ALK fusion transcript was performed by reverse transcription PCR analysis, as described previously.26 A clear band indicating the presence of a cell population bearing the NPM–ALK rearrangement was detected in both samples.
The final diagnosis was anaplastic large cell lymphoma (small cell variant) with a T cytotoxic phenotype expressing the NPM–ALK protein (ALKoma).
DISCUSSION
T cell PTLDs are relatively rare: only 76 cases of have been reported to date. They include a broad morphological spectrum of lesions.4–24 Clinically, these T cell proliferations range from indolent oligoclonal expansions of T cells (large granular lymphocytes, CD8+ and CD57+)14 to aggressive EBV negative T cell or natural killer cell lymphomas, consisting of intermediate to large sized lymphoid cells that usually occur late after transplantation and do not respond to a decrease in or withdrawal from immunosuppression.3,9,18,20–22 Only 15 of these 76 cases were EBV positive.4,5,7,9,12,16,19 In most cases (48 of 76) these lymphoproliferations occurred after renal transplantation. We only found two reported cases in patients after liver transplantation: an aggressive large granular lymphocytic leukaemia14 and a liver localised T cell lymphoma without other specification.27 In a recent study on PTLD, using univariate analysis, Leblond et al found that T cell origin, monoclonality, and the lack of EBV detection were adverse prognostic factors.28
Anaplastic large cell lymphoma was first described in 1985 as a previously unrecognised lymphoid tumour in which the neoplastic cells were labelled by the monoclonal antibody Ki-1 (this cell surface antigen is now known as the CD30 molecule).29 It was subsequently shown that many (31–85% of cases) of these tumours are associated with a recurrent translocation, the t(2;5) (p23;q35) translocation.25,30,31 Expression of the ALK protein in these tumours is usually the result of the (2;5)(p23;q35) chromosome translocation, which fuses the ALK gene at 2p23 with the NPM gene at 5q35.26 The ALK gene encodes a tyrosine kinase receptor belonging to the insulin growth factor receptor superfamily, which is normally expressed in nerve cells but silent in normal lymphoid cells.32 In contrast, the NPM gene encodes a nucleolar phosphoprotein (involved in shuttling ribonucleoproteins from the cytoplasm to the nucleus), which is widely expressed. Its ubiquitous presence in normal lymphoid cells means that the NPM–ALK gene will be transcribed if cells acquire the (2;5) translocation. Recently, Pulford et al reported some evidence that the ALK protein is immunogenic.33 Except for rare cases of diffuse large B cell lymphoma expressing the full length ALK protein,34 the hybrid NPM–ALK protein is only expressed by anaplastic large cell lymphoma of T or null phenotype, sometimes referred to as ALKoma.26 These ALK positive lymphomas show a broad spectrum of morphological features, including cases with a predominant population of small sized cells (also referred to as small cell variant anaplastic large cell lymphoma), as in our present case. Some cases may mimic inflammatory lesions of lymph nodes, and such a diagnosis was originally considered in our patient.35 As in our present case, anaplastic large cell lymphomas are of T or null phenotype and often express the cytotoxic molecules perforin, granzyme B, and TIA-1.
Take home messages.
To our knowledge, this is the first case of anaplastic lymphoma kinase (ALK) positive lymphoma occurring after organ transplantation
The neoplastic cells were of the T/cytotoxic phenotype
The NPM–ALK fusion transcript associated with the t(2;5) translocation was detected by reverse transcription polymerase chain reaction
Accurate immunostaining is necessary for the diagnosis of this rare, and apparently aggressive, lymphoma in immunosuppressed patients
“In immunosuppressed patients ALK positive anaplastic large cell lymphomas seem to be unusually aggressive because our patient died two months after diagnosis, whereas in immunocompetent patients the expression of the ALK protein is associated with a favourable prognosis”
To our knowledge, this is the first case of ALK positive lymphoma occurring after organ transplantation, although previously reported ALCL cases were not investigated for ALK protein expression.5,9,23,24
Because this lymphoma was EBV negative and did not respond to decreased immunosuppression we cannot definitely say that it was related to the immune suppressed state. Fortuitous occurrence of this type of lymphoma in a patient after liver transplantation is a remote possibility. Nonetheless, in immunosuppressed patients ALK positive anaplastic large cell lymphomas seem to be unusually aggressive because our patient died two months after diagnosis, whereas in immunocompetent patients the expression of the ALK protein is associated with a favourable prognosis (five year survival, 70%).30,31,36 Immunosuppressive treatment probably interferes with the specific B cell immune response to the NPM–ALK fusion kinase described by Pulford et al.33
Acknowledgments
This work was supported by the Projet Hospitalier de Recherche Clinique (PHRC 98), the Ligue Nationale contre le Cancer (Equipe Labellisée Ligue), the Ligue du Gers, and the Ligue du Lot.
