Abstract
A 72 year old woman presented complaining of nasal obstruction, rhinorrea, and epistaxis. At examination, a polypoid mass on the right posterior choana was revealed and subsequently removed. Light microscopic findings consisted of a diffuse proliferation of spindle shaped, pleomorphic cells with eosinophilic cytoplasm and blunt ended nuclei in a prominent myxoid background. The presence of numerous plurinucleate, bizarre cells made it very difficult to determine the malignant potential. Immunohistochemical evidence for leiomyogenic markers coupled with the low mitotic rate, the lack of an infiltrating growth pattern, and the indolent clinical course led to the diagnosis of atypical leiomyoma with prominent myxoid change. A literature survey confirmed that such a tumour is extremely rare at this site, but the biological behaviour seems to be similar to its uterine counterpart. Clinicians should be aware of this occurrence to prevent misdiagnosis because a conservative therapeutic approach is necessary in this disease.
Keywords: sinonasal cavity, atypical leiomyoma, bizarre cells, myxoid change
Primary leiomyogenic tumours have been reported in unusual locations where smooth muscle cells are usually absent, such as the sinonasal cavity.1 A recent literature review found only 22 cases of benign leiomyogenic tumours arising in the nose and paranasal sinuses.2 So far, the different variants include classic leiomyoma,1,2 leiomyoblastoma,3 and vascular leiomyoma,4 this last tumour being the most common histotype. Only one case of atypical leiomyoma has been described previously at this location,5 although it did not display myxoid change.
“Primary leiomyogenic tumours have been reported in unusual locations where smooth muscle cells are usually absent, such as the sinonasal cavity”
The finding of pronounced nuclear pleomorphism associated with prominent myxoid matrix in an otherwise leiomyogenic tumour has not been previously reported at this site and must be distinguished from other neoplasms and non-neoplastic processes. Here, we report such a case, focusing on the importance of its unusual morphological features, and discuss the differential diagnosis with other similar lesions.
CASE REPORT
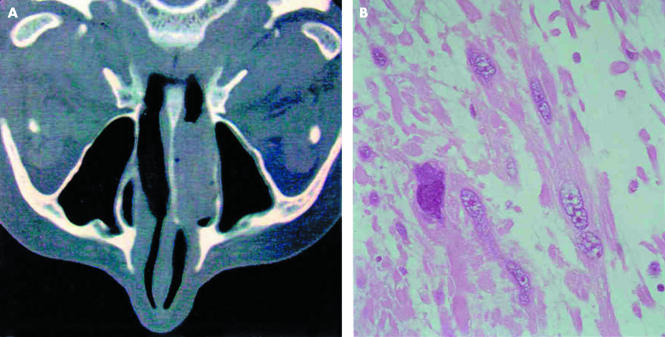
A previously healthy 72 year old woman was referred to the division of otorhinolaryngology because of a two month history of nasal obstruction, rhinorrea, and recurrent episodes of epistaxis. Rhinofibroscopic examination displayed a reddish polypoid mass involving the right inferior portion of the nasal fossa. A computed tomography (CT) scan confirmed a lesion occupying the whole inferior nasal fossa, whereas it excluded local invasion of the maxillary sinus and rhinopharynx (fig 1A). The lesion was completely excised with a small cuff of adjacent normal tissue. The intraoperative pathological sampling of the underlying bone rim was negative. No further treatment was given. The patient has not shown evidence of recurrence or metastases after 21 months of follow up as confirmed by a rhinofibroscopic examination and CT scan.
Figure 1.
(A) Axial computed tomography scan of the nasal sinuses shows a polypoid lesion occupying the whole inferior nasal fossa. (B) At histological examination, the tumour consisted of a proliferation of spindle shaped, pleomorphic cells with eosinophilic cytoplasm in a prominent myxoid background. Haematoxylin and eosin stained; original magnification, ×250.
Pathological findings
The specimen consisted of a mass measuring 3 cm at its greatest axis. Grossly, the lesion appeared as a firm, well demarcated, grey/whitish polypoid mass with gelatinous areas.
Histologically, a diffuse intersecting fascicle of spindle shaped cells with deeply eosinophilic cytoplasm and blunt ended nuclei covered by a partially eroded respiratory epithelium was seen. Many of these cells, widely distributed throughout the tumour, showed pleomorphic, pyknotic, and multilobated nuclei with prominent intranuclear invaginations. A large amount of dispersed, prominent basophilic acellular stroma was seen between the tumour cells, determining a loss of the predominant fascicular pattern (fig 1B). Perivascular cuffs of smooth muscle cells were noted around the vessels wall. The acellular matrix stained strongly with Alcian blue at pH 2.5 (digested by hyaluronidase), indicating that it consisted of myxoid material (fig 2A). Tumour cell necrosis or haemorrhagic areas were absent. The mitotic rate was low (0–1 mitosis/10 high power fields), and atypical figures were not found.