Abbreviations
ALK, anaplastic lymphoma kinase
EBV, Epstein-Barr virus
HTLV, human T cell lymphotropic virus
NPM, nucleophosmin
PCR, polymerase chain reaction
PTLD, post transplantation lymphoproliferative disorders
REFERENCES
- 1.Nalesnik MA, Jaffe R, Starzl TE, et al. The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol 1988;133:173–92. [PMC free article] [PubMed] [Google Scholar]
- 2.Knowles D, Cesarman E, Chadburn A, et al. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of postransplantation lymphoproliferative disorders. Blood 1995;85:552–65. [PubMed] [Google Scholar]
- 3.Hanson MN, Morrison VA, Peterson BA, et al. Posttransplant T-cell lymphoproliferative disorders—an aggressive, late complication of solid organ transplantation. Blood 1996;88:3626–33. [PubMed] [Google Scholar]
- 4.Hoshida Y, Li T, Dong Z, et al. Lymphoproliferative disorders in renal transplant patients in Japan. Int J Cancer 2001;91:869–75. [DOI] [PubMed] [Google Scholar]
- 5.Van Gorp J, Doornewaard H, Verdonck L, et al. Post-transplant T-cell lymphoma: report of three cases and a review of the literature. Cancer 1994;73:3064–72. [DOI] [PubMed] [Google Scholar]
- 6.Labouyrie E, Morel D, Boiron JM, et al. Peripheral T-cell lymphoma in a chronically immuno suppressed renal transplant patient. Mod Pathol 1995;8:355–9. [PubMed] [Google Scholar]
- 7.Wu T, Swerdlow S, Locker J, et al. Recurrent Epstein-Barr virus-associated lesions in organ transplant recipients. Hum Pathol 1996;27:157–64. [DOI] [PubMed] [Google Scholar]
- 8.Ghorbani RP, Shokouh-Amiri H, Gaber LW. Intragraft angiotropic large-cell lymphoma of T cell type in a long-term renal allograft recipient. Mod Pathol 1996;9:671–6. [PubMed] [Google Scholar]
- 9.Harris N, Ferry J, Swerdlow S. Posttransplant lymphoproliferative disorders: summary of society for haematopathology workshop. Semin Diagn Pathol 1997;14:8–14. [PubMed] [Google Scholar]
- 10.Kim JY, Kim CW, Ahn C, et al. Rapidly developing T-cell postransplantation lymphoproliferative disorder. Am J Kidney Dis 1999;34:pE3. [DOI] [PubMed] [Google Scholar]
- 11.Berho M, Viciana A, Weppler D, et al. T cell lymphoma involving the graft of a multivisceral organ recipient. Transplantation 1999;68:1135–9. [DOI] [PubMed] [Google Scholar]
- 12.Yufu Y, Kimura M, Kawano R, et al. Epstein-Barr virus associated T cell lymphoproliferative disorder following autologous blood stem cell transplantation for relapsed Hodgkin’s disease. Bone Marrow Transplant 2000;26:1339–41. [DOI] [PubMed] [Google Scholar]
- 13.Wang LC, Lu MY, Yu J, et al. T cell lymphoproliferative disorder following bone marrow transplantation for severe aplastic anemia. Bone Marrow Transplant 2000;26:893–7. [DOI] [PubMed] [Google Scholar]
- 14.Feher O, Barilla D, Locker J, et al. T-cell large granular lymphocytic leukemia following orthotopic liver transplantation. Am J Hematol 1995;49:216–20. [DOI] [PubMed] [Google Scholar]
- 15.Gentile TC, Hadlock KG, Uner AH, et al. Large granular lymphocyte leukaemia occurring after renal transplantation. Br J Haematol 1998;101:507–12. [DOI] [PubMed] [Google Scholar]
- 16.Kwong YL, Lam CC, Chan TM. Post-transplantation lymphoproliferative disease of natural killer cell lineage: a clinicopathological and molecular analysis. Br J Haematol 2000;110:197–202. [DOI] [PubMed] [Google Scholar]
- 17.Nelson BP, Nalesnik MA, Bahler DW, et al. Epstein-Barr virus negative post-transplant lymphoproliferative disorders. Am J Surg Pathol 2000;24:375–85. [DOI] [PubMed] [Google Scholar]
- 18.Hsi ED, Picken MM, Alkan S. Post transplantation lymphoproliferative disorder of the NK-cell type: a case report and review of the literature. Mod Pathol 1998;11:479–84. [PubMed] [Google Scholar]
- 19.Mukai HY, Kojima H, Suzukawa K, et al. Nasal natural killer cell lymphoma in a post-renal transplant patient. Transplantation 2000;69:1501–3. [DOI] [PubMed] [Google Scholar]
- 20.Ross CW, Schnitzer B, Sheldon S, et al. Gamma/delta T-cell post-transplantation lymphoproliferative disorder primarily in the spleen. Am J Clin Pathol 1994;102:310–15. [DOI] [PubMed] [Google Scholar]
- 21.Kraus MD, Crawford DF, Kaleem Z, et al. T Gamma/delta hepatosplenic lymphoma in a heart transplant patient after an Epstein-Barr virus positive lymphoproliferative disorder: a case report. Cancer 1998;82:983–92. [DOI] [PubMed] [Google Scholar]
- 22.Wu H, Wasik MA, Przybylski G, et al. Hepatosplenic gamma-delta T cell lymphoma as a late-onset posttransplant lymphoproliferative disorder in renal transplant recipients. Am J Clin Pathol 2000;113:487–96. [DOI] [PubMed] [Google Scholar]
- 23.Audouin J, Le Tourneau A, Diebold J, et al. Primary intestinal lymphoma of Ki-1 large cell anaplastic type with mesenteric lymph node and spleen involvement in a renal transplant recipient. Hematol Oncol 1989;7:441–9. [DOI] [PubMed] [Google Scholar]
- 24.Jimenez-Heffernan JA, Viguer JM, Vicandi B, et al. Posttransplant CD30 (Ki-1)-positive anaplastic large cell lymphoma. Report of a case with presentation as a pleural effusion. Acta Cytol 1997;41:1519–24. [DOI] [PubMed] [Google Scholar]
- 25.Benharroch D, Megurian-Bedoyan Z, Lamant L, et al. ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood 1998;91:2076–84. [PubMed] [Google Scholar]
- 26.Lamant L, Meggetto F, al Saati T, et al. High incidence of the t(2;5)(p23;q35) translocation in anaplastic large cell lymphoma and its lack of detection in Hodgkin’s disease. Comparison of cytogenetic analysis, reverse transcriptase-polymerase chain reaction, and P80 immunostaining. Blood 1996;87:284–91. [PubMed] [Google Scholar]
- 27.Nuckols J, Baron P, Stenzel T, et al. The pathology of liver-localized post-transplant lymphoproliferative disease. A report of three cases and review of the literature. Am J Surg Pathol 2000;24:733–41. [DOI] [PubMed] [Google Scholar]
- 28.Leblond V, Dhedin N, Mamzer Bruneel MF, et al. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol 2001;19:772–8. [DOI] [PubMed] [Google Scholar]
- 29.Stein H, Mason DY, Gerdes J, et al. The expression of the Hodgkin disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 1985;66:848–56. [PubMed] [Google Scholar]
- 30.Falini B, Pileri S, Zinzani L, et al. ALK+ lymphoma: clinico-pathological findings and outcome. Blood 1999;93:2697–706. [PubMed] [Google Scholar]
- 31.ten Berge R, Dukers D, Oudejans J, et al. Adverse effects of activated cytotoxic T lymphocytes on the clinical outcome of nodal anaplastic large cell lymphoma. Blood 1999,93:2688–96. [PubMed] [Google Scholar]
- 32.Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non Hodgkin’s lymphoma. Science 1994;263:1281–8. [DOI] [PubMed] [Google Scholar]
- 33.Pulford K, Falini B, Banham A, et al. Immune response to the ALK oncogenic kinase in patients with anaplastic large-cell lymphoma. Blood 2000;96:1605–7. [PubMed] [Google Scholar]
- 34.Delsol G, Lamant L, Mariamé B, et al. A new subtype of large B-cell lymphoma expressing the ALK kinase and lacking the 2;5 translocation. Blood 1997;89:1483–9. [PubMed] [Google Scholar]
- 35.Cheuk W, Hill RW, Bacchi C, et al. Hypocellular anaplastic large cell lymphoma mimicking inflammatory lesions of lymph nodes. Am J Surg Pathol 2000;24:1537–43. [DOI] [PubMed] [Google Scholar]
- 36.Gascoyne R, Aoun P, Wu D, et al. Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood 1999;93:3913–21. [PubMed] [Google Scholar]