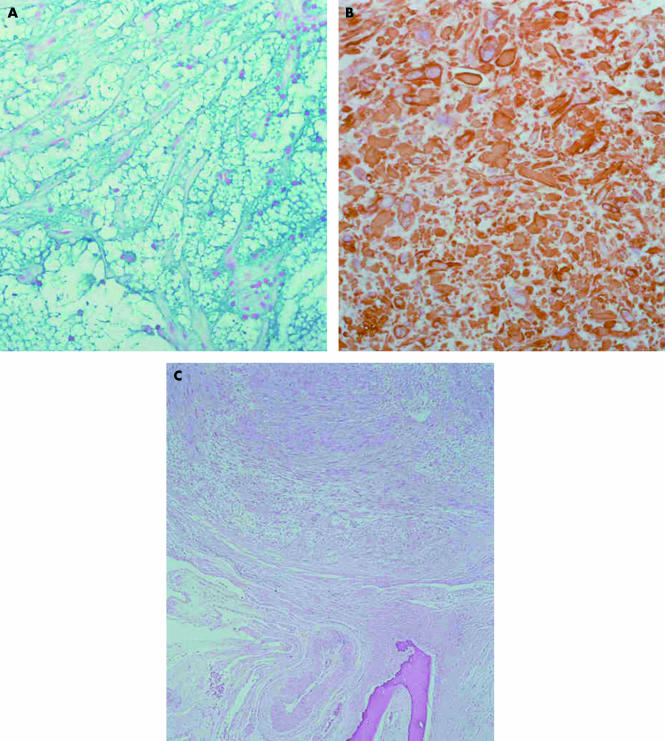
Figure 2.
(A) The prominent acellular myxoid stroma is positive for Alcian blue at pH 2.5. Histochemistry; original magnification, ×100. (B) The tumour cells display strong immunoreactivity with desmin. Immunohistochemistry; original magnification, ×100. (C) The tumour displays expansive growth margins and does not infiltrate the underlying bone of the nasal septum. Haematoxylin and eosin stained; original magnification, ×40.
The tumour cells stained strongly for vimentin, muscle specific actin, α smooth muscle actin, and desmin (fig 2B), but negatively for cytokeratins, CD34, glial fibrillary acid protein, and S-100 protein. The labelling index (Mib-1) was 3%, and p53 staining by means of immunohistochemistry was very low (about 3%). The tumour was diploid, as assessed by quantitative DNA analysis (FACScan, flow cytometer; Becton Dickinson, San Jose, California, USA). The margins between the lesion and the adjacent non-neoplastic tissue were well circumscribed but not encapsulated (fig 2C).
DISCUSSION
Several benign and malignant entities appear as a polypoid mass in the nasal cavity, with inflammatory polyps being by far the most common. A large study of 256 non-epithelial tumours of the nasal cavity, paranasal sinuses, and nasopharynx included only eight cases of smooth muscle origin (two classic leiomyomas and six leiomyosarcomas).1
Unlike previous reports of sinonasal leiomyogenic tumours, our case had pronounced pleomorphic cells coupled with a prominent myxoid background.
These morphological features may pose some difficulties in defining the malignant potential and in the differential diagnosis. Pathologists would probably recognise the reactive nature of the atypical mesenchymal cells in inflammatory or antrochoanal polyps. These bizarre cells are haphazardly dispersed in an oedematous stroma, typically concentrated in the submucosa or near vascular structures, and stain for fibrohistiocytic markers.6
“The careful assessment of mitotic activity, infiltrating margins, and coagulative tumour necrosis still remains the main criteria to determine the malignant behaviour of leiomyogenic neoplasms”
Therefore, the main differential diagnosis included a sarcomatoid carcinoma, a myxoma, and a myxoid leiomyosarcoma. The lack of surface epithelial alterations and of a high mitotic activity, and the negative immunostaining for cytokeratins, excluded a sarcomatoid/spindle cell carcinoma. The large amount of Alcian blue positive myxoid material indicated a loss of the fascicular pattern, giving the impression of a myxoid lesion. However, myxomas usually involve the maxillary sinus and appear as a uniform proliferation of scanty spindle/stellate cells floating in a myxoid background and do not stain for myogenic markers.7
In approaching this case, our main difficulty was in defining whether the lesion was benign or malignant. As pointed out by Enzinger et al,8 pronounced nuclear pleomorphism and myxoid change are the degenerative aspects of a long standing lesion, and are often seen in so called ancient schwannomas. Furthermore, as stated for uterine smooth muscle tumours, the careful assessment of mitotic activity, infiltrating margins, and coagulative tumour necrosis still remains the main criteria to determine the malignant behaviour of leiomyogenic neoplasms.9 In contrast to myxoid leiomyosarcoma, which shows at least one of these histological criteria,10 our case had none of the above findings, except for the presence of prominent nuclear atypia. In addition, the low labelling index and the indolent clinical course of the disease helped to rule out a diagnosis of malignancy. Finally, we would like to give some suggestions that might help with the diagnosis and characterisation of myofibroblastic lesions, which encompass a large spectrum of pathologies, ranging from reactive proliferations or benign tumours to overtly true sarcomas, and which can be reported at almost any anatomical site, as recently reviewed by Mentzel.11 Myofibroblastic lesions mainly consist of spindle shaped cells with a paler and less fibrillary cytoplasm than that of smooth muscle cells. Moreover, myofibroblasts usually are intersected by hyalinised collagenous, rather than myxoid, stroma and do not stain uniformly for actin or desmin, as is often seen in leiomyogenic tumours. We would like particularly to focus attention on myxoid nodular fasciitis, a lesion also included in the update of myxoid tumours by Graadt van Roggen et al.12 As in the present case, it is usually well circumscribed, displaying great cellularity in a prominent myxoid background and stains (at least weakly) for leiomyogenic markers. However, unlike the lesion reported here, myxoid nodular fasciitis occurs in the head and neck region of children and shows a rapid growth consisting of fibromyofibroblasts arranged in short fascicles without evidence of cytological atypia.12
Take home messages.
We report a case of atypical leiomyoma of the nasal cavity with prominent myxoid change
Immunohistochemical evidence for leiomyogenic markers coupled with the low mitotic rate, the lack of an infiltrating growth pattern, and the indolent clinical course led to the diagnosis
This tumour is extremely rare at this site, and its biological behaviour seems to be similar to its uterine counterpart
Clinicians should be aware of this occurrence to prevent misdiagnosis because a conservative therapeutic approach is needed
The histogenesis of atypical leiomyoma remains controversial, and whether it arises from the smooth muscle wall of blood vessels or from multipotential mesenchymal cells is still unresolved. Although the clinical behaviour of smooth muscle tumours is related to classic histological criteria and varies depending on different sites of origin, we suppose that extrauterine atypical leiomyomas, including those arising from the nasal cavity, have an outcome similar to their uterine counterpart, as recently suggested by Mahalingam and Goldberg in atypical pilar leiomyoma.13 Finally, atypical leiomyoma with myxoid change is a diagnostic challenge because of its heterogeneous appearance. Careful evaluation of the above described morphological features should enable the differential diagnosis to be made so that the correct surgical approach can be taken.
Acknowledgments
We thank Professor L Michaels for reviewing this case in consultation. This work was supported by a grant of the University of Modena and Reggio Emilia (found ex 60%).
REFERENCES
- 1.Fu YS, Perzin KH. Non-epithelial tumours of the nasal cavity, paranasal sinuses, and nasopharynx; a clinicopathologic study. IV. Smooth muscle tumours (leiomyoma, leiomyosarcoma). Cancer 1975;35:1300–8. [DOI] [PubMed] [Google Scholar]
- 2.Llorente JL, Suarez C, Seco M, et al. Leiomyoma of the nasal septum: report of a case and review of the literature. J Laryngol Otol 1996;110:65–8. [DOI] [PubMed] [Google Scholar]
- 3.Papavasiliou A, Michaels L. Unusual leiomyoma of the nose (leiomyoblastoma): report of a case. J Laryngol Otol 1981;95:1281–6. [DOI] [PubMed] [Google Scholar]
- 4.Murono S, Ohmura T, Sugimori S, et al. Vascular leiomyoma with abundant adipose cells of the nasal cavity. Am J Otolaryngol 1998;19:50–3. [DOI] [PubMed] [Google Scholar]
- 5.Van Ingen G, Stel HV, Tiwari RM. Atypical leiomyoma of the choana. J Laryngol Otol 1991;105:1065–7. [DOI] [PubMed] [Google Scholar]
- 6.Kindblom LG, Angervall L. Nasal polyps with atypical stromal cells: a pseudosarcomatous lesion. Acta Pathol Microbiol Immunol Scand 1984;92:65–72. [PubMed] [Google Scholar]
- 7.Gregor RT, Loftus-Coll B. Myxoma of the paranasal sinuses. J Laryngol Otol 1994;108:679–81. [DOI] [PubMed] [Google Scholar]
- 8.Enzinger FM, Weiss SW. Benign tumors of smooth muscle. In: Enzinger FM, Weiss SW, eds. Soft tissue tumors, 4th ed. St Louis, MO: Mosby, 2001:695–726.
- 9.Downes KA, Hart WR. Bizarre leiomyomas of the uterus: a comprehensive pathologic study of 24 cases with long-term follow-up. Am J Surg Pathol 1997;21:1261–70. [DOI] [PubMed] [Google Scholar]
- 10.Rubin BP, Fletcher CDM. Myxoid leiomyosarcoma of soft tissue, an underrecognized variant. Am J Surg Pathol 2000;24:927–36. [DOI] [PubMed] [Google Scholar]
- 11.Mentzel T. Myofibroblastic sarcomas: a brief review of sarcomas showing a myofibroblastic line of differentiation and discussion of the differential diagnosis. Curr Diagn Pathol 2001;7:17–24. [Google Scholar]
- 12.Graadt van Roggen JF, Hogendoorn PCW, Fletcher CDM. Myxoid tumours of soft tissue. Histopathology 1999;35:291–312. [DOI] [PubMed] [Google Scholar]
- 13.Mahalingam M, Goldberg LJ. Atypical pilar leiomyoma. Cutaneous counterpart of uterine symplastic leiomyoma? Am J Dermatopathol 2001;23:299–303. [DOI] [PubMed] [Google Scholar